Abstract
Type 1A diabetes (T1D) is an autoimmune disorder the risk of which is increased by specific HLA DR/DQ alleles [e.g., DRB1*03-DQB1*0201 (DR3) or DRB1*04-DQB1*0302 (DR4)]. The genotype associated with the highest risk for T1D is the DR3/4-DQ8 (DQ8 is DQA1*0301, DQB1*0302) heterozygous genotype. We determined HLA-DR and -DQ genotypes at birth and analyzed DR3/4-DQ8 siblings of patients with T1D for identical-by-descent HLA haplotype sharing (the number of haplotypes inherited in common between siblings). The children were clinically followed with prospective measurement of anti-islet autoimmunity and for progression to T1D. Risk for islet autoimmunity dramatically increased in DR3/4-DQ8 siblings who shared both HLA haplotypes with their diabetic proband sibling (63% by age 7, and 85% by age 15) compared with siblings who did not share both HLA haplotypes with their diabetic proband sibling (20% by age 15, P < 0.01). 55% sharing both HLA haplotypes developed diabetes by age 12 versus 5% sharing zero or one haplotype (P = 0.03). Despite sharing both HLA haplotypes with their proband, siblings without the HLA DR3/4-DQ8 genotype had only a 25% risk for T1D by age 12. The risk for T1D in the DR3/4-DQ8 siblings sharing both HLA haplotypes with their proband is remarkable for a complex genetic disorder and provides evidence that T1D is inherited with HLA-DR/DQ alleles and additional MHC-linked genes both determining major risk. A subset of siblings at extremely high risk for T1D can now be identified at birth for trials to prevent islet autoimmunity.
Keywords: haplotype, human leukocyte antigen, major histocompatibility complex
A large body of evidence indicates that type 1A diabetes (T1D) is an autoimmune disorder with important genetic determinants, and it has become one of the most intensively studied complex genetic disorders (1–3). The major histocompatibility complex (MHC) is reported to account for ≈40% of the familial aggregation of T1D (4, 5). The HLA-DR and -DQ genes (linked HLA genes in the class II region of the MHC) are well established as being associated with risk for T1D. Although studies have implicated loci other than the HLA-DR and -DQ loci (i.e., HLA-DPB1, HLA-A, HLA-B, and various non-HLA genes) with diabetes risk and earlier age of onset, no loci with contribution to risk equivalent to that of the HLA-DR and -DQ alleles have been identified (6–10).
The insulin, PTPN22, and CTLA4 genes are non-HLA diabetes-susceptibility loci with allelic odds ratios of 1.9, 1.7, and 1.2, respectively (11–14). However, even in combination with HLA alleles, none of these identified loci confer a risk >25% in prospective studies. Nevertheless, a remaining fundamental question is whether there are genetic polymorphisms other than the HLA-DR and -DQ alleles that confer major risk for T1D. If such loci existed, they could be linked to the MHC and would thus be “hidden” in most linkage studies by the dramatic influence of the HLA-DR and -DQ alleles.
Haplotypes are defined by sets of closely linked genetic variants making chromosomal segments. In a family, the inheritance of these chromosomal segments can be readily defined, and, in particular, the number of haplotypes identical by descent (shared) between siblings (none, one, or two) determined. Well over a decade ago, Glenys Thomson and others pioneered the concept that affected sibling pairs are more likely to share both HLA haplotypes than unaffected sibling pairs (15–19). It was shown that children sharing two haplotypes with an affected sibling have a risk of 16%, versus 9% for those sharing one, and <1% for those sharing none (20). This excess sharing of haplotypes in affected sibling pairs could reflect the influence of alleles at the DRB1 and DQB1 loci and, possibly, other MHC loci (21, 22). In the nonobese diabetic mouse model, class I alleles in addition to class II alleles contribute to diabetes (23, 24).
The Diabetes Autoimmunity Study of the Young (DAISY) enrolled its first newborn subjects in 1993. Since then, cord blood from more than 33,000 newborns has been screened for HLA-DR and -DQ alleles associated with diabetes risk and protection (25). Those at increased risk, including 1,058 unaffected young relatives of patients with T1D, were enrolled for follow-up detection of anti-islet autoantibodies and diabetes. DAISY and other studies (26–28) have found that risk for T1D in early childhood is higher in siblings than in offspring of individuals with T1D, despite all having the same high-risk HLA-DR and -DQ alleles.
We hypothesized that siblings of a child with diabetes have a higher diabetes risk compared with offspring of a parent with diabetes, even though siblings and offspring both share approximately half of their genome with their diabetic proband, because siblings can share both HLA haplotypes identical to their proband, whereas offspring inherit only one haplotype from their single diabetic parent. Specifically, the sharing of multiple genetic polymorphisms of DR, DQ genes and non-DR, DQ genes linked to the MHC region on both copies of chromosome 6 could cause the increased sibling risk. We determined that we could characterize the amount of risk contributed by non-DR, DQ loci by analyzing the prospective risk associated with HLA haplotype sharing in siblings that all had the same high-risk DR3/4-DQ8 (DQ8 is DQA1*0301, DQB1*0302) genotype. We analyzed genotypes of sibling families (proband, sibling, and parents) at the HLA class II region and, if needed, at additional polymorphisms within the MHC to determine the number of HLA haplotypes shared between siblings and their diabetic probands.
Here, we report a remarkable high risk for DR3/4-DQ8 children who share both HLA haplotypes identical by descent with their affected sibling. These results are consistent with the effect of a gene(s) that confers major risk for T1D that is linked to, but distinct from, the HLA-DR and -DQ alleles.
Results
We studied a group of children with the highest risk HLA genotype, DR3/4-DQ8 heterozygotes. By life table analysis, DR3/4-DQ8 siblings in the study had a 41% (±8% SEM) overall risk for developing islet autoantibodies by age 7, compared with a 16% (±6%) risk in DR3/4-DQ8 offspring (P = 0.01) and a 5% (±1.6%) risk in DR3/4-DQ8 children from the general population.
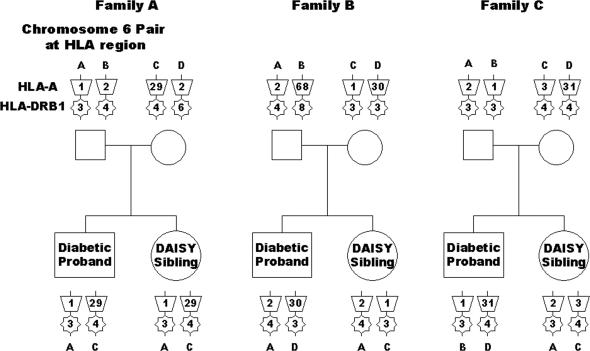
Fig. 1 illustrates three representative DAISY families where a DAISY sibling was prospectively monitored for islet autoimmunity and development of diabetes. All three DAISY siblings have the DR3/4-DQ8 genotype. The DAISY sibling in family A shares both haplotypes with the sibling proband, with the proband defined as the first child in the family diagnosed with T1D. The DAISY sibling in family B shares one haplotype with the sibling proband, and the DAISY sibling in family C does not share any haplotypes with the sibling proband. DAISY siblings being followed in this study of 48 families always have the DR3/4-DQ8 alleles, but the diabetic proband sibling does not always have the DR3, DQ2 or DR4, DQ8 alleles. Even if the diabetic proband has DR3, DQ2 and DR4, DQ8 alleles, they may be inherited from different parental haplotypes than those inherited by the DR3/4-DQ8 sibling.
Fig. 1.
Haplotype sharing analysis. Haplotype sharing is illustrated in three representative families in which the DR3/4-DQ8 DAISY sibling shares both (family A), one (family B), or no (family C) haplotypes with the diabetic proband.
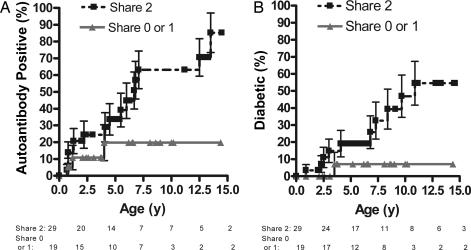
The majority of the DR3/4-DQ8 DAISY siblings shared both HLA haplotypes with the proband (n = 29), 17 siblings shared one haplotype, one sibling shared no haplotypes, and haplotype sharing was either zero or one for 1 sibling (family was not further informative with the markers analyzed). We performed survival-curve analysis to compare progression to islet autoantibody positivity (Fig. 1, A) and diabetes (Fig. 1, B) in DR3/4-DQ8 DAISY siblings, stratified by the number of haplotypes they shared with the sibling proband (Fig. 2). The survival curves in Fig. 2A show that DR3/4-DQ8 siblings sharing both HLA haplotypes with their diabetic proband sibling have a 63% (±11% SEM) risk of developing persistent anti-islet autoantibodies by age 7 and an 85% (±12%) risk by age 15, versus a 20% (±11%) risk by age 15 for those not sharing both haplotypes. To date, 55% (16 of 29) sharing both haplotypes versus 16% (3 of 19) sharing zero or one haplotype are autoantibody positive (P = 0.008). DR3/4-DQ8 siblings sharing both HLA haplotypes with their diabetic proband sibling have a 55% (±13%) risk for diabetes (Fig. 2B) by age 12, compared with a 7% (±7%) risk for those not sharing both haplotypes (Fig. 2B). To date, 34% (10 of 29) versus 5% (1 of 19) have type 1 diabetes (P = 0.03). The eight remaining nondiabetic DR3/4-DQ8 DAISY siblings that are persistently positive for anti-islet autoantibodies are at high risk for diabetes given their high-risk HLA alleles and their expression of multiple anti-islet autoantibodies, with two or more anti-islet autoantibodies expressed in five of these siblings (Table 1).
Fig. 2.
Haplotype sharing survival curves. Shown is progression to anti-islet autoimmunity (A) and type 1A diabetes (B) in DR3/4-DQ8 siblings stratified by the number of HLA haplotypes shared with their proband siblings. n = 48 for A and B; error bars for all panels represent the SEM.
Table 1.
Summary of Phenotype, Haplotype Sharing, and HLA Genotypes
| ID | T1D | Positive AutoAb | DQ | DRB1*04 | DQ | DR | No. shared | Haplotypes shared | DPB1 | HLA-A |
|---|---|---|---|---|---|---|---|---|---|---|
| 483-0 | Yes | All 3 | 8 | 0401 | 2 | 3 | 2 | Both | 0101/0401 | 03/32 |
| 471-0 | Yes | All 3 | 8 | 0401 | 2 | 3 | 2 | Both | 0401/0201 | 01/02 |
| 667-0 | Yes | All 3 | 8 | 0402 | 2 | 3 | 2 | Both | 0201/0401 | 24/66 |
| 60-0 | Yes | All 3 | 8 | 0404 | 2 | 3 | 2 | Both | 0201/0401 | 01/02 |
| 539-0 | Yes | All 3 | 8 | 0402 | 2 | 3 | 2 | Both | 0401/0501 | 02/03 |
| 533-0 | Yes | IAA, IA-2 | 8 | 0401 | 2 | 3 | 2 | Both | 0101/0301 | 02/03 |
| 132-0 | Yes | IAA, GAD | 8 | 0401 | 2 | 3 | 2 | Both | 0301/1601 | 01/02 |
| 330-0 | Yes | IAA, GAD | 8 | 0401 | 2 | 3 | 2 | Both | 0301/1601 | 01/02 |
| 45–0 | Yes | IAA, IA-2 | 8 | 0401 | 2 | 3 | 2 | Both | 0201/0401 | 01/02 |
| 275-0 | Yes | IAA | 8 | 0405 | 2 | 3 | 2 | Both | 0101/0201 | 29/01 or 36 |
| 343-0 | Yes | IAA | 8 | 0405 | 2 | 3 | 1 | DQ2 | 0301/0401 | 02/03 |
| 40-0 | No | All 3 | 8 | 0401 | 2 | 3 | 2 | Both | 0202/0401 | 01/03 |
| 399-0 | No | All 3 | 8 | 0401 | 2 | 3 | 2 | Both | 0301/0401 | 02/30 |
| 41-0 | No | GAD, IA-2 | 8 | 0401 | 2 | 3 | 2 | Both | 0401/0401 | 02/02 |
| 18-0 | No | GAD | 8 | 0401 | 2 | 3 | 2 | Both | 0201/0401 | 01/01 |
| 133-0 | No | IA-2 | 8 | 0401 | 2 | 3 | 2 | Both | 0301/0401 | 01/02 |
| 656-0 | No | IAA | 8 | 0404 | 2 | 3 | 2 | Both | 0401/0201 | 02/02 |
| 409-0 | No | All 3 | 8 | 0401 | 2 | 3 | 1 | DQ2 | 0401/0401 | 01/02 |
| 732-0 | No | All 3 | 8 | 0401 | 2 | 3 | 1 | DQ8 | 0201/0201 | 02/29 |
| 21-0 | No | 0 | 8 | 0401 | 2 | 3 | 2 | Both | 0101/0401 | 02/32 |
| 178-0 | No | 0 | 8 | 0401 | 2 | 3 | 2 | Both | 0301/0401 | 03/24 |
| 182-0 | No | 0 | 8 | 0401 | 2 | 3 | 2 | Both | 0401/0401 | 01/02 |
| 263-0 | No | 0 | 8 | 0401 | 2 | 3 | 2 | Both | 0101/0401 | 01/11 |
| 325-0 | No | 0 | 8 | 0401 | 2 | 3 | 2 | Both | 0101/0402 | 02/25 |
| 707-0 | No | 0 | 8 | 0401 | 2 | 3 | 2 | Both | 0101/0201 | 02/24 |
| 764-0 | No | 0 | 8 | 0401 | 2 | 3 | 2 | Both | 0201/0401 | 01/ 03 or 11 |
| 811-0 | No | 0 | 8 | 0401 | 2 | 3 | 2 | Both | 0202/0401 | 02/30 |
| 815-0 | No | 0 | 8 | 0401 | 2 | 3 | 2 | Both | 0101/0401 | 01/11 |
| 810-0 | No | 0 | 8 | 0404 | 2 | 3 | 2 | Both | 0202/0402 | 02/30 |
| 858-0 | No | 0 | 8 | 0404 | 2 | 3 | 2 | Both | ||
| 958-0 | No | 0 | 8 | 0404 | 2 | 3 | 2 | Both | 0301/0401 | 11/23 |
| 1020-0 | No | 0 | 8 | 0404 | 2 | 3 | 2 | Both | 0101/2001 | 03/26 |
| 352-0 | No | 0 | 8 | 0401 | 2 | 3 | 1 | DQ2 | 0101/0101 | 02/25 |
| 938-0 | No | 0 | 8 | 0401 | 2 | 3 | 1 | DQ2 | 0201/0401 | 02/02 |
| 922-0 | No | 0 | 8 | 0401 | 2 | 3 | 1 | DQ2 | 0301/0402 | 01/29 |
| 457-0 | No | 0 | 8 | 0401 | 2 | 3 | 1 | DQ8 | 0401/0901 | 02/03 |
| 731-0 | No | 0 | 8 | 0401 | 2 | 3 | 1 | DQ8 | 0201/0201 | 02/29 |
| 736-0 | No | 0 | 8 | 0401 | 2 | 3 | 1 | DQ8 | 0201/0401 | 03/31 |
| 800-0 | No | 0 | 8 | 0401 | 2 | 3 | 1 | DQ8 | 0401/0401 | 01/02 |
| 580-0 | No | 0 | 8 | 0401 | 2 | 3 | 1 | DQ8 | 0401/0401 | 02/25 |
| 255-0 | No | 0 | 8 | 0404 | 2 | 3 | 1 | DQ8 | 0301/0401 | 01/24 |
| 925-0 | No | 0 | 8 | 0405 | 2 | 3 | 1 | DQ8 | 0401/0401 | 02/02 |
| 397-0 | No | 0 | 8 | 0404 | 2 | 3 | 0 or 1 | Unknown | 0301/0401 | 01/01 |
| 281-0 | No | 0 | 8 | 0403 | 2 | 3 | 1 | DQ8 | 0401/0401 | 02/03 |
| 759-0 | No | 0 | 8 | 0407 | 2 | 3 | 1 | DQ2 | 0301/0402 | 01/33 |
| 767-0 | No | 0 | 8 | 0407 | 2 | 3 | 1 | DQ2 | 0401/0401 | 02/68 |
| 321-0 | No | 0 | 8 | 0408 | 2 | 3 | 1 | DQ8 | 1301/1501 | 01/03 |
| 538-0 | No | 0 | 8 | 0401 | 2 | 3 | 0 | Neither | 0101/0201 | 02/03 |
Abbreviations: T1D, type 1A diabetes; Positive AutoAb, positive islet autoantibodies; # Shared, number of haplotypes shared between siblings.
For DAISY siblings sharing both haplotypes with their proband but lacking the DR3/4-DQ8 genotype (n = 27), risk by survival-curve analysis is only 23% for anti-islet autoantibodies at age 15 and 25% for diabetes at age 12. These results indicate that high-risk DR-DQ alleles (e.g., DR3-DQ2 and DR4-DQ8) and HLA haplotype sharing with the proband sibling are both essential for extremely high diabetes risk.
We summarize the disease status of each DAISY DR3/4-DQ8 sibling and list the DRB1 and DQB1 alleles (including DRB1*04 subtypes), haplotypes shared with probands, HLA-DPB1, and HLA-A alleles in Table 1. When there was only one haplotype inherited in common by the siblings, the DR3-DQ2 haplotype was shared in 41% of the siblings (n = 7) and the DR4-DQ8 haplotype was shared in 59% (n = 10) (Table 1, rightmost column). The DRB1*04 subtypes did not differ significantly between the groups stratified by haplotype sharing. Because specific alleles at the HLA-DPB1 locus have an association with diabetes risk (29, 30), we analyzed genotypes of the DR3/4-DQ8 siblings at the DPB1 locus. The high-risk DPB1 alleles were not significantly associated with diabetic autoimmunity in this group (n = 47; Table 2). Similarly, the distribution of the HLA-A alleles (HLA-A*0201 and HLA-A*24 alleles) did not significantly differ between groups (6). Thus, MHC genes previously reported as being associated with T1D do not explain the magnitude of increased risk observed with HLA haplotype sharing.
Table 2.
DPB1 allele frequencies
| DPB1 | Haplotypes, n | Haplotypes, % | Ab− subjects, % | Ab+ subjects, % | Diabetic patients, % |
|---|---|---|---|---|---|
| 0101 | 12 | 12.8 | 14.0 | 7.9 | 13.6 |
| 0201* | 16 | 17.0 | 12.3 | 23.7 | 22.7 |
| 0202* | 3 | 3.2 | 3.5 | 2.6 | 0.0 |
| 0301* | 12 | 12.8 | 10.5 | 15.8 | 18.2 |
| 0401 | 40 | 42.6 | 45.6 | 42.1 | 31.8 |
| 0402** | 4 | 4.3 | 7.0 | 0.0 | 0.0 |
| 1601 | 2 | 2.1 | 0.0 | 5.3 | 9.1 |
| Other | 5 | 5.3 | 7.0 | 2.6 | 4.5 |
| Total | 94.0 | 100.0 | 100.0 | 100.0 | 100.0 |
Reported as higher (*) and lower (**) risk. DPB1 allele frequencies in 47 DR3/4-DQ8 siblings of diabetic probands. Ab−, islet autoantibody negative; Ab+, islet autoantibody positive.
Higher maternal age at delivery, male gender, and younger proband sibling age at diagnosis are risk factors that were associated with sibling recurrence risk for diabetes in a study of >10,000 sibling pairs in the Finnish population (31). We performed Cox proportional hazards regression analysis on the DR3/4-DQ8 sibling data to evaluate risk for islet autoimmunity and diabetes, using HLA haplotype sharing, maternal age at delivery, gender, and age of proband at diagnosis as potential explanatory variables. Only HLA haplotype sharing was associated with risk in the DR3/4-DQ8 siblings using the likelihood ratio statistic (n = 48, P = 0.03 for islet autoimmunity, P = 0.04 for diabetes) and none of the other variables contributed significantly. However, the results of this analysis of the DAISY siblings do not refute the findings of the Finnish study, because the Finnish study (of >10,000 siblings pairs) had more power to detect factors associated with minor risk than our more selective study of DAISY siblings with the DR3/4-DQ8 genotype (n = 48).
The DR3/4-DQ8 siblings were also genotyped at HLA-B (n = 47) to identify alleles associated with extended haplotypes (e.g., HLA-B*08 and -B*15). The presence of alleles associated with the HLA-DR3-B8-A1 extended haplotype in DR3/4-DQ8 siblings that shared both HLA haplotypes with their proband sibling did not significantly add to their risk (n = 28, 31% with alleles B8 and A1 affected versus 25% unaffected, P = 1). In a similar way, the presence of the HLA B15 allele was not significantly increased in affected siblings that shared both haplotypes (n = 28, 13% affected versus 33% unaffected, P = 0.36).
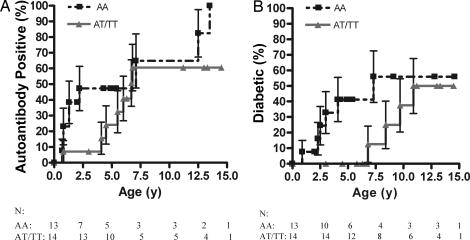
We analyzed the HphI insulin gene polymorphism to assess its effect on risk in the DR3/4-DQ8 siblings that shared both haplotypes. Of the DR3/4-DQ8 siblings with the highest-risk insulin genotype (AA) who shared both HLA haplotypes with the proband, 47% (±14%) progressed to autoantibody positivity by age 2.5 (Fig. 3A) and 56% (±17%) to diabetes by age 7.5 (Fig. 3B). By age 5, a significantly higher number of DR3/4-DQ8 siblings with the high-risk insulin genotype who shared both HLA haplotypes with the proband developed T1D versus siblings with the lower-risk insulin genotypes, excluding nondiabetic siblings younger than age 5 (5 of 11 versus 0 of 12, P = 0.01). Thus, the high-risk insulin genotype may be associated with a lower age-of-onset of T1D in DR3/4-DQ8 children sharing both haplotypes with a diabetic sibling. We also analyzed risk associated with the PTPN22 gene, but the high-risk allele, T, did not significantly influence risk for anti-islet autoantibody development in the DAISY siblings studied [CT or TT genotype in 16% of affected (n = 19) and 21% of unaffected (n = 28) siblings].
Fig. 3.
Haplotype sharing and insulin genotype survival curves. Shown is progression to anti-islet autoantibody positivity (A) and type 1 diabetes (B) in DR3/4-DQ8 siblings sharing both HLA haplotypes with their sibling probands stratified by genotypes of the insulin gene at the HphI polymorphism. The survival curves represent the high-risk genotypes (AA; n = 13) versus the lower-risk genotypes (AT, TT; n = 14). Error bars represent the SEM.
We sought a different population of DR3/4 siblings for initial confirmation of the added importance of HLA haplotype sharing to the DR3-DQ2/DR4-DQ8 genotype. The Human Biological Data Interchange database has families selected a priori for multiple diabetic siblings; however, unlike the DAISY siblings, the Human Biological Data Interchange siblings were not prospectively followed to diabetes. The proband was defined as the first sibling in the family to develop diabetes. We found that 83% (35 of 42) of the DR3/4-DQ8 siblings sharing both haplotypes by descent with their diabetic proband were diabetic versus 58% (18 of 31) not sharing both haplotypes with their diabetic proband (n = 73, P = 0.03).
Discussion
We report the discovery of a genetic risk for type T1D three to four times higher than previously reported (32). A feature of this study is the simple combined analysis of the HLA-DR and -DQ highest-risk alleles with analysis of HLA haplotype sharing. The DAISY study has followed children prospectively from birth for development of anti-islet autoantibodies and diabetes with HLA typing of >33,000 newborns to aid in risk stratification (26–28). Among those HLA typed, only 29 siblings have the DR3/4-DQ8 alleles and share both HLA haplotypes identical by descent with their diabetic proband sibling. Nevertheless, ≈20% of the children currently diagnosed with T1D from the DAISY population are in this tiny group of 29 children. By survival-curve analysis, this group has a 63% risk of progressing to persistent anti-islet autoimmunity by age 7 and a 55% risk of overt diabetes by age 12. The eventual risk for anti-islet autoimmunity for this subgroup of siblings with further follow up is unknown but will almost certainly exceed 80% (Fig. 2). This subgroup not only has an enormous risk for T1D but also comprises a significant percentage of the cases of early childhood anti-islet autoimmunity identified in all participants of the large-scale DAISY study. Our preliminary results indicate that an even more rapid onset of islet autoimmunity may occur in DR3/4-DQ8 siblings that have the high-risk genotype for the insulin gene in addition to sharing both HLA haplotypes with their diabetic proband.
This study also illuminates the most probable explanation for the “relative paradox” of siblings having higher diabetes risk compared with offspring and the general population, despite all having matched high-risk DR-DQ genotypes. One possible explanation for the increased risk of siblings has been that “family based” shared environmental factors are a major contributor to disease. However, even though environmental factors may be crucial, if they are common (for instance, all individuals exposed to a triggering virus or dietary factor), they would not significantly contribute to familial aggregation of disease. Another proposed explanation has been that siblings have increased risk due to the polygenic inheritance of T1D, with multiple loci outside of the MHC contributing to risk. However, neither of these two initial explanations accounts for the dramatic difference in risk for DAISY DR3/4-DQ8 siblings sharing both HLA haplotypes versus those not sharing both haplotypes with their affected probands and confirmed by our independent analysis of DR3/4-DQ8 Human Biological Data Interchange siblings. Our results provide strong evidence to support the hypothesis that siblings, unlike offspring, can inherit both HLA haplotypes identical by descent with their sibling proband, implicating MHC-linked non-DR, DQ genes plus high-risk DR and DQ alleles.
The higher risk in children that share both HLA haplotypes with their diabetic siblings also suggests that the susceptibility allele or alleles may be inherited in a recessive manner (33). Regardless of the model of inheritance, it is likely that HLA-DR3-DQ2 and -DR4-DQ8 haplotypes shared by descent between affected siblings contain a higher proportion of high-risk alleles than HLA-DR3-DQ2 and -DR4-DQ8 haplotypes not shared by descent. We believe that the search for additional polymorphisms contributing to young-onset anti-islet autoimmunity should concentrate on detailed analysis of genes within or linked to the MHC.
It may be much easier to prevent anti-islet autoimmunity than to prevent progression to diabetes once anti-islet autoimmunity is initiated, as indicated by studies of animal models (34). Infants and children who have the highest genetic risk for diabetes as defined by this study are a major group that may now be considered for initial clinical trials to prevent childhood diabetes before the development of anti-islet autoantibodies.
Methods
Population.
DAISY is a prospective study in which blood samples from >33,000 young first-degree relatives of patients with T1D and general population newborns were obtained with informed parental consent and institutional review board oversight at the University of Colorado Health Sciences Center (27, 35). Survival curves in this article are based on prospective follow-up of both siblings and general population newborns from birth.
First-degree relatives (n = 1,058) and a subset of the general population children (n = 1,411) were followed prospectively for the development of anti-islet autoantibodies. Of children enrolled for follow-up, 49 full siblings and 51 offspring of T1D relatives and 398 general population children carried the highest-risk HLA-DR3/4-DQ8 genotype. The DAISY study also enrolled 319 non-HLA-DR3/4-DQ8 siblings of T1D relatives. Insulin, GAD65, and IA-2 autoantibody levels were measured at follow-up visits at 9, 15, and 24 months of age and annually thereafter in children with normal autoantibody levels (27). We obtained and analyzed parental, proband, and sibling DNA from 48 families of the 49 DR3/4-DQ8 siblings of patients with T1D to directly determine the number of HLA haplotypes shared identical by descent between siblings.
To further test the influence of haplotype sharing for DR3/4-DQ8 siblings, we analyzed available data from families of the Human Biological Data Interchange collection. From 273 families, we identified 73 DR 3/4-DQ8 siblings of diabetic proband children. The number of DR3/4-DQ8 children with diabetes sharing both haplotypes with their diabetic proband siblings (n = 42) was compared with those sharing zero or one haplotypes (n = 31).
Genotyping.
HLA-DRB1, HLA-DQB1, HLA-B, HLA-A, and HLA-DP genotyping of the DAISY siblings was performed with immobilized sequence-specific oligonucleotide genotyping similar to a previously described methodology (36). Family members other than the DAISY siblings were genotyped at the HLA-A locus only if one or more parents were homozygous at the HLA-DR and HLA-DQ loci to determine which HLA haplotypes were inherited and shared.
Siblings of probands from these families were also genotyped at the insulin and PTPN22 loci by using previously published methods (12, 37). The insulin polymorphism was detected by sequencing at the HphI locus. The PTPN22 polymorphism was detected by XcmI restriction enzyme digestion of amplified DNA.
Statistical Analysis.
The statistical difference between the survival curves for progression to anti-islet autoantibody positivity or diabetes in DAISY children was evaluated with the log-rank test with Prism software (Prism Software Corporation, Irvine, CA). The Fisher two-sided exact test was used to compare the number of individuals who developed persistent anti-islet autoantibodies and diabetes in the DR3/4-DQ8 siblings who shared both haplotypes versus those who did not share both haplotypes. The Fisher two-sided exact test was also used to evaluate data for uneven distribution of DRB1*04 subtypes, HLA-DP alleles, HLA-A alleles, and HLA-B alleles, and for risk associated with the insulin and PTPN22 loci in the DAISY siblings being studied. The alpha level for significance for the Fisher exact test was set at 0.05.
Cox proportional hazards regression analysis was performed on the DAISY sibling data with HLA haplotype sharing, maternal age at delivery, gender, and age at diagnosis of the proband as predictor variables. The likelihood ratio statistic was used to test the significance of the variables with an alpha level of significance set at 0.05.
Acknowledgments
We thank Brooke Hensley and Derrick Houser for laboratory assistance. This work was supported by National Institutes of Health Diabetes Autoimmunity Study of the Young (DAISY) Grants DK32083 and DK32493, Autoimmunity Prevention Center Grant AI50964, Diabetes Endocrine Research Center Grant P30DK57516, Clinical Research Centers Grants M01 RR00069 and M01 RR00051, Immune Tolerance Network Grant AI 15416, the American Diabetes Association, the Juvenile Diabetes Research Foundation, and the Children's Diabetes Foundation.
Abbreviations
- T1D
type 1A diabetes
- DR3
DRB1*03-DQB1*0201
- DR4
DRB1*04-DQB1*0302; DRB1*03-DQB1*0201/DRB1*04-DQB1*0302 (DR3/4-DQ8)
- DAISY
The Diabetes Autoimmunity Study of the Young.
Footnotes
The authors declare no conflict of interest.
References
- 1.Onengut-Gumuscu S, Concannon P. Immunol Rev. 2002;190:182–194. doi: 10.1034/j.1600-065x.2002.19014.x. [DOI] [PubMed] [Google Scholar]
- 2.Anjos S, Polychronakos C. Mol Genet Metab. 2004;81:187–195. doi: 10.1016/j.ymgme.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Pugliese A. Endocrinol Metab Clin North Am. 2004;33:1–16, vii. doi: 10.1016/S0889-8529(03)00082-3. [DOI] [PubMed] [Google Scholar]
- 4.Noble JA, Valdes AM, Cook M, Klitz W, Thomson G, Erlich HA. Am J Hum Genet. 1996;59:1134–1148. [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert AP, Gillespie KM, Thomson G, Cordell HJ, Todd JA, Gale EA, Bingley PJ. J Clin Endocrinol Metab. 2004;89:4037–4043. doi: 10.1210/jc.2003-032084. [DOI] [PubMed] [Google Scholar]
- 6.Nakanishi K, Kobayashi T, Murase T, Nakatsuji T, Inoko H, Tsuji K, Kosaka K. Diabetes. 1993;42:1086–1098. doi: 10.2337/diab.42.7.1086. [DOI] [PubMed] [Google Scholar]
- 7.Dawkins RL, Christiansen FT, Kay PH, Garlepp M, McCluskey J, Hollingsworth PN, Zilko PJ. Immunol Rev. 1983;70:1–22. doi: 10.1111/j.1600-065x.1983.tb00707.x. [DOI] [PubMed] [Google Scholar]
- 8.Valdes AM, Erlich HA, Noble JA. Hum Immunol. 2005;66:301–313. doi: 10.1016/j.humimm.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson WP, Barbosa J, Rich SS, Thomson G. Genet Epidemiol. 1993;10:273–288. doi: 10.1002/gepi.1370100502. [DOI] [PubMed] [Google Scholar]
- 10.Johansson S, Lie BA, Todd JA, Pociot F, Nerup J, Cambon-Thomsen A, Kockum I, Akselsen HE, Thorsby E, Undlien DE. Genes Immun. 2003;4:46–53. doi: 10.1038/sj.gene.6363917. [DOI] [PubMed] [Google Scholar]
- 11.Concannon P, Erlich HA, Julier C, Morahan G, Nerup J, Pociot F, Todd JA, Rich SS. Diabetes. 2005;54:2995–3001. doi: 10.2337/diabetes.54.10.2995. [DOI] [PubMed] [Google Scholar]
- 12.Bottini N, Muscumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, MacMurray J, Meloni G, Lucarelli P, Pellechia M, et al. Nature Genet. 2004;36:337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 13.Smyth D, Cooper JD, Collins JE, Heward JM, Franklyn JA, Howson JM, Vella A, Nutland S, Rance HE, Maier L, et al. Diabetes. 2004;53:3020–3023. doi: 10.2337/diabetes.53.11.3020. [DOI] [PubMed] [Google Scholar]
- 14.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, et al. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 15.Thomson G. Tissue Antigens. 1983;21:81–104. doi: 10.1111/j.1399-0039.1983.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 16.Thomson G, Robinson WP, Kuhner MK, Joe S, MacDonald MJ, Gottschall JL, Barbosa J, Rich SS, Bertrams J, Baur MP, et al. Am J Hum Genet. 1988;43:799–816. [PMC free article] [PubMed] [Google Scholar]
- 17.Valdes AM, Thomson G, Erlich HA, Noble JA. Diabetes. 1999;48:1658–1661. doi: 10.2337/diabetes.48.8.1658. [DOI] [PubMed] [Google Scholar]
- 18.Cudworth AG, Woodrow JC. Br Med J. 1975;3:133–135. doi: 10.1136/bmj.3.5976.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deschamps I, Lestradet H, Busson M, Hors J. Diabetes Res. 1986;3:391–396. [PubMed] [Google Scholar]
- 20.Tarn AC, Thomas JM, Dean BM, Ingram D, Schwarz G, Bottazzo GF, Gale EA. Lancet. 1988;1:845–850. doi: 10.1016/s0140-6736(88)91601-7. [DOI] [PubMed] [Google Scholar]
- 21.Blomhoff A, Lie BA, Myhre AG, Kemp EH, Weetman AP, Akselsen HE, Huseby ES, Undlien DE. J Clin Endocrinol Metab. 2004;89:3474–3476. doi: 10.1210/jc.2003-031854. [DOI] [PubMed] [Google Scholar]
- 22.Hanifi MP, de Knijf P, Roep BO, van der AB, Naipal A, Gorus F, Schuit F, Giphart MJ. Diabetes. 1998;47:263–269. [PubMed] [Google Scholar]
- 23.Inoue K, Ikegami H, Fujisawa T, Noso S, Nojima K, Babaya N, Itoi-Babaya M, Makimo S, Ogihara T. Diabetologia. 2004;47:739–747. doi: 10.1007/s00125-004-1370-2. [DOI] [PubMed] [Google Scholar]
- 24.Hattori M, Yamato E, Itoh N, Senpuku H, Fujisawa T, Yoshino M, Fukuda M, Matsumoto E, Toyonaga T, Nakagawa I, et al. J Immunol. 1999;163:1721–1724. [PubMed] [Google Scholar]
- 25.Barker JM, Goehrig SH, Barriga K, Hoffman M, Slover R, Eisenbarth GS, Norris JM, Klingensmith GJ, Rewers M. Diabetes Care. 2004;27:1399–1404. doi: 10.2337/diacare.27.6.1399. [DOI] [PubMed] [Google Scholar]
- 26.Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler AG, Bonifacio E. J Clin Invest. 2004;114:589–597. doi: 10.1172/JCI21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barker JM, Barriga KJ, Yu L, Miao D, Erlich HA, Norris JM, Eisenbarth GS, Rewers M. J Clin Endocrinol Metab. 2004;89:3896–3902. doi: 10.1210/jc.2003-031887. [DOI] [PubMed] [Google Scholar]
- 28.Hermann R, Bartsocas CS, Soltesz G, Vazeou A, Paschou P, Bozas E, Malamitsi-Puchner A, Simell O, Knip M, Ilonen J. Diabetes Metab Res Rev. 2004;20:322–329. doi: 10.1002/dmrr.455. [DOI] [PubMed] [Google Scholar]
- 29.Noble JA, Valdes AM, Thomson G, Erlich HA. Diabetes. 2000;49:121–125. doi: 10.2337/diabetes.49.1.121. [DOI] [PubMed] [Google Scholar]
- 30.Nishimaki K, Kawamura T, Inada H, Yagawa K, Nose Y, Nabeya N, Isshiki G, Tatsumi N, Niihira S. Diabetes Res Clin Pract. 2000;47:49–55. doi: 10.1016/s0168-8227(99)00103-5. [DOI] [PubMed] [Google Scholar]
- 31.Harjutsalo V, Podar T, Tuomilehto J. Diabetes. 2005;54:563–569. doi: 10.2337/diabetes.54.2.563. [DOI] [PubMed] [Google Scholar]
- 32.Tarn AC, Thomas JM, Dean BM, Ingram D, Schwarz G, Bottazzo GF, Gale EA. Lancet. 1988;1:845–850. doi: 10.1016/s0140-6736(88)91601-7. [DOI] [PubMed] [Google Scholar]
- 33.Larsen CE, Alper CA. Curr Opin Immunol. 2004;16:660–667. doi: 10.1016/j.coi.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Atkinson MA, Leiter EH. Nat Med. 1999;5:601–604. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- 35.Rewers M, Bugawan TL, Norris JM, Blair A, Beaty B, Hoffman M, McDuffie RS, Jr, Hamman RF, Klingensmith G, Eisenbarth GS, et al. Diabetologia. 1996;39:807–812. doi: 10.1007/s001250050514. [DOI] [PubMed] [Google Scholar]
- 36.Bugawan TL, Erlich HA. Immunogenetics. 1991;33:163–170. doi: 10.1007/BF01719235. [DOI] [PubMed] [Google Scholar]
- 37.Pugliese A, Miceli D. Diabetes Metab Res Rev. 2002;18:13–25. doi: 10.1002/dmrr.261. [DOI] [PubMed] [Google Scholar]