Abstract
The prototypic arenavirus lymphocytic choriomeningitis virus (LCMV) is a formidable battle horse for the study of viral immunology, as well as viral persistence and associated diseases. Investigations with LCMV have uncovered basic mechanisms by which viruses avoid elimination by the host adaptive immune response. In this study we show that LCMV also disables the host innate defense by interfering with beta interferon (IFN-β) production in response to different stimuli, including infection with Sendai virus and liposome-mediated DNA transfection. Inhibition of IFN production in LCMV-infected cells was caused by an early block in the IFN regulatory factor 3 (IRF-3) activation pathway. This defect was restored in cells cured of LCMV, indicating that one or more LCMV products are responsible for the inhibition of IRF-3 activation. Using expression plasmids encoding individual LCMV proteins, we found that expression of the LCMV nucleoprotein (NP) was sufficient to inhibit both IFN production and nuclear translocation of IRF-3. To our knowledge, this is the first evidence of an IFN-counteracting viral protein in the Arenaviridae family. Inhibition of IFN production by the arenavirus NP is likely to be a determinant of virulence in vivo.
Arenaviruses merit significant attention both as tractable model systems to study acute and persistent viral infections (28, 51) and as clinically important human pathogens, including several causative agents of severe hemorrhagic fever, chiefly Lassa fever virus (LFV) (8, 14). In addition, the prototypic arenavirus lymphocytic choriomeningitis virus (LCMV) has proven to be a Rosetta stone in the fields of viral immunology and pathogenesis (28, 51).
LCMV is an enveloped virus with a bisegmented RNA genome (8, 25). Each segment, designated L (ca 7.2 kb) and S (ca 3.4 kb), expresses two viral gene products using an ambisense coding strategy. The S RNA directs the synthesis of the nucleoprotein NP and the glycoprotein precursor GPC. The NP, the most abundant viral protein, encapsidates viral genomes and antigenomic replicative intermediates. GPC is posttranslationally cleaved by the cellular subtilase S1P into mature viral glycoproteins, GP-1 and GP-2 (5, 34). Noncovalently associated GP-1/GP-2 complexes make up the spikes on the virion envelope and mediate virus interaction with the host cell receptor (20). The L segment codes for the virus RNA-dependent RNA polymerase (L) and a small (11-kDa) RING finger protein called Z that functions as the arenavirus counterpart of the matrix protein found in many negative-strand RNA viruses (32, 42). Additional roles of Z in the arenavirus life cycle have been proposed on the basis of its interaction with several host cell proteins (8, 25) and its ability to inhibit RNA synthesis mediated by the virus polymerase (17, 25).
The mechanisms underlying arenavirus hemorrhagic fever disease are not understood. Individuals succumbing to LF generate only minimal or no anti-LFV immune response, while those recovering from LF disease show evidence of both T- and B-cell responses against LFV (18, 24). Histological examination of tissues from LF patients shows minimal cellular damage and only very modest immune cell infiltrates (44). These findings suggest that the host's inability to mount an effective antiviral immune response contributes to LFV morbidity and lethality. Accordingly, the extent of viremia is a good predictor for the outcome of LFV infection (18).
The adaptive immune response provides the host with a robust and long-term antiviral defense, but it does not reach full efficacy for days or weeks. In contrast, the host innate response is elicited very rapidly upon infection and provides the host with early protection and critically influences the subsequent adaptive immune response (4). The balance between the quality and magnitude of the host innate immune responses and the corresponding viral counteracting activities often influences viral pathogenicity. Type I interferons (IFNs) play key roles in both the innate and adaptive immune response of the host against viral infections (7). Expression of type I IFN is controlled by latent transcription factors including the IFN regulatory factor 3 (IRF-3). Upon activation via cellular “sensors,” such as Toll-like receptors or cytoplasmic RNA helicases (49), IRF-3 becomes phosphorylated and undergoes homodimerization and nuclear translocation (38). Once in the nucleus, IRF-3 interacts with IRF-3-responsive promoters and the transcriptional coactivator histone acetyltransferase CBP/p300, leading to the transcription of IRF-responsive genes, and together with NF-κB and AP-1, IRF-3 also promotes transcription of beta IFN (IFN-β). The mechanisms by which viruses activate the IκB kinases and TANK-binding kinase 1 that activate IRF-3 are little understood, but double-stranded RNA (dsRNA) generated during viral infection is thought to be one of the main elements responsible for the transcriptional induction of type I IFNs. Recently, the cytoplasmic RNA helicases retinoic acid inducible gene I (RIG-I) and melanome differentiation-associated gene 5 have been proposed to bind to viral dsRNA, resulting in activation of the IRF-3 kinases. Binding of RIG-I to dsRNA stimulates its ATPase/helicase activity, resulting in exposure of its N-terminal caspase recruitment domain (CARD), which recruits other cellular factors including IPS-1, also known as VISA, CARDIF, and MAVS (mitochondrial antiviral signaling protein) (19, 26, 39, 47), thus leading to the activation of the IRF-3 kinases and production of IFN-α/β. Secreted IFN-β binds to its cell surface receptor and activates the JAK/STAT signaling pathway, leading to transcription activation of IFN-stimulated genes (ISGs), including those with antiviral activities like PKR and Mx. Many viruses have developed mechanisms that antagonize the IFN response by inhibiting IFN induction, its signaling, or both (46). Here we show that LCMV NP blocks the nuclear translocation and transcriptional activity of IRF-3, which results in a robust inhibition of type I IFN production. This IFN-counteracting activity of the arenavirus NP may contribute to the failure of the host innate antiviral response to control the multiplication of pathogenic arenaviruses.
MATERIALS AND METHODS
Cells and viruses.
BHK-21, 293T, and Vero cells were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum and penicillin/streptomycin. A549 cells and A549/LCMV-Pi cells (A549 cells persistently infected with LCMV) were maintained in Dulbecco modified Eagle medium with 10% fetal bovine serum and penicillin/streptomycin. LCMV titers were determined by plaque assay on Vero cells as described previously (10). Sendai virus (SeV) and Newcastle disease virus (NDV) expressing green fluorescent protein (GFP) (NDV-GFP) were grown in 10-day-old embryonated eggs (3, 31). Vesicular stomatitis virus (VSV) expressing GFP (VSV-GFP) was grown in BHK-21 cells (41).
Plasmids.
pHISG54-GFP/CAT, pIFNβ-GFP/CAT, pHISG54-RFP/CAT, and pIFNβ-RFP/CAT express the green fluorescent protein or the monomeric red fluorescence protein (RFP) cDNA (9) fused to chloramphenicol acetyltransferase (CAT) under the control of the interferon-stimulated gene 54 (ISG54) or IFN-β promoters. These plasmids were made by introducing the GFP or RFP open reading frame upstream of the CAT open reading frame in pHISG54-CAT (6) or pIFNβ-CAT (45). p55C1B-FF has been described previously (48). Plasmids pC-L, pC-NP, pC-Z, and pC-GP expressing the polymerase (L), nucleoprotein (NP), Z and glycoprotein (GP) of LCMV-ARM, respectively, have been described previously (32). pEGFP-C1-hIRF3 and pCAGGs firefly luciferase expression plasmid have been described previously (2). pC-IRF3(DN) expresses a dominant-negative form of IRF-3 (ΔN IRF-3) corresponding to an N-terminal truncation of 133 residues that was generated by PCR using as a template pCAGGs IRF-3 (2). Plasmids expressing influenza A/PR/8/34 virus NS1 (3) and Nipah virus W (31) have been described previously.
Persistent infection of A549 cells with LCMV and ribavirin treatment.
To generate A549/LCMV-Pi cells, we infected A549 cells with LCMV-ARM (multiplicity of infection [MOI] of 0.1). At 72 h postinfection (p.i.), we subcultured the cells to generate A549/LCMV-Pi p1. After two additional passages, A549/LCMV-Pi p3 contained a majority of cells that expressed viral antigens (>95%), as determined by immunofluorescence (IF), and harbored infectious virus (>90%), as determined by infectious center assay (11). A549/LCMV-Pi cells were cured of LCMV infection by treatment with ribavirin (RB) as described previously (10, 36).
Quantitative reverse transcription-PCR (qRT-PCR) analysis.
RNA was isolated from A549 cells or A549/LCMV-Pi cells by TRIzol following the manufacturer's conditions (Invitrogen) at 24 h postinfection or post-IFN treatment. RNA (100 ng) was used to measure mRNA levels of MxA, IFN-β, IFI56, and actin by using specific primers (see the legend to Fig. 2) and SYBR green in an ABI7900 HT instrument as described previously (50).
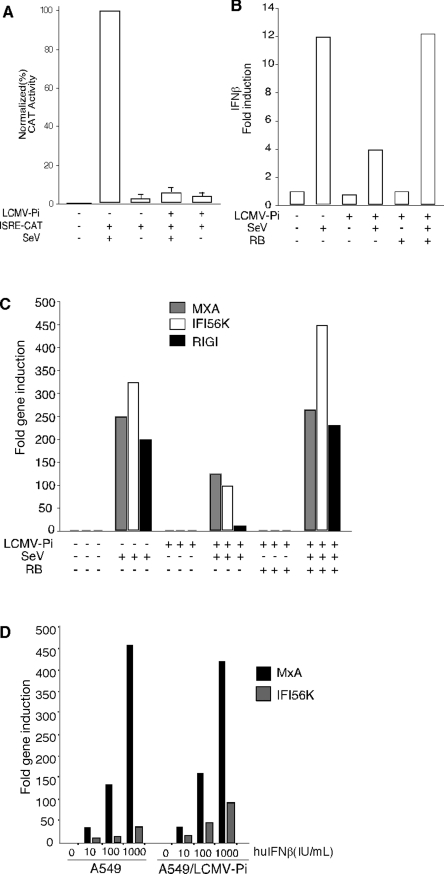
FIG. 2.
SeV-mediated activation of an ISRE promoter, as well as induction of IFN and ISGs, but not the IFN-β induced antiviral state, are inhibited in A549/LCMV-Pi cells. (A) A549 and A549/LCMV-Pi cells were transfected with 1 μg of the ISRE-CAT reporter plasmid (+). After transfection, cells were mock infected (−) or infected with SeV (+). Twenty-four hours later, cell lysates were prepared for CAT assays. (B, C) A549, A549/LCMV-Pi, and RB-cured A549/LCMV-Pi cells were mock infected (−) or infected with SeV (+). At 24 h p.i., total RNA was isolated and quantitative RT-PCR was performed by using specific primers (shown below) to determine the levels of IFN-β mRNA (B), as well as MxA, IFI56K, and RIG-I mRNA (C). (D) Persistence of LCMV in A549 cells does not prevent the type I IFN-induced antiviral state. A549 and A549/LCMV-Pi cells were treated with human IFN-β (huIFNβ) (0, 10, 100, and 1,000 U/ml). Twenty-four hours after IFN treatment, total RNA was isolated and mRNA levels for MxA and IFI56K were determined by qRT-PCR. RT was done using random hexamers as primers. The gene-specific primers used for qPCR were as follows: IFN-β (sense, 5′-GTCAGAGTGGAATCCTAAG-3′; antisense, 5′-ACAGCATCTGCTGGTTGAAG-3′); Mx1 (sense, 5′-CGTGGTGATTTAGCAGGAAG-3′; antisense, 5′-TGCAAGGTGGAGCGATTCTG-3′); RIG-I (sense, 5′-AAAGCCTTGGCATGTTACAC-3′; antisense, 5′-GGCTTGGGATGTGGTCTACT-3′); IFI56K (sense, 5′-TCGGAGAAAGGCATTAGATC-3′; antisense, 5′-GACCTTGTCTCACAGAGTTC-3′); and actin (sense, 5′-ACTGGAACGGTGAAGGTGAC-3′; antisense, 5′-GTGGACTTGGGAGAGGACTG-3′).
Immunofluorescence.
SeV-infected cells were fixed in 2.5% formaldehyde and permeabilized with 0.1% NP-40. Cells were incubated with SeV monoclonal antibodies 11F3 and 5F5 (1 μg/ml) (gift from C. Lopez) for 1 h, followed by incubation with a fluorescein isothiocyanate (FITC)-conjugated anti-mouse antibody (DAKO Laboratories) (1:100 in 1% bovine serum albumin) for 30 min. Detection of LCMV antigens by IF was done using a guinea pig serum to LCMV as described previously (7). Samples were mounted using Mowiol and analyzed by fluorescence microscopy, and images were digitized using Adobe Photoshop and Canvas software.
NDV-GFP assay for inhibitors of IFN signaling.
Vero cells were transfected with 2 μg of the indicated expression plasmids using Lipofectamine (LF) 2000 (Invitrogen). Fourteen hours later, cells were treated (1,000 IU/ml) with human IFN-β (Calbiochem) for 24 h and then infected with NDV-GFP (MOI of 2), and 16 h later, GFP expression was examined by fluorescence microscopy (27).
Nuclear translocation of IRF-3.
Vero cells were transfected with 1 μg of pEGFP-C1-hIRF3 (1 μg) together with the indicated expression plasmids (2 μg each) using LF 2000 (2). Fourteen hours later, cells were washed twice with phosphate-buffered saline and infected with SeV for 1 hour at 37°C. After removal of virus inoculum, fresh medium was added, and nuclear translocation of IRF-3 was visualized by epifluorescence at 12 to 16 h p.i.
Reporter assays.
293T cells were cotransfected by calcium phosphate with 0.5 μg of GFP/CAT or RFP/CAT reporter plamids and 4 μg of the indicated expression plasmids together with a luciferase-expressing plasmid (1 μg). Fourteen hours later, cells were washed with phosphate-buffered saline and infected with SeV. At 24 h p.i., GFP or RFP expression was detected by epifluorescence and cell lysates were prepared for luciferase and CAT assay. CAT activity was normalized using luciferase values. The same protocol was used to transfect 293T cells with the IRF3 promoter reporter plasmid, p55C1B-FF, but using as a control an expression plasmid encoding Renilla luciferase under a simian virus 40 promoter. For reporter assays in A549 cells, transfections were done using the Amaxa nucleofection technology. A549 cells (106 cells) in 100 μl of solution T (nucleofector kit) were mixed with plasmid DNA (2 μg), and nucleofection was done using program A-31 in an Amaxa Nucleofector apparatus.
NDV-GFP and VSV-GFP bioassays.
Supernatants from transfected or virus-infected cells or cells both transfected and infected with virus were inactivated for 10 min under UV light and added to fresh Vero cells. Sixteen hours later, cells were infected with NDV-GFP (MOI of 2) or VSV-GFP (MOI of 2), and at 24 h p.i., GFP expression was monitored by epifluorescence. As positive controls, we used Vero cells treated with the indicated amounts of IFN-β. The assay was validated by examining the effect of a sheep polyclonal antibody against human IFN-β (Biomedical Laboratories) (dilution of 1/100) in control experiments.
RESULTS
Effect of LCMV infection on the cell antiviral state induced by liposome-mediated DNA transfection.
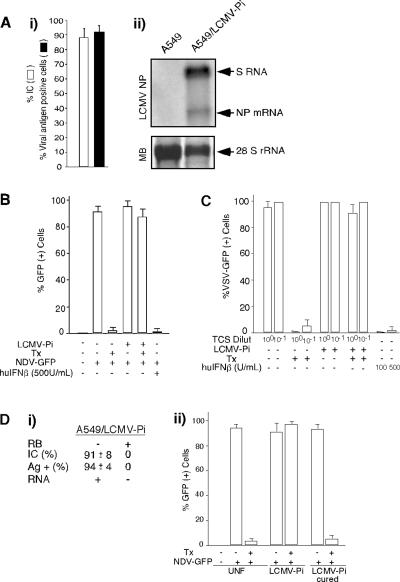
To investigate whether LCMV could inhibit the type I IFN response, we used an NDV-GFP complementation assay based on the ability of Lipofectamine/DNA transfection (Tx) to induce a robust IFN-mediated antiviral state in several cell lines, which prevents replication of NDV-GFP (30, 31). For these studies we use A549 cells derived from a human lung adenocarcinoma, as these cells are widely used to study virus-IFN interactions and support long-term LCMV persistent infection (A549/LCMV-Pi) (Fig. 1A). Both nontransfected and LF/DNA-transfected A549/LCMV-Pi cells supported similarly high levels of NDV-GFP multiplication (Fig. 1B), whereas as predicted, LF/DNA transfection of A549 cells induced an antiviral state that prevented NDV-GFP replication (Fig. 1B). This suggested that the type I IFN response induced by LF/DNA transfection was blocked in A549/LCMV-Pi cells. Accordingly, treatment of Vero cells with tissue culture supernatant (TCS) from LF/DNA-transfected A549 cells induced an antiviral state that inhibited VSV-GFP replication (Fig. 1C). In contrast, treatment of Vero cells with TCS from transfected A549/LCMV-Pi cells or nontransfected A549 or A549/LCMV-Pi cells did not inhibit VSV-GFP multiplication (Fig. 1C). Treatment with sheep serum to human IFN-β eliminated the VSV inhibitory activity associated with the TCS from transfected A549 cells (not shown). Moreover, A549/LCMV-Pi cells cured of LCMV by treatment with ribavirin (Fig. 1D, panel i) recovered the ability to create an antiviral state in response to LF/DNA transfection (Fig. 1D, panel ii).
FIG. 1.
Induction of type I IFN is inhibited in A549/LCMV-Pi cells. (A) Persistence of LCMV in A549 cells. A549 cells were infected with LCMV (MOI of 0.1), and 72 h later, cells were subcultured to establish a persistently infected line (A549/LCMV-Pi). (i) Numbers of cells expressing viral antigen and harboring infectious virus in A549/LCMV-Pi cells were assessed by IF and infectious center assay, respectively. The percentage of infectious centers (IC) was determined as described previously (11). (ii) Levels of viral RNA were determined by Northern blotting using a NP probe to detect S RNA (replication) and NP mRNA (transcription). MB, methylene blue staining of the membrane to detect 28S rRNA. (B) A549 or A549/LCMV-Pi cells were mock transfected (Tx −) or transfected (Tx +) with 2 μg of empty pC plasmid (2, 32). Twenty-four hours posttransfection, cells were infected with NDV-GFP (MOI of 2) (+), and at 24 h p.i., GFP expression was assessed. For a control, A549 cells were treated with 500 IU/ml of human IFN-β (huIFNβ) (+). (C) Vero cells treated (12 h) with TCS from A549 cells or A549/LCMV-Pi cells that had been mock transfected or transfected with empty plasmid. Treated Vero cells were infected with VSV expressing GFP (MOI of 1). TCS from A549 cells treated with human IFN-β were used as controls. Dilut, dilutions. (D) Cells cured of LCMV infection restored their ability to produce type I IFN in response to LF-mediated DNA transfection. (i) Characterization of cells cured of LCMV infection by RB treatment. Ag +, antigen positive. (ii) A549, A549/LCMV-Pi, and RB-treated A549/LCMV-Pi were mock transfected or transfected with empty plasmid. Sixteen hours after transfection, cells were infected with NDV-GFP (MOI of 2), and GFP expression was determined at 24 h p.i. UNF, uninfected.
Effect of LCMV persistence on SeV-mediated activation of an ISRE promoter and production of IFN-β and ISG mRNAs.
We next investigated whether LCMV persistence interfered with IFN-β production triggered by SeV infection. For this, we used a nucleofection protocol, which does not induce an IFN response, to transfect A549 and A549/LCMV-Pi cells with plasmid pHISG54-CAT. At 24 h posttransfection, we infected the cells with SeV to cause IRF-3 activation and subsequent induction of type I interferon-responsive element (ISRE) promoters, and at 24 h p.i., we prepared cell lysates for CAT activity. SeV-mediated activation of the ISRE promoter was blocked in A549/LCMV-Pi cells (Fig. 2A). Both A549 and A549/LCMV-Pi cells were equally susceptible to SeV infection (data not shown), indicating that the inhibition of the ISRE promoter was not due to reduced levels of SeV replication in A549/LCMV-Pi cells.
We next used qRT-PCR to determine mRNA levels of IFN-β (Fig. 2B) and ISGs MxA, IFI56K, and RIG-I (Fig. 2C) in A549 and A549/LCMV-Pi cells upon SeV infection. As predicted, SeV-infected A549 cells showed upregulation of these mRNAs. In contrast, induction of these mRNAs was drastically reduced in A549/LCMV-Pi cells, whereas normal levels of SeV induction of ISGs were restored in A549/LCMV-Pi cells cured of LCMV by RB treatment. A549/LCMV-Pi cells exposed to exogenous IFN-β exhibited increased levels of MxA and IFI56K mRNAs (Fig. 2D), indicating that LCMV persistence does not prevent the type I IFN signaling but rather blocks production of endogenous IFN-β in response to infection.
Effects of individual LCMV polypeptides on SeV-induced activation of IFN-β and ISRE promoters.
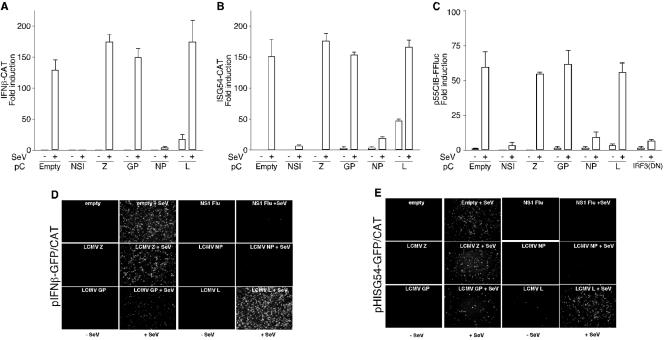
To determine whether a specific LCMV gene product was responsible for the inhibition of IFN-β production in A549/LCMV-Pi cells, we transfected 293T cells with plasmids expressing each of the LCMV proteins individually together with pIFNβ-GFP/CAT or pHISG54-GFP/CAT reporter plasmid. Transfections were done using CaPO4, which does not induce IFN-β production and the subsequent antiviral state (23). We used transfection of the influenza A virus NS1 as a control of a gene that effectively inhibits the activation of these two promoters. Twenty-four hours after transfection, cells were mock or SeV infected, and at 24 h p.i., cells were analyzed for CAT activity (Fig. 3A and B) and GFP expression by epifluorescence (Fig. 3D and E). Cells transfected with pC plasmids expressing LCMV Z, GP, or L protein did not block SeV-mediated activation of the IFN-β or ISRE promoters. In contrast, expression of LCMV-NP protein blocked the activation of these two promoters to levels similar to those of the influenza A virus NS1 protein. We confirmed the expression and function of LCMV Z, GP, and L proteins by Western blotting and production of virus-like particles in an LCMV minigenome rescue assay (32), respectively (not shown).
FIG. 3.
LCMV NP inhibits SeV-mediated activation of IFN-β, ISRE, and IRF promoters. Cells (293T) were cotransfected (+) with 0.5 μg of the different reporter plasmids, together with 4 μg of the indicated LCMV expression plasmids, and 24 h later, cells were mock infected (−) or infected with SeV (+). CAT (A and B), luciferase (C), and GFP (D and E) expression was determined 24 h p.i.
LCMV-NP and NS1, as well as a dominant-negative form of IRF-3, exhibited a similar inhibitory activity on the expression of a luciferase reporter gene under an IRF-3-dependent promoter (p55C1B-FF luc) (Fig. 3C). Influenza virus NS1 and LCMV NP did not inhibit SeV multiplication (data not shown). These data uncovered NP as the sole virus gene product responsible for the IFN antagonist activity in LCMV-infected cells and indicated that LCMV-NP can specifically inhibit IRF-3-dependent transcription.
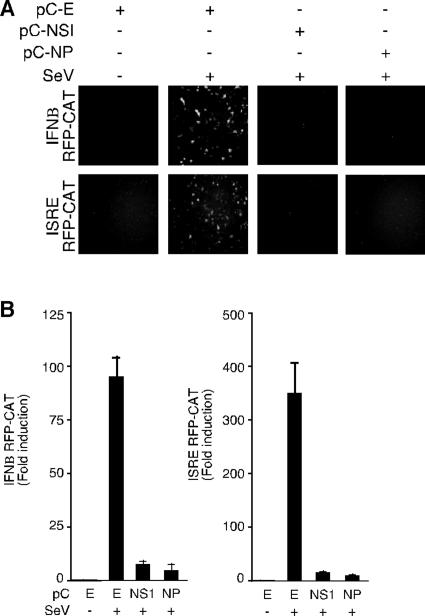
Effect of LCMV-NP on SeV-induced IFN-β production.
To examine the effect of LCMV NP on transcriptional activation of endogenous IFN-β, we transfected 293T cells with pIFNβ-RFP/CAT or pHISG54-RFP/CAT alone or together with pC-NP or pC-NS1. Twenty-four hours later, cells were infected with SeV, and at 24 h p.i., cells were analyzed for RFP and CAT expression (Fig. 4A and B, respectively). Both LCMV NP and influenza virus NS1 gene products inhibited both RFP and CAT expression with similar efficiencies. Consistent with the reporter gene expression results, TCS from cells infected with SeV and transfected with either NS1 or NP had dramatically reduced levels of IFN compared with SeV-infected and mock-transfected cells, as determined by their respective effects on the NDV-GFP bioassay in Vero cells (Fig. 5). Incubation with sheep anti-human IFN-β polyclonal serum eliminated the inhibitory activity in the NDV-GFP bioassay of TCS from cells transfected with empty plasmid and infected with SeV (data not shown). Together, these findings indicated that LCMV-NP inhibited the cellular production of IFN-β in response to SeV infection.
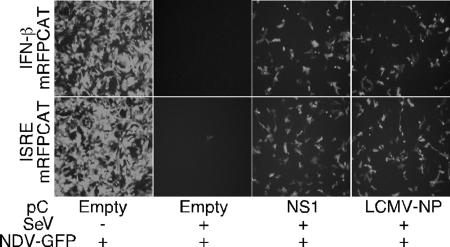
FIG. 4.
SeV-mediated activation of IFN-β and ISRE promoters is inhibited in LCMV-NP-transfected cells. 293T cells were transfected as described in the legend to Fig. 3, but using the monomeric red fluorescence protein fused to CAT under the IFN-β (IFN-β mRFP-CAT) or ISRE (ISRE mRFP-CAT) promoter. Twenty-four hours later, cells were mock infected (−) or infected with SeV (+), and 16 h later, activation of the reporter promoters was assessed by epifluorescene and CAT assay. (A) Expression of monomeric RFP was examined by epifluorescence microscopy. (B) Normalized CAT expression levels are shown as changes in induction compared to the values obtained for uninfected cells that were transfected with an empty (E) control plasmid.
FIG. 5.
NDV-GFP bioassay. Supernatants from transfected and infected 293T cells shown in Fig. 4 were treated with UV and added to fresh Vero cells. Sixteen hours later, cells were infected with the NDV-GFP (MOI of 2), and at 24 h p.i., GFP expression was detected by epifluorescence.
Effect of LCMV-NP on nuclear translocation of IRF-3.
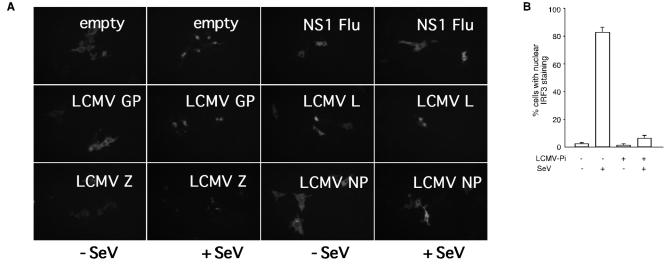
Nuclear translocation is a hallmark of IRF-3 activation. Therefore, we examined the ability of LCMV-NP to prevent IRF-3 nuclear activation using a GFP-tagged IRF-3 expression plasmid. We transfected Vero cells with GFP-tagged hIRF-3 together with either an empty plasmid or plasmids expressing LCMV NP, L, GP, or Z protein. Twenty-four hours after transfection, cells were infected with SeV, and 14 h later GFP-IRF-3 subcellular localization was examined by fluorescence microcopy. Cells transfected with empty plasmid and infected with SeV, but not mock-infected controls, showed a nuclear localization of GFP-tagged IRF-3. Likewise, cells transfected with plasmids expressing LCMV L, Z, or GP protein exhibited IRF-3 nuclear localization upon SeV infection. In contrast, cells transfected with either LCMV-NP or with influenza virus NS1 exhibited a severe inhibition of nuclear translocation of IRF-3 in response to SeV infection (Fig. 6A). SeV-induced nuclear translocation of IRF-3 was also inhibited in A549/LCMV-Pi, but not in A549 control, cells (Fig. 6B).
FIG. 6.
Nuclear translocation of IRF-3 is inhibited in both LCMV-PI and LCMV-NP-transfected cells. (A) Vero cells were cotransfected with GFP-tagged IRF-3 (1 μg) together with 4 μg of the indicated LCMV expression plasmids. Twenty-four hours later, cells were mock infected or infected with SeV, and at 16 h p.i. nuclear translocation of GFP-tagged IRF-3 was assessed by epifluorescence. (B) LCMV persistently infected (LCMV-Pi) and control Vero cells were transfected with GFP-tagged IRF-3, and 24 h later, cells were mock infected or infected with SeV. At 16 h p.i. we determined the percentage of cells showing nuclear translocation of GFP-IRF3 in mock-infected or LCMV-Pi Vero cells that were also either mock infected or infected with SeV.
Effects of LCMV gene products on the antiviral state induced by interferon.
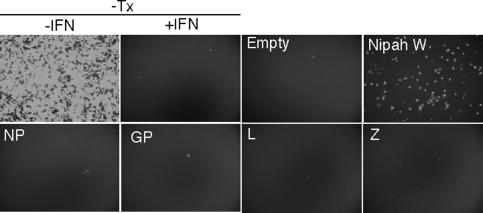
To examine whether LCMV proteins could also prevent the antiviral state induced by cell exposure to IFN, we transfected Vero cells with plasmids expressing each of the LCMV gene products and examined their effects on the antiviral state induced upon IFN treatment (Fig. 7). We selected Vero cells for this assay because they do not produce IFN but maintain a functional response to exogenously added type I IFN. For controls, we transfected Nipah W, a well-known IFN signaling antagonist (40). Twenty-four hours after transfection, cells were treated with human IFN-β, and 12 h later, they were infected with NDV-GFP. Replication of NDV-GFP was monitored by fluorescence microscopy. Proteins that inhibit IFN signaling, like Nipah W, facilitate replication of the IFN-sensitive NDV-GFP in the transfected cells. Cells that were not treated with IFN showed high levels of NDV multiplication as assessed by GFP expression. Treatment of nontransfected cells or cells transfected with empty plasmid with IFN prior to infection completely inhibited NDV replication. Consistent with previous data, cells expressing Nipah W promoted NDV-GFP replication regardless of IFN treatment. NDV-GFP replication was inhibited in cells transfected with each individual LCMV gene product, L, NP, GP, or Z. These results correlated with our observation that A549/LCMV-Pi cells were not able to inhibit the antiviral state after treatment with human IFN-β (data not shown). Together, these findings indicate that under the experimental conditions assayed, none of the LCMV individual gene products counteracts the type I IFN signaling leading to the establishment of an antiviral cell state.
FIG. 7.
Expression of LCMV proteins does not prevent the antiviral state induced by interferon. Vero cells were transfected with 2 μg of the indicated expression plasmids, and 24 h later, the cells were treated with human IFN-β (1000 U/ml). Twenty-four hours after IFN treatment, cells were infected with NDV-GFP. A negative control (empty plasmid) and a positive control (Nipah W) were used to validate the assay. Additional controls included Vero cells (top panels) not treated (−IFN) or pretreated (+IFN) with human IFN-β (1,000 U/ml). −Tx, nontransfected cells.
DISCUSSION
We document here for the first time an arenavirus IFN antagonist. LCMV infection impaired the cell's ability to induce transcriptional activation of the IFN-β promoter in response to LF/DNA transfection or SeV infection. This resulted in highly reduced levels of IFN-β secretion and subsequent low levels of transcriptional activation of ISGs. This inhibition of IFN-β induction was mediated by the virus NP (Fig. 3, 4, and 5), which blocked SeV-induced IRF-3 activation (Fig. 6). IRF-3 is a key transcriptional factor in induction of type I IFN, and thereby NP-mediated inhibition of IRF-3 activation likely interferes with IFN-β promoter activation in LCMV-infected cells. Consistent with an NP-mediated inhibition of IRF-3 activation, NP caused transcriptional inhibition of several other IRF-3-inducible promoters (Fig. 3).
A variety of viruses, including both RNA and DNA viruses, express gene products that target IRF-3 to inhibit IFN production by the infected cells (15). Virus inhibition of IRF-3 can be achieved by targeting steps downstream of IRF-3 activation, as well as upstream activators of IRF-3 phosphorylation. For example, the P protein of Borna disease virus inhibits IRF-3 phosphorylation by the upstream kinase TANK-binding kinase 1 (43). Likewise, inhibition of the activation of IRF-3 kinases at the level of the upstream activators IPS-1 and melanome differentiation-associated gene 5 has been reported for hepatitis C virus and paramyxoviruses, respectively (13, 35). The plethora of strategies used by viruses to counteract IRF-3 activation likely reflects a key role of IRF-3 in the host innate response to viruses. We have now extended this concept to the arenaviruses. Additional studies will be needed to determine at what level LCMV NP inhibits IRF-3 activation. Nevertheless, the lack of IRF-3 nuclear translocation in the presence of LCMV NP would suggest the inhibition of upstream processes associated with IRF-3 phosphorylation. To our knowledge, this is the first example of a viral nucleoprotein from a negative-strand RNA virus with IRF-3 inhibitory properties. This observation is in contrast to the IRF-3 activating properties associated with ribonucleoprotein complexes of other negative-strand RNA viruses, such as measles virus and VSV (38, 41).
Type I IFN is strongly induced at early times during LCMV infection of mice (21, 33). However, this response is thought to be driven mainly by mechanisms intrinsic to the biology of the host, whereas LCMV appears to be a poor direct inducer of type I IFN. The latter may be related to the virus's ability to selectively curtail some IFN signaling pathways, including the IRF-3-dependent type I IFN production upon poly(IC) challenge (12). We have obtained preliminary evidence of an impaired type I IFN production following poly(IC) stimulation in LCMV-infected mice, which would be consistent with our data showing a strong NP-mediated inhibition of IRF-3 activation (Fig. 6). It will be important to determine whether the NP from different members of the Arenaviridae differ in their ability to inhibit the induction of type I IFN and whether such differences are related to virulence. In this respect, LFV, but not the less pathogenic Mopeia virus, was shown to be an efficient inhibitor of type I IFN and cytokine production (1, 22, 29). Inhibition of type I IFN induction is likely to have important consequences not only for virus evasion of innate immune responses but also in modulating the quality and magnitude of the host adaptive immunity (16). A detailed knowledge of the mechanisms underlying the IFN-counteracting activity of the LCMV NP will contribute to a better understanding of the pathogenicity and immunogenicity of arenavirus infections. We have recently documented the establishment of reverse genetics procedures for the rescue of infectious LCMV entirely from cloned cDNAs (37). This development would facilitate the generation of recombinant LCMV to examine the biological implications of the IFN-counteracting activity of NP in the context of the natural course of both acute and persistent LCMV infections. Knowledge derived from such studies may uncover new insights about arenavirus virulence and could open new avenues for the generation of highly attenuated arenaviruses that could be considered as vaccine candidates.
Acknowledgments
We thank J. Hiscott, D. Levy, T. Fujita, M. Shaw, and R. Tsien for providing plasmids; C. Lopez for SeV monoclonal antibodies; R. Cadagan for technical support; A. Fernandez-Sesma and S. Sealfon for technical help with the qRT-PCR measurements; and W. Cardenas for advice and technical support.
The work of A.G.-S. and L.M.-S. was partially supported by CIVIA, a NIH center grant (U19 AI62623), and the DoD. The work of J.C.D.L.T, E.I.Z., and D.R. was supported by a NIH grant (AI47140) to J.C.D.L.T.
REFERENCES
- 1.Baize, S., J. Kaplon, C. Faure, D. Pannetier, M. C. Georges-Courbot, and V. Deubel. 2004. Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J. Immunol. 172:2861-2869. [DOI] [PubMed] [Google Scholar]
- 2.Basler, C. F., A. Mikulasova, L. Martinez-Sobrido, J. Paragas, E. Muhlberger, M. Bray, H. D. Klenk, P. Palese, and A. Garcia-Sastre. 2003. The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J. Virol. 77:7945-7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basler, C. F., X. Wang, E. Muhlberger, V. Volchkov, J. Paragas, H. D. Klenk, A. Garcia-Sastre, and P. Palese. 2000. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc. Natl. Acad. Sci. USA 97:12289-12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutler, B. 2004. Innate immunity: an overview. Mol. Immunol. 40:845-859. [DOI] [PubMed] [Google Scholar]
- 5.Beyer, W. R., D. Popplau, W. Garten, D. von Laer, and O. Lenz. 2003. Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P. J. Virol. 77:2866-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bluyssen, H. A., R. J. Vlietstra, A. van der Made, and J. Trapman. 1994. The interferon-stimulated gene 54 K promoter contains two adjacent functional interferon-stimulated response elements of different strength, which act synergistically for maximal interferon-alpha inducibility. Eur. J. Biochem. 220:395-402. [DOI] [PubMed] [Google Scholar]
- 7.Bonjardim, C. A. 2005. Interferons (IFNs) are key cytokines in both innate and adaptive antiviral immune responses—and viruses counteract IFN action. Microbes Infect. 7:569-578. [DOI] [PubMed] [Google Scholar]
- 8.Buchmeier, M. J., M. D. Bowen, and C. J. Peters. 2001. Arenaviridae: the viruses and their replication, p. 1635-1668. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 9.Campbell, R. E., O. Tour, A. E. Palmer, P. A. Steinbach, G. S. Baird, D. A. Zacharias, and R. Y. Tsien. 2002. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99:7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Torre, J. C., and M. B. Oldstone. 1992. Selective disruption of growth hormone transcription machinery by viral infection. Proc. Natl. Acad. Sci. USA 89:9939-9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle, M. V., and M. B. Oldstone. 1978. Interactions between viruses and lymphocytes. I. In vivo replication of lymphocytic choriomeningitis virus in mononuclear cells during both chronic and acute viral infections. J. Immunol. 121:1262-1269. [PubMed] [Google Scholar]
- 12.Doyle, S., S. Vaidya, R. O'Connell, H. Dadgostar, P. Dempsey, T. Wu, G. Rao, R. Sun, M. Haberland, R. Modlin, and G. Cheng. 2002. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17:251-263. [DOI] [PubMed] [Google Scholar]
- 13.Foy, E., K. Li, C. Wang, R. Sumpter, Jr., M. Ikeda, S. M. Lemon, and M. Gale, Jr. 2003. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 300:1145-1148. [DOI] [PubMed] [Google Scholar]
- 14.Geisbert, T. W., and P. B. Jahrling. 2004. Exotic emerging viral diseases: progress and challenges. Nat. Med. 10:S110-S121. [DOI] [PubMed] [Google Scholar]
- 15.Haller, O., G. Kochs, and F. Weber. 2006. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344:119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoebe, K., E. Janssen, and B. Beutler. 2004. The interface between innate and adaptive immunity. Nat. Immunol. 5:971-974. [DOI] [PubMed] [Google Scholar]
- 17.Jacamo, R., N. Lopez, M. Wilda, and M. T. Franze-Fernandez. 2003. Tacaribe virus Z protein interacts with the L polymerase protein to inhibit viral RNA synthesis. J. Virol. 77:10383-10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, K. M., J. B. McCormick, P. A. Webb, E. S. Smith, L. H. Elliott, and I. J. King. 1987. Clinical virology of Lassa fever in hospitalized patients. J. Infect. Dis. 155:456-464. [DOI] [PubMed] [Google Scholar]
- 19.Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6:981-988. [DOI] [PubMed] [Google Scholar]
- 20.Kunz, S., P. Borrow, and M. B. Oldstone. 2002. Receptor structure, binding, and cell entry of arenaviruses. Curr. Top. Microbiol. Immunol. 262:111-137. [DOI] [PubMed] [Google Scholar]
- 21.Le Bon, A., N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D. F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009-1015. [DOI] [PubMed] [Google Scholar]
- 22.Lukashevich, I. S., R. Maryankova, A. S. Vladyko, N. Nashkevich, S. Koleda, M. Djavani, D. Horejsh, N. N. Voitenok, and M. S. Salvato. 1999. Lassa and Mopeia virus replication in human monocytes/macrophages and in endothelial cells: different effects on IL-8 and TNF-alpha gene expression. J. Med. Virol. 59:552-560. [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Sobrido, L., and A. Garcia-Sastre. Virus complementation assays to identify interferon antagonists. In R. O. Donis and J. M. Katz (ed.), Viral immunity: methods and protocols, in press. Humana Press, Totowa, N.J.
- 24.McCormick, J. B., and S. P. Fisher-Hoch. 2002. Lassa fever. Curr. Top. Microbiol. Immunol. 262:75-109. [DOI] [PubMed] [Google Scholar]
- 25.Meyer, B. J., J. C. de La Torreqq, and P. J. Southern. 2002. Arenaviruses: genomic RNAs, transcription, and replication. Curr. Top. Microbiol. Immunol. 262:139-149. [DOI] [PubMed] [Google Scholar]
- 26.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437:1167-1172. [DOI] [PubMed] [Google Scholar]
- 27.Munoz-Jordan, J. L., M. Laurent-Rolle, J. Ashour, L. Martinez-Sobrido, M. Ashok, W. I. Lipkin, and A. Garcia-Sastre. 2005. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J. Virol. 79:8004-8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oldstone, M. B. 2002. Biology and pathogenesis of lymphocytic choriomeningitis virus infection. Curr. Top. Microbiol. Immunol. 263:83-117. [DOI] [PubMed] [Google Scholar]
- 29.Pannetier, D., C. Faure, M. C. Georges-Courbot, V. Deubel, and S. Baize. 2004. Human macrophages, but not dendritic cells, are activated and produce alpha/beta interferons in response to Mopeia virus infection. J. Virol. 78:10516-10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park, M. S., A. Garcia-Sastre, J. F. Cros, C. F. Basler, and P. Palese. 2003. Newcastle disease virus V protein is a determinant of host range restriction. J. Virol. 77:9522-9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park, M. S., M. L. Shaw, J. Munoz-Jordan, J. F. Cros, T. Nakaya, N. Bouvier, P. Palese, A. Garcia-Sastre, and C. F. Basler. 2003. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J. Virol. 77:1501-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez, M., R. C. Craven, and J. C. de la Torre. 2003. The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies. Proc. Natl. Acad. Sci. USA 100:12978-12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pien, G. C., K. B. Nguyen, L. Malmgaard, A. R. Satoskar, and C. A. Biron. 2002. A unique mechanism for innate cytokine promotion of T cell responses to viral infections. J. Immunol. 169:5827-5837. [DOI] [PubMed] [Google Scholar]
- 34.Pinschewer, D. D., M. Perez, A. B. Sanchez, and J. C. de la Torre. 2003. Recombinant lymphocytic choriomeningitis virus expressing vesicular stomatitis virus glycoprotein. Proc. Natl. Acad. Sci. USA 100:7895-7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poole, E., B. He, R. A. Lamb, R. E. Randall, and S. Goodbourn. 2002. The V proteins of simian virus 5 and other paramyxoviruses inhibit induction of interferon-beta. Virology 303:33-46. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Jarabo, C. M., C. Ly, E. Domingo, and J. C. de la Torre. 2003. Lethal mutagenesis of the prototypic arenavirus lymphocytic choriomeningitis virus (LCMV). Virology 308:37-47. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez, A., and J. C. de la Torre. 2006. Rescue of the prototypic Arenavirus LCMV entirely from plasmid. Virology 350:370-380. [DOI] [PubMed] [Google Scholar]
- 38.Servant, M. J., B. ten Oever, C. LePage, L. Conti, S. Gessani, I. Julkunen, R. Lin, and J. Hiscott. 2001. Identification of distinct signaling pathways leading to the phosphorylation of interferon regulatory factor 3. J. Biol. Chem. 276:355-363. [DOI] [PubMed] [Google Scholar]
- 39.Seth, R. B., L. Sun, C. K. Ea, and Z. J. Chen. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF3. Cell 122:669-682. [DOI] [PubMed] [Google Scholar]
- 40.Shaw, M. L., A. Garcia-Sastre, P. Palese, and C. F. Basler. 2004. Nipah virus V and W proteins have a common STAT1-binding domain yet inhibit STAT1 activation from the cytoplasmic and nuclear compartments, respectively. J. Virol. 78:5633-5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stojdl, D. F., B. D. Lichty, B. R. tenOever, J. M. Paterson, A. T. Power, S. Knowles, R. Marius, J. Reynard, L. Poliquin, H. Atkins, E. G. Brown, R. K. Durbin, J. E. Durbin, J. Hiscott, and J. C. Bell. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263-275. [DOI] [PubMed] [Google Scholar]
- 42.Strecker, T., R. Eichler, J. Meulen, W. Weissenhorn, H. Dieter Klenk, W. Garten, and O. Lenz. 2003. Lassa virus Z protein is a matrix protein and sufficient for the release of virus-like particles. J. Virol. 77:10700-10705. (Erratum, 77:12927.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unterstab, G., S. Ludwig, A. Anton, O. Planz, B. Dauber, D. Krappmann, G. Heins, C. Ehrhardt, and T. Wolff. 2005. Viral targeting of the interferon-β-inducing Traf family member-associated NF-κB activator (TANK)-binding kinase-1. Proc. Natl. Acad. Sci. USA 102:13640-13645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker, D. H., J. B. McCormick, K. M. Johnson, P. A. Webb, G. Komba-Kono, L. H. Elliott, and J. J. Gardner. 1982. Pathologic and virologic study of fatal Lassa fever in man. Am. J. Pathol. 107:349-356. [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, X., M. Li, H. Zheng, T. Muster, P. Palese, A. A. Beg, and A. Garcia-Sastre. 2000. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J. Virol. 74:11566-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber, F., G. Kochs, and O. Haller. 2004. Inverse interference: how viruses fight the interferon system. Viral Immunol. 17:498-515. [DOI] [PubMed] [Google Scholar]
- 47.Xu, L. G., Y. Y. Wang, K. J. Han, L. Y. Li, Z. Zhai, and H. B. Shu. 2005. VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol. Cell 19:727-740. [DOI] [PubMed] [Google Scholar]
- 48.Yoneyama, M., and T. Fujita. 2004. RIG-I: critical regulator for virus-induced innate immunity. Tanpakushitsu Kakusan Koso 49:2571-2578. (In Japanese.) [PubMed] [Google Scholar]
- 49.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 50.Yuen, T., E. Wurmbach, R. L. Pfeffer, B. J. Ebersole, and S. C. Sealfon. 2002. Accuracy and calibration of commercial oligonucleotide and custom cDNA microarrays. Nucleic Acids Res. 30:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zinkernagel, R. M. 2002. Lymphocytic choriomeningitis virus and immunology. Curr. Top. Microbiol. Immunol. 263:1-5. [DOI] [PubMed] [Google Scholar]