Abstract
We report here the isolation of a novel acid-labile yellow chromophore from the enzymatic digest of human lens proteins and the identification of its chemical structure by LC-MS and NMR. This new chromophore exhibited a UV absorbance maximum at 343 nm and a molecular mass of 370 Da. One- and two-dimensional NMR analyses elucidated the structure as being 1-(5-amino-5-carboxypentyl)-4-(5-amino-5-carboxypentyl-amino)-3-hydroxy-2, 3-dihydropyridinium, a cross-link between the _-amino groups of two lysine residues and a five-carbon atom ring. We assigned it the trivial name of K2P. Quantitative determinations of K2P in individual normal human lens or cataract lens water-soluble and water-insoluble protein digests revealed a significant enhancement of K2P in the early stage of brunescent cataract lens proteins (type I/II, 613 ± 362 pmol/mg of water-insoluble sonicate supernatant (WISS) protein or 85 ± 51 pmol/mg of water-soluble [WS] protein) when compared with aged normal human lens proteins (261 ± 93 pmol/mg of WISS protein or 23 ± 15 pmol/mg of WS protein). Furthermore, a gradual decrease of K2P in the late stages of brunescent cataract lenses with the development of the browning color in the lens argues different coloration mechanisms during the processes of normal aging and cataract development. This new cross-link may serve as a quantitatively significant biomarker for assessing the role of lens protein modifications during aging and in the pathogenesis of cataract.
Keywords: human lens, brunescent cataract, cross-link, advanced glycation end product, yellow chromophore, fluorophore, UVA sensitizer
INTRODUCTION
The aging of the human lens is characterized by increasing levels of water-insoluble (WI) proteins in association with high levels of yellow chromophores and nontryptophan fluorescence.1 These biochemical changes are all enhanced in senile and brunescent cataractous lenses. Glycation of lens proteins has been suggested to be a major protein modification in older lenses; and, indeed, diabetic patients have an increased risk of cataract formation.2 Due to the lifelong stability of the lens crystallins, they can continuously accumulate damaging modifications.
In the past two decades, enormous progress has been made in our understanding of the Maillard reaction and its contribution to age-related changes and cataract development. Some cross-links, such as pentosidine,3, 4 LM-1 (vesperlysine A),5, 6 MOLD/GOLD,7, 8 and glucosepane,9 have been detected in human lens tissues; however, their quantities are rather low even in cataracts. Therefore, the major crosslinks may not yet have been isolated, possibly because they do not survive acid hydrolysis of the proteins.
In this study, we report the isolation and characterization of a novel lysinelysine cross-link, 1-(5-amino-5-carboxypentyl)-4-(5-amino-5-carboxypentyl-amino)-3-hydroxy-2,3-dihydropyridinium (K2P), from enzymatic digests of aged and cataract human lens proteins. In addition, quantitative determination of this cross-link in individual lenses was performed in both normal human lenses and brunescent cataract lenses. The age-dependent increase of this cross-link in normal human lenses and its significant enhancement in cataract lenses support the possibility that glycation plays an important role in lens protein insolubilization and precipitation during aging and cataract development.
MATERIALS AND METHODS
Materials
All reagents were obtained in the highest purity available from Sigma Chemical Co. (St. Louis, MO). Deionized water (18 megaohm resistance or greater) was used for all experiments. Phosphate buffers were treated with 10 g/L chelex resin (200–400 mesh, Bio-Rad Laboratories, Richmond, CA) overnight to remove trace metal ion contaminants as described by Beyer and Fridovich,10 and were filtered through a 0.2-∝m nitrocellulose filter before use. Normal human lenses were obtained as a kind gift from the Heartland Lions Eye Tissue Bank (Columbia, MO) following cornea removal. The lenses were stored individually, frozen at −70° C until use. Brunescent cataracts were collected from hospitals in Rajkot, India and stored frozen at the Patney Eye Clinic. They were transported by hand to Columbia, Missouri, where they were stored individually and frozen at −70° C until use.
Isolation and Purification of K2P from Human Lenses
WI lens proteins were isolated from 150 pooled aged normal human lenses and from 350 early stage brunescent cataract lenses from India, and subjected to extensive enzymatic digestion as described in detail in our previous reports.11–13 The digest was applied to a Bio-Gel P-2 size exclusion chromatography, the early-eluting peak at 330 nm (peak 1) was concentrated, and multiple aliquots were subjected to reversed-phase high-performance liquid chromatography (RP-HPLC). The final product was judged pure by virtue of a single ninhydrin-positive spot that corresponded to a single UV and fluorescent spot on Silica Gel 60 F254 aluminum thinlayer chromatography (Merck) and a single UV HPLC peak under various chromatographic conditions.
Bio-Gel P-2 Size-Exclusion Chromatography
The total digest from γ↔2 g of either aged or cataract water-insoluble sonicate supernatant (WISS) protein was concentrated and subjected to gel filtration chromatography on a Bio-Gel P-2 column (5 · 76 cm, bed volume 1.5 L), with 25 mM formic acid as eluant. Fractions of 14.5 ml were collected at a flow rate of 1 mL per minute and monitored for absorbance at 330 nm and for fluorescence at Ex/Em = 350/450 nm. An enzymatic digest of calf lens protein (control) was run under the same conditions. Peak fractions were pooled according to the A330 readings. Five peaks (designated P1 to P5) were obtained, and peak 1 was used for the isolation of the novel cross-link in this study.
High-Performance Liquid Chromatography
Peak 1 was concentrated and injected into a preparative C18 reversed-phase column (Prodigy 21.2 · 250 mm, Phenomenex, Torrance, CA) using a Shimadzu HPLC system (Shimadzu Scientific Instruments, Lenexa, KS) with Model LC-6AD pumps, SCL-10A vp controller, and SPD-M10A vp photodiode array detector. The column was eluted for 2 minutes with 5% (v/v) acetonitrile in water containing 0.1% (v/v) heptafluorobutyric acid (HFBA), at a flow rate of 8 mL per minute and then with a linear gradient from 5% to 45% (v/v) acetonitrile in 0.1% HFBA over 40 minutes, followed by a linear increase to 100% acetonitrile over the next 5 minutes. The eluant from the column was monitored with an online diode array detector.
One peak, which had maximum absorbance at 343 nm, was collected and further purified over a semipreparative reversed-phase Prodigy column (10 · 250 mm). The column was eluted for 2 minutes with 25 mM formic acid-water (buffer A), at a flow rate of 1.8 mL per minute, and then with a linear gradient to 25% buffer B (water/acetonitrile = 1/1, vol/vol, with 25 mM formic acid) over 25 minutes, followed by a linear increase to 50% buffer B over the next 8 minutes and continued to 100% buffer B over the next 5 minutes. The eluant from the column was monitored with an online photodiode array detector. The A343 peak was concentrated and purified once again with the same solvent system but with an analytical Prodigy RP-HPLC column. After extensive drying with a speed vacuum, 1.3 mg of K2P was obtained.
Spectroscopy
Absorption spectra were recorded with a Cary 1E spectrophotometer (Varian Analytical Instruments, CA) connected to a Dell PC computer. Fluorescence spectra were recorded with a Hitachi F-2500 fluorescence spectrophotometer (Hitachi Instruments, MO).
Samples for NMR spectroscopy were dissolved in 300 ∝L of H2O/D2O (9/1, vol/vol) or 100% D2O after two exchanges with D2O (Cambridge Isotope Laboratories Inc.) and transferred to a 5-mm Advanced Microtube (Shigemi, Allison Park, PA) and scanned for 1H-NMR, 13C-NMR, distortionless enhancement by polarization transfer (DEPT), correlation spectroscopy (COSY), heteronuclear multiple quantum correlation (HMQC), and heteronuclear multiple bond correlation (HMBC) at 25°C with a Bruker (Karlsruhe, Germany) model ARX-500 MHz spectrometer. For an external standard, 99.9% D2O with 0.75% 3-(trimethylsilyl)-propionic-2, 2, 3, 3-d4 acid sodium salt was used. LC-MS and LC-MS/MS were performed with an in-line HPLC-TSQ 7000 mass spectrometer (Thermo Finnigan, San Jose, CA) equipped with an electrospray interface. The LC system consists of quaternary pump P4000, auto sampler AS3000, and photo diode array detector UV6000LP. A Phenomenex LUNA 5 ∝ C18 (2) 250 · 4.6 mm column was used for sample analysis. The column was eluted for 2 minutes with 25 mM formic acid-water (buffer A), at a flow rate of 0.6 mL per minute, and then with a linear gradient to 40% buffer B (100% acetonitrile in 25 mM formic acid) over 40 minutes, followed by a linear increase to 100% buffer B over the next 5 minutes. The collision energy used for MS/MS experiments was either 25 or 70 eV.
Quantitative Determination of K2P in Individual Normal Human Lenses and Brunescent Cataract Lenses
Thirty normal human lenses, ranging in age from 2 to 75 year old, and 19 single brunescent cataract lenses (type I to V, classified based on the extent of browning of the lens) were decapsulated and homogenized individually in 0.5 mL of deionized water. The homogenate was centrifuged at 27,000 · g for 20 minutes, the supernatant was removed, and the pellet was washed twice with 0.5 mL of deionized water. The 27,000-g pellet obtained was washed once again with 1 mL of deionized water and resuspended in 2.5 mL of deionized water. It was sonicated in a water bath for 6 minutes with a Heat Systems-Ultrasonic Inc. Sonicator (Model W-375) at a power setting of 4 and a duty cycle of 40%. The solubilized protein was recovered after centrifugation at 27,000 · g, representing at least 95% of the total WI fraction. The supernatants were combined and considered the water-soluble (WS) fraction. The protein content of both WS and WI fractions was determined using a BCA assay kit (Pierce, Rockford, IL).
Both the WS and WISS fractions were submitted individually to enzymatic digestion, as described above. The final digests of each single lens were applied to small size-exclusion Bio-Gel P-2 chromatography (1 · 25 cm, bed volume 19.6 mL), with 25 mM formic acid as eluant. Fractions of 0.5 mL were collected and monitored for absorbance at 330 nm and for fluorescence at 450 nm when excited at 350 nm. Based on 330-nm readings, peak 1 was pooled and concentrated to 0.5 mL. Peak 1, 100 ∝L (20% of the total), was injected onto an analytical Prodigy HPLC column (4.6 · 250 mm, Phenomenex, Torrance, CA) for quantitative determination of K2P based on the absorbance at 343 nm. The peak area was converted to pmol/mg lens protein according to a standard calibration curve of purified K2P.
RESULTS
Isolation and Purification of K2P from Human Lens Protein Digests
Figure 1
FIGURE 1.
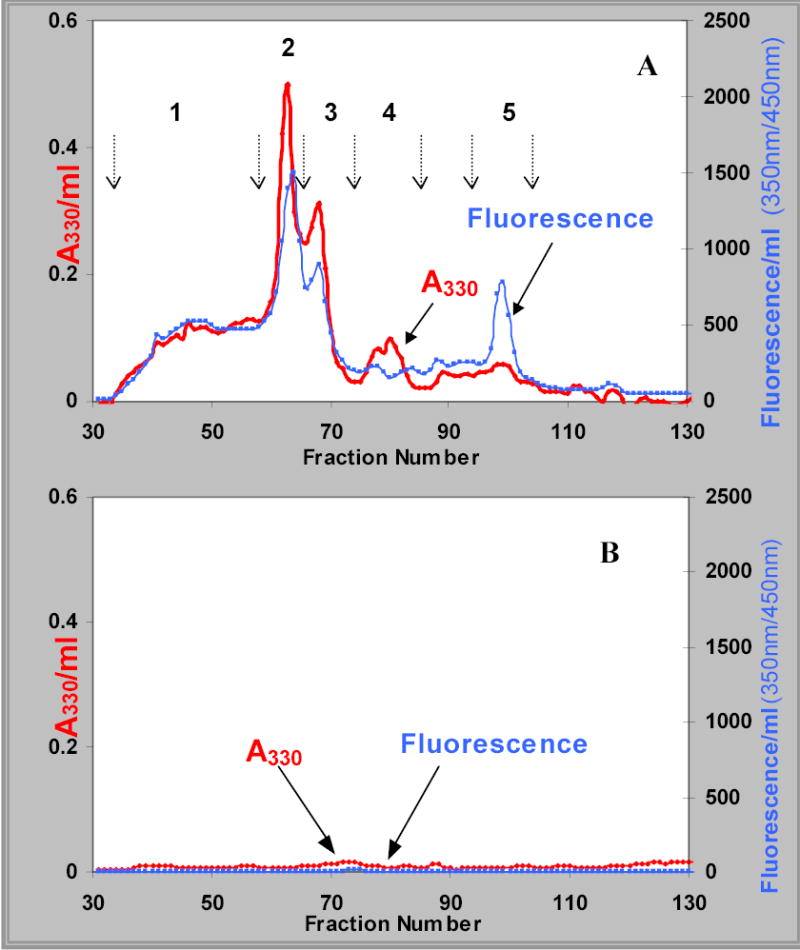
Bio-Gel P-2 elution profile of a typical proteolytic digest of the water-insoluble sonicate supernatant (WISS) fraction from brunescent cataract (panel A) and calf lens protein control (panel B). The profiles for absorbance at 330 nm and 350/450 nm fluorescence are labeled in each panel. The numbers and arrows in panel A identify the peaks, which were pooled based upon 330 nm absorbance.
A pooled cataract lens WISS protein digest (panel A) and a control of calf lens protein digest (panel B) were fractionated by Bio-Gel P-2 size-exclusion gel filtration chromatography (Fig. 1). Five peaks were pooled from the cataract lens WISS protein digest. Very few chromophores or fluorophores were detected from the calf lens protein digest, indicating that the cataract lens chromophores were not produced during the lengthy enzymatic digestion process or contributed by the added enzymes per se.
Figure 2
FIGURE 2.
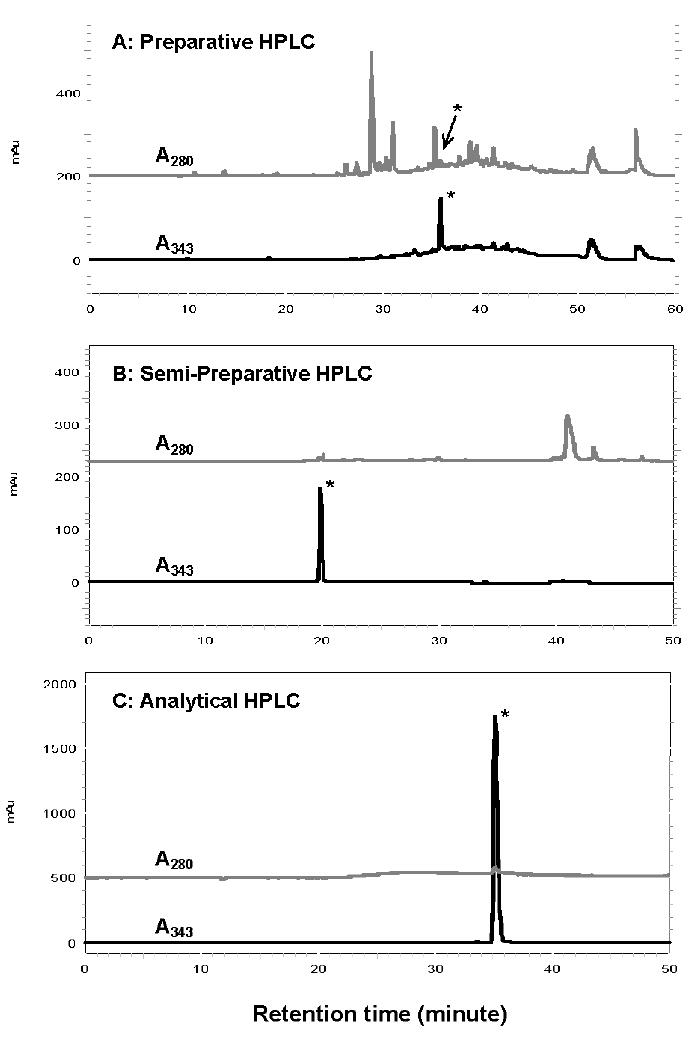
Reverse-phase HPLC profiles showing the purification of K2P. Peak 1 was fractionated by preparative (panel A), semipreparative (panel B). and analytical (panel C) Prodigy ODS columns. The absorbance at 280 nm and 330 nm were monitored and labeled in each panel. The peak marked with an asterisk is K2P.
Peak 1 was concentrated and submitted to three additional purification steps by using preparative (panel A), semipreparative (panel B), and analytical (panel C) reversed-phase HPLC (Fig. 2). The absorbance profiles at 280 nm and 343 nm are presented and labeled in each panel. The A343 peak marked by an asterisk is K2P. The final purified K2P recovery was 1.3 milligrams from 8–9 g of protein digest.
Figure 3: Structure Elucidation of K2P
FIGURE 3.
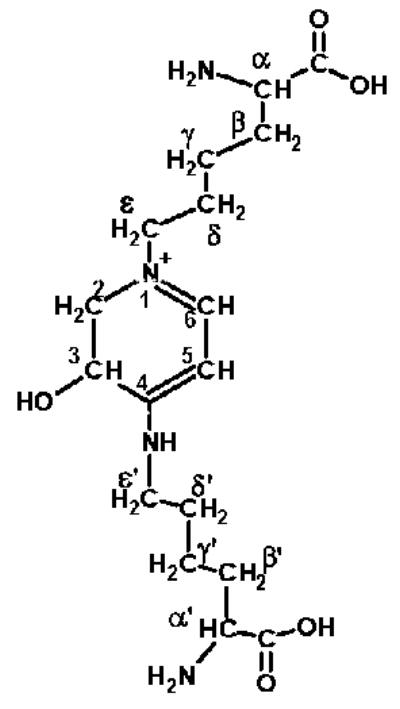
Chemical structure of K2P.
The chemical structure of this novel chromophore, K2P, was characterized by its absorption (⌊max = 343 nm), fluorescence (Ex/Em = 343/410 nm), 1H-NMR, 13CNMR, COSY, HMQC, HMBC, NOESY, and TOCSY two-dimensional spectra, and its mass spectroscopic properties (data not shown). Its structure was determined to be 1-(5-amino-5-carboxypentyl)-4-(5-amino-5-carboxypentyl-amino)-3-hydroxy-2, 3-dihydropyridinium, to which we assigned the trivial name lysine-lysine pyridinium (K2P) (Fig. 3).
Figure 4: Quantification of K2P in Normal Human Lenses and Brunescent Cataract Lenses
FIGURE 4.
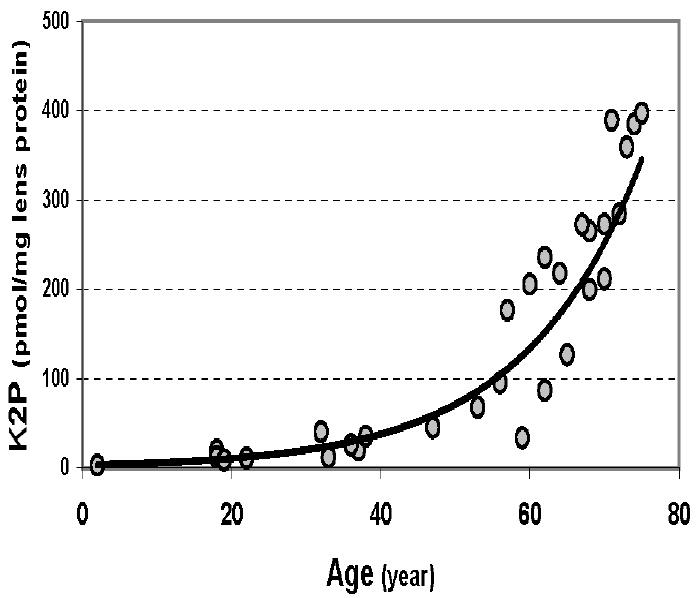
Age-dependent increase of K2P in normal human lens WISS proteins.
The acid stability of K2P was tested by analyzing the samples before and after the acid hydrolysis. Although K2P was stable in acid solution (pH ~2) during our purification process, it was totally decomposed by acid hydrolysis with 6 M HCl at 110°C for 20 hours. Therefore, analyses of all the protein samples prepared from individual human lenses were carried out using our enzymatic digestion procedure.
The quantity of K2P in individual normal human lenses of different ages and brunescent (type I to V, classified based on the extent of browning) cataract lenses was determined. The enzymatic digests of WS and WISS proteins from each individual lens were prepared. Peak 1, isolated from a small-scale Bio-Gel P-2 column, was analyzed by an analytical RP-HPLC with an online photodiode array (PDA) detector.
Twenty percent of the pooled peak 1 by volume was subjected to HPLC, and the quantity of K2P in each lens was calculated as pmol per mg lens protein, based on the peak area compared to a standard curve.
Thirty individual normal human lenses of different age were analyzed (Fig. 4).
The results revealed an age-dependent increase in K2P, especially after 40 years. The highest samples from aged lenses reached approximately 400 pmol per mg of lens protein.
Figure 5
FIGURE 5.
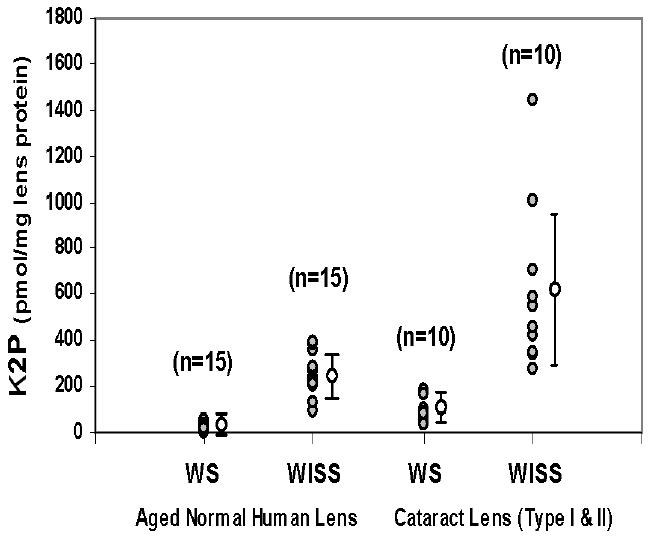
Quantitative determination of K2P in WS and WISS proteins from individual aged normal human and brunescent cataract lenses.
Figure 5 shows the K2P levels in both WS and WISS protein digests of 15 aged normal human lenses ranging from 62 to 75 years old (average 68 years) and 10 early-stage (type I/II) brunescent cataract lenses. The average levels of K2P in aged normal human lens WS and WISS protein digests were 22 ± 15 and 260 ± 93 pmol/mg of lens protein, respectively. Cataract lens WS and WISS protein digests contained 85 ± 51 and 613 ± 363 pmol/mg of lens protein, respectively. A large variation of K2P content was seen in the cataract WISS protein digests. In one particular case, the K2P level reached 1400 pmol/mg of lens protein.
Figure 6
FIGURE 6.
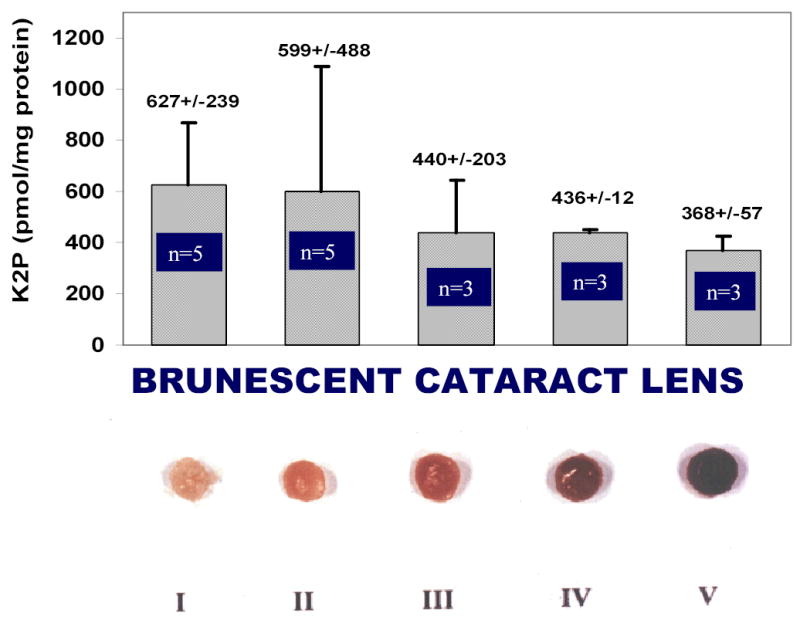
Quantitative determination of K2P in WISS protein digests of type I to V brunescent cataract lens.
Figure 6 shows the K2P levels in WISS protein digests of type I to V brunescent cataract lenses. The number of lenses used for this analysis is labeled in the graph. A gradual decrease of K2P level contrasted with the development of browning color in the lens was observed.
DISCUSSION
As the human lens ages, protein-bound yellow chromophores accumulate in the lens, particularly in the lens core. Many of these browning compounds are proposed to be advanced glycation end products (AGEs), which can absorb light in the UVA region (320–400 nm) and which contribute to lens protein cross-linking. Our enzymatic digestion procedure released at least 95% free amino acids from both aged normal human lens proteins and cataract lens proteins.11–13 To minimize oxidative degradation of modified amino acids, the digestion was carried out under argon and in the dark at all times.
Bio-Gel P2 chromatography separated the yellow chromophores into five A330-absorbing peaks. A broad early-eluting peak, which may contain protein cross-links, represented 25 to 30 % of the total A330 absorbance. The major peak in this fraction was identified, and sufficient purified material allowed the isolation of 1.3 mg of K2P, which was sufficient for NMR spectral measurements.
LC-MS/MS and NMR spectroscopy determined the structure of K2P to be a glycation cross-link between the Σ-amino groups of two lysine residues by a pyridinium ring (Fig. 3). Structurally, K2P has two unusual features. One lysine Σ-amino group is incorporated into the pyridinium ring, as seen with pentosidine, MOLD, GOLD, crossline, and vesperlysine A; however, the second lysine Σ-amino group in K2P is attached off the ring. Also, the ring structure is not fully unsaturated. It is possible that the second lysine attaches to carbon 3 prior to ring closure, preventing the final ring dehydration step. The ⌊ max of 343 nm of K2P is consistent with the structure of the conjugate and depends upon the presence of the nitrogens as part of the resonance structure.
K2P level in individual human lens WS and WISS protein digests was quantitatively determined. K2P levels in normal human lenses increased with age, especially after the age of 40 years (Fig. 4). The same pattern of human lens coloration12 and the increases of other posttranslational modifications of lens proteins, such as by UV filters,14–15 after middle age have been previously reported. A lens barrier hypothesis proposed by Truscott allows an explanation for these phenomena.16 Significantly higher levels of K2P were detected in cataract lenses and in water-insoluble proteins as compared to water-soluble proteins (Fig. 5) suggesting that this cross-link may have a role in the formation of the water-insoluble proteins. K2P was present at 260 pmol/mg of lens protein in aged normal human lenses; it increased to 610 pmol/mg of lens protein in the early stage of brunescent cataract lens, which is remarkable compared to the well-known cross-links, such as pentosidine or LM-1 (2-10 pmol/mg protein in aged normal human lens or γ→20 pmol/mg protein in cataract lens). Therefore, as with yellow chromophores in the human lens, K2P appears to be a major UVA-absorbing chromophore and protein cross-link. Indeed, K2P can be oxidized by UVA irradiation (data not shown). The gradual decrease of K2P level in brunescent cataract lenses shown in Figure 6 reveals that one possibility of this decrease may be due to UVA light–mediated photooxidation. Determining the photochemistry of K2P will be one of our future objectives.
K2P, therefore, may serve as a useful biomarker for assessing the role of lens protein modification and coloration in aging and in the pathogenesis of cataract. There is no clear data now showing the formation pathway of K2P, even though we found an identical signal of m/z and retention time by LC-MS from an enzymatic digest of ascorbic acid–modified calf lens proteins. The origin of K2P is currently being pursued.
Acknowledgments
The authors would like to express their sincere gratitude to Dr. S. A. Patney for her tireless efforts in the collection of the cataract lenses used in this research. This work was supported in part by NIH Grant EY07070 and in part by a department grant from Research to Prevent Blindness, Inc.
References
- 1.Kurzel RB, Wolbarsht ML, Yamanashi BS. Spectral studies on normal and cataractous intact human lenses. Exp Eye Res. 1973;17:65–71. doi: 10.1016/0014-4835(73)90168-1. [DOI] [PubMed] [Google Scholar]
- 2.Cotlier E. Senile cataracts: evidence for acceleration by diabetes and deceleration by salicylate. Can J Ophthalmol. 1981;16:113–118. [PubMed] [Google Scholar]
- 3.Sell DR, Monnier VM. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989;264:21,597–21,602. [PubMed] [Google Scholar]
- 4.Grandhee SK, Monnier VM. Mechanism of formation of the Maillard protein cross-link pentosidine. Glucose, fructose, and ascorbate as pentosidine precursors. J Biol Chem. 1991;266:11,649–11,653. [PubMed] [Google Scholar]
- 5.Nagaraj RH, Monnier VM. Isolation and characterization of a blue fluorophore from human eye lens crystallins: in vitro formation from Maillard reaction with ascorbate and ribose. Biochim Biophys Acta. 1992;1116:34–42. doi: 10.1016/0304-4165(92)90125-e. [DOI] [PubMed] [Google Scholar]
- 6.Tessier F, Obrenovich M, Monnier VM. Structure and mechanism of formation of human lens fluorophore LM-1. Relationship to vesperlysine A and the advanced Maillard reaction in aging, diabetes, and cataractogenesis. J Biol Chem. 1999;274:20,796–20,804. doi: 10.1074/jbc.274.30.20796. [DOI] [PubMed] [Google Scholar]
- 7.Nagaraj RH, Shipanova IN, Faust FM. Protein cross-linking by the Maillard reaction. Isolation, characterization, and in vivo detection of a lysine-lysine cross-link derived from methylglyoxal. J Biol Chem. 1996;271:19,338–19,345. doi: 10.1074/jbc.271.32.19338. [DOI] [PubMed] [Google Scholar]
- 8.Frye EB, et al. Role of the Maillard reaction in aging of tissue proteins. Advanced glycation end product-dependent increase in imidazolium cross-links in human lens proteins. J Biol Chem. 1998;273:18,714–18,719. doi: 10.1074/jbc.273.30.18714. [DOI] [PubMed] [Google Scholar]
- 9.Biemel KM, Friedl DA, Lederer MO. Identification and quantification of major Maillard cross-links in human serum albumin and lens protein. Evidence for glucosepane as the dominant compound. J Biol Chem. 2002;277:24,907–24,915. doi: 10.1074/jbc.M202681200. [DOI] [PubMed] [Google Scholar]
- 10.Beyer WF, JR, Fridovich I. Characterization of a superoxide dismutase mimic prepared from desferrioxamine and MnO2. Arch Biochem Biophys. 1989;271:76–85. doi: 10.1016/0003-9861(89)90265-8. [DOI] [PubMed] [Google Scholar]
- 11.Cheng R, et al. Similarity of the yellow chromophores isolated from human cataracts with those from ascorbic acid-modified calf lens proteins: evidence for ascorbic acid glycation during cataract formation. Biochim Biophys Acta. 2001;1537:14–26. doi: 10.1016/s0925-4439(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 12.Cheng R, et al. Rate of formation of AGEs during ascorbate glycation and during aging in human lens tissue. Biochim Biophys Acta. 2002;1587:65–74. doi: 10.1016/s0925-4439(02)00069-8. [DOI] [PubMed] [Google Scholar]
- 13.Cheng R, et al. Separation of the yellow chromophores in individual brunescent cataracts. Exp Eye Res. 2003;77:313–325. doi: 10.1016/s0014-4835(03)00131-3. [DOI] [PubMed] [Google Scholar]
- 14.Hood BD, Garner B, Truscott RJW. Human lens coloration and aging. Evidence for crystallin modification by the major ultraviolet filter, 3-hydroxykynurenine O-beta-D-glucoside. J Biol Chem. 1999;274:32547–32550. doi: 10.1074/jbc.274.46.32547. [DOI] [PubMed] [Google Scholar]
- 15.Bova LM, et al. Major changes in human ocular UV protection with age. Invest Ophthalmol Vis Sci. 2001;42:200–205. [PubMed] [Google Scholar]
- 16.Truscott RJW. Age-related nuclear cataract: a lens transport problem. Ophthal Res. 2000;32:185–194. doi: 10.1159/000055612. [DOI] [PubMed] [Google Scholar]


