Abstract
An aversive tobacco abstinence syndrome, thought to reflect an underlying level of nicotine dependence, contributes to cigarette smokers’ failed quit attempts. Nicotine replacement therapy (NRT) suppresses tobacco abstinence, but high relapse rates suggest room for improvement. Improving NRT’s efficacy might begin with identifying factors that influence tobacco abstinence symptom suppression. Two such factors are smokers’ gender and NRT dose. The purpose of this study was to determine the dose-related effects of transdermal nicotine (TN) on tobacco abstinence symptoms in 75 men and 53 women who regularly smoked cigarettes but who had abstained from smoking for at least 8-12 hr. Participants completed 4 double-blind, randomized 6.5-hr laboratory sessions that differed by TN dose (0, 7, 21, or 42 mg). Each session included blood sampling for plasma nicotine level, measurement of heart rate, participants’ ratings of tobacco abstinence symptoms and effects of nicotine, and psychomotor performance. Increases in plasma nicotine level were related to TN dose and were independent of gender. TN-induced abstinence symptom suppression was dose-related for items assessing craving and urge to smoke and largely was independent of gender. TN increased heart rate and ratings of aversive side effects (e.g., nausea, lighthead-edness) in a dose-related manner, and women were more sensitive at higher doses. Results from this laboratory study support the continued use of TN as a pharmacotherapy. Higher doses may ameliorate some abstinence symptoms, although the side effect profile, at least in the short term, may limit effectiveness, especially for women.
Keywords: nicotine, abstinence, symptoms, gender, dose
In the United States, over 23% of men and 18% of women smoke tobacco cigarettes (Centers for Disease Control and Prevention [CDC], 2005). Of these, about 40% try to quit each year, but failure rates are high (CDC, 2005). One factor that contributes to failed quit attempts is an aversive tobacco abstinence syndrome (John, Meyer, Hapke, Rumpf, & Schumann, 2004; Piasecki et al., 2000). Tobacco abstinence includes urges to smoke, anxiety, rest-lessness, and difficulty concentrating (e.g., Buchhalter, Acosta, Evans, Breland, & Eissenberg, 2005; Hughes & Hatsukami, 1986; Hughes, Higgins, & Hatsukami, 1990). These effects are thought to reflect an underlying level of dependence on tobacco-delivered nicotine (e.g., U.S. Department of Health and Human Services [U.S. DHHS], 1988), giving rise to the notion that pharmacologically pure nicotine might suppress them (e.g., Eissenberg, Stitzer, & Henningfield, 1999; Shiffman, Fant, Buchhalter, Gitchell, & Henningfield, 2005). Indeed, numerous studies have demonstrated that, relative to placebo, active nicotine replacement therapy (NRT; including gum, inhaler, lozenge, nasal spray, and transdermal patch) suppresses tobacco abstinence symptoms (Hurt et al., 1998; Killen, Fortmann, Newman, & Varady, 1990; Molander, Lunell, & Fagerström, 2000; Muramoto, Ranger-Moore, & Leischow, 2003; Shiffman, Ferguson, Gwaltney, Balabanis, & Shadel, 2005) and roughly doubles quit rates (Fiore et al., 2000; Hughes, Goldstein, Hurt, & Shiffman, 1999; Silagy, Mant, Fowler, & Lodgy, 1994). However, even with NRT, relapse rates as high as 92% suggest room for improvement (Hughes, Shiffman, Callas, & Zhang, 2003; Winchell et al., 1997). One strategy for improving NRT’s efficacy might involve identifying factors that influence its ability to suppress tobacco abstinence effects. Two such factors are smokers’ gender and dose of NRT.
While NRT-induced suppression of tobacco abstinence symptoms is well-established, most studies that demonstrate it have not addressed gender differences clearly (e.g., Hughes, Gust, Keenan, & Fenwick, 1990; Jorenby et al., 1996) and/or have not been designed to assess these differences optimally (Fagerström, Schneider, & Lunell, 1993; Hajek, Jarvis, Belcher, Sutherland, & Feyerabend, 1989; Hurt et al., 1998; Leischow et al., 1997; Levin et al., 1994; Lunell, Molander, Leischow, & Fagerström, 1995; Perkins, Grobe, Stiller, Fonte, & Goettler, 1992; Pickworth, Fant, Butschky, & Henningfield, 1996; Rose, Herskovic, Trilling, & Jarvik, 1985; Schneider & Jarvik, 1984; Schneider, Jarvik, & Forsythe, 1984; West, Jarvis, Russell, Carruthers, & Feyerabend, 1984). One concern involves statistical sensitivity: Few studies have included the approximately 120 participants required to have a 70% chance of detecting a moderately sized gender effect (Cohen, 1988). Another concern, particularly relevant to lengthy clinical trials, involves infrequent, retrospective withdrawal assessments that may introduce recall bias (see Shiffman, Ferguson, et al., 2005). Nonetheless, two small sample studies are consistent with the notion that, relative to men, women report less NRT-induced suppression of abstinence symptoms (e.g., impatience; Hatsukami, Skoog, Allen, & Bliss, 1995) or signs (i.e., sleep disturbance; Wetter, Kenford, et al., 1999). Also, one clinical trial (Killen et al., 1990) indicated that nicotine gum did not produce the reduction in subjective cigarette craving in women that was observed for men. The need for more research on this topic, especially studies sensitive to gender differences, is frequently noted (e.g., Killen et al., 1990; Kinnunen, Nordstrom, Utman, & Garvey, 1998; Perkins, 2001; Wetter, Fiore, Young, McClure, & deMoor, 1999).
Dose may also be an important factor in NRT-induced suppression of tobacco abstinence symptoms, and may interact with gender. Several studies suggest that higher NRT doses produce better treatment outcome (Daughton et al., 1999; Sachs, Sawe, & Leischow, 1993; Tønnesen & Mikkelsen, 2000; Tønnesen et al., 1999) and/or greater abstinence symptom suppression (e.g., Dale et al., 1995), at least in some individuals and on some measures. The need for data to generate NRT dose effect curves on tobacco abstinence measures has been noted (Shiffman, Ferguson, et al., 2005), and ideally, these data should be collected in a way that reduces the likelihood of confounding through recall bias. Given the possibility that women may respond to nicotine differently than men (see reviews by Perkins, 1995,2001), and the potential for differential nicotine-induced abstinence symptom suppression in men and women, these dose-effect curve data should be generated with large samples of men and women.
The purpose of this study is to determine the dose-related effects of transdermal nicotine (TN) on tobacco abstinence symptoms in men and women who smoke cigarettes. Accordingly, 128 overnight-abstinent smokers (53 of whom were women) completed four double-blind, randomized 6.5-hr laboratory sessions that differed by transdermal nicotine dose (0, 7, 21, or 42 mg). Each session included regular assessment of plasma nicotine level, cardiovascular response, participant-rated tobacco abstinence and effects of nicotine, and psychomotor performance. We hypothesized that TN-induced abstinence symptom suppression would increase as dose increased and that, at least on some measures, suppression would be less for women than for men.
Method
Recruitment and Inclusion-Exclusion Criteria
Individuals were recruited for this study (approved by an institutional review board) with advertisements and word-of-mouth, and they were included if they were between the ages of 18 to 55 years and were self-reported smokers of ≥15 cigarettes per day for the past 2 years. In addition, each participant provided an afternoon expired air carbon monoxide (CO) sample of ≥15 parts/million (ppm) at screening and was healthy according to medical history and physical examination. Individuals were excluded if they were pregnant or breast-feeding or had any self-reported history of chronic health problems or psychiatric conditions. Any individuals who reported that they were currently trying to quit or reduce their cigarette intake were excluded. In addition, individuals who scored greater than 17 on the Beck Depression Inventory (Beck, Steer, & Brown, 1996) were excluded, because moderate depression could influence tobacco abstinence symptoms and might be exacerbated by smoking cessation (Covey, 1999).
Menstrual Cycle Phase
Several studies have suggested that menstrual cycle phase has little influence on tobacco abstinence symptomatology (e.g., Marks, Pomerleau, & Pomerleau, 1999; Masson & Gilbert, 1999; Pomerleau, Mehringer, Marks, Downey, & Pomerleau, 2000; Pomerleau, Tate, Lumley, & Pomerleau, 1994). Nonetheless, the potential effects of menstrual cycle phase were minimized by scheduling women’s participation during the follicular to early luteal phase of their menstrual cycle (i.e., Days 2-16) to control for the influence of premenstrual symptoms (participation across two cycles was permitted). Thus, although extant data suggest no need to control for menstrual cycle phase in short-term studies (Allen, Hatsukami, Christianson, & Nelson, 2000), careful scheduling of experimental sessions was used to limit any influence of hormonal cycle on study outcomes.
Demographic Summary
A total of 157 community volunteers consented to participate in this study, passed the in-person screening, and began their first scheduled session. Of these 157 participants, 6 withdrew because of nausea/vomiting (4 after receiving 42 mg nicotine, 1 after 21 mg, and 1 after 7 mg), while 16 were disqualified because of noncompliance (e.g., with presession smoking restrictions). In addition, participation was stopped for 3 individuals because their blood pressure was elevated and for 3 individuals because of difficulty obtaining blood samples. Finally, because of computer error, the data set for 1 participant who completed all sessions was substantially incomplete, and this individual’s data were excluded from all analyses.
As shown in Table 1, 75 men (37 non-White; mean age = 35.4 years, SD = 10.2) and 53 women (27 non-White; mean age = 32.3 years, SD = 2.1) completed the study with a complete data set. For these participants, mean afternoon expired CO levels were 24.8 (SD = 8.8) and self-reported daily cigarette intake was 22.9 (SD = 8.6). All participants were moderately dependent, as indicated by a mean score 5.5 (SD = 2.1) on the Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991). On average, smokers reported 3.4 (SD = 5.9) quit attempts. Thirty-five participants (18 men) reported previous use of nicotine-dependence pharmacotherapy, including 26 (13 men) who had used TN.
Table 1.
Statistical Analysis Results for Completed Participants’ Demographs Data by Gender
| Demographic variables | Men (N = 75) | Women (N = 53) | F | p |
|---|---|---|---|---|
| Generala | ||||
| %Caucasian | 50.7 | 49.1 | 0.0 | ns |
| % employed | 60.0 | 58.5 | 0.0 | ns |
| % not married | 74.7 | 69.8 | 0.4 | ns |
| Age in years | 35.4 (10.2) | 32.3 (10.4) | 2.9 | ns |
| Years education completed | 12.8 (1.8) | 13.2 (2.1) | 1.4 | ns |
| Body mass index | 26.3 (4.8) | 27.2 (7.2) | 0.8 | ns |
| Beck Depression Inventory | 4.8 (4.6) | 4.7 (4.1) | 0.0 | ns |
| Screening CO | 25.0 (8.4) | 24.6 (9.5) | 0.0 | ns |
| Cigarette smoking related | ||||
| Cigarettes per daya | 24.1 (10.0) | 21.3 (5.8) | 3.4 | ns |
| Duration of use in years a | 13.0 (9.3) | 9.1 (7.4) | 6.2 | <.01 |
| FTC tar yieldb | 14.6 (3.3) | 13.7 (3.9) | 1.6 | ns |
| FTC nicotine yieldc | 1.0 (0.3) | 1.0 (0.3) | 0.8 | ns |
| FTC CO yieldb | 14.8 (2.8) | 14.5 (3.5) | 0.3 | ns |
| No. quit attemptsa | 4.0 (7.4) | 2.6 (2.5) | 1.9 | ns |
| Fagerströma | 5.8 (2.2) | 5.1 (2.0) | 3.0 | ns |
| % nicotine medication naivea | 76.0 | 67.9 | 1.0 | ns |
| Alcohol relateda | ||||
| % use | 71.0 | 74.0 | 0.1 | ns |
| Use past 30 days | 5.0 (5.9) | 5.3 (7.4) | 0.1 | ns |
| Marijuana smoking relateda | ||||
| % use ever | 82.7 | 84.9 | 0.1 | ns |
| Use part 30 days | 2.2 (6.7) | 1.5 (4.4) | 0.4 | ns |
| Other drug relateda | ||||
| % used other drugs | 1.3 | 7.6 | 3.2 | ns |
Note. Data presented as means (SD) except where noted. CO = carbon monoxide; FTC = Federal Trade Commission.
df = 1, 126; all participants, N = 128.
df = 1, 115; data available for only 117 participants.
df = 1, 116; data available for only 118 participants.
Men and women did not differ significantly in terms of general characteristics (e.g., educational level, body mass index) or those related specifically to cigarettes, alcohol, or marijuana use, except for an observed difference in duration of use of cigarettes, with women smoking for about 4 years less than men, on average. The impact on study outcomes of this apparent difference in duration of use is uncertain, given that on the other demographic measures shown in Table 1 there were no differences between men and women (including measures more sensitive to potential differences in nicotine/tobacco dependence, such as the Fagerström Test for Nicotine Dependence). In fact, with 21 demographic variables in Table 1, one difference may reflect a chance outcome, rather than a population difference. Additionally, when duration of use was correlated with all area under the curve (AUC) data, only 7 of 104 possible correlations attained statistical significance (ps < .05, |rs| < .24). Because of the fact that the groups did not differ on other demographic measures, the likelihood of Type I error, and the generally unreliable correlation between duration of use and out-comes, this variable was not entered as a covariate in any analysis.
Procedure
After providing informed consent, participants scheduled four, approximately 6.5-hr long experimental conditions, with each condition corresponding to a TN dose (0, 7, 21, or 42 mg). Conditions were conducted double-blind and separated by at least 48 hr. On each condition day, participants reported to the laboratory at approximately 8 a.m. (time of day varied across participants, but was constant within-participant). Immediately after arrival, expired air CO level was measured (BreathCO; Vitalograph, Lenexa, KS) to verify prestudy cigarette abstinence: CO levels had to be less than 10 ppm (e.g., Breland, Buchhalter, Evans, & Eissenberg, 2002; Buchhalter, Schrinel, & Eissenberg, 2001). If the CO level did not meet criterion, participants waited until it did or had the option of rescheduling that day’s condition.
Once the CO criterion was met, women in the study provided a urine sample to test for pregnancy; a positive test led to exclusion from further participation. At session onset, participants were connected to equipment that recorded and monitored heart rate and blood pressure continuously. Then, a nurse placed a catheter in a forearm vein, and 10 ml of blood was sampled, centrifuged, and plasma was frozen for later determination of baseline nicotine levels. Participants then responded to a battery of computerized questionnaires and performance tasks to assess baseline responding. After completion of the tasks, a randomized TN dose was administered.
After TN administration, blood samples (10 ml each) were obtained at 30-min intervals for exactly 6 hr. Subjective measures were assessed every hour throughout each condition; physiological responses were recorded continuously. At the end of each 6-hr, postmedication period, the catheter was removed and the participant was paid $100 for his or her time; an additional $100 was paid after completing the entire study. Thus, participants who completed the entire study earned $500.
Transdermal Nicotine and Placebo Patches
NicoDerm CQ (GlaxoSmithKline Consumer Health Care, L.P.) TN was used for all active nicotine doses. Blood levels of nicotine delivered from this product peak within approximately 4 hr and then remain at a steady state (Henningfield & Keenan, 1993; Shiffman, Khayrallah, & Nowak, 2000), thus making it appropriate for a short-term study of TN-induced abstinence symptom suppression. The 7- and 21-mg TN doses were chosen because they covered the dose range currently approved for cessation. The 42-mg dose was chosen to ensure that dose effect functions covered the highest doses that could be delivered safely in a short-term study (Benowitz, Zevin, & Jacob, 1998; Frederickson et al., 1995).
TN dose is controlled, in part, by patch size (i.e., the 7-mg patch is 70 mm × 70 mm; the 21-mg patch is 220 mm × 220 mm). Participants always received one 7-mg-sized and two 21-mg-sized patches: None, one, or two could be active. Placebo patches (1-800-Patches, Salt Lake City, UT) were the same size but contained no nicotine. To strengthen the blinding procedures, patches were placed on each participant’s upper back by staff with minimal patient contact and were covered with taped gauze. The same staff member removed and disposed of the patches and gauze at the end of each condition.
Outcome Measures
Plasma nicotine level. Blood samples were centrifuged and the plasma was separated and stored at -70 °C. The plasma was analyzed for nicotine and cotinine with high performance liquid chromatography and liquid chromatography mass spectrometry (a modified version of that reported by Naidong, Shou, Chen, & Jiang, 2001; see Breland, Kleykamp, & Eissenberg, in press). This assay had a limit of quantitation of 2.0 ng/ml.
Heart rate. During each session, heart rate was measured every 20 s (and, for safety, blood pressure was measured every 5 min) by noninvasive computerized equipment (Noninvasive Patient Monitor Model 507E, Criticare Systems, Waukesha, WI). Data collection began immediately prior to patch administration and ended immediately after the last blood sample. Heart rate data were grouped into 30-min bins and averaged.
Participant-rated tobacco abstinence effects and direct effects of nicotine. All participant-rated measures were computerized (Plowshare Technologies, Baltimore), and volunteers responded to them using a computer mouse. The Hughes-Hatsukami questionnaire (adapted from Hughes & Hatsukami, 1986) consists of 11 visual analogue score (VAS) items that are sensitive to tobacco abstinence effects (e.g., Buchhalter et al., 2005): urges to smoke, irritability/frustration/anger, anxious, difficulty concentrating, rest-lessness, hunger, impatient, craving a cigarette/nicotine, drowsiness, depression/feeling blue, and desire for sweets. Each word or phrase is centered above a horizontal line; the left anchor is not at all and the right is extremely. Clicking a mouse-controlled cursor produces a vertical mark whose position on the line can be adjusted. The score is the distance of the vertical mark from the left anchor, expressed as a percentage of line length.
Tiffany and Drobes’s (1991) Questionnaire of Smoking Urges (QSU) is sensitive to tobacco abstinence effects (see also Buchhalter et al., 2005) and consists of 32 items rated on a 7-point scale (0 = strongly disagree, 6 = strongly agree) that yield two factors: Factor 1 (Intention to Smoke) and Factor 2 (Anticipation of Relief From Withdrawal).
Direct effects of transdermal nicotine were assessed after with-drawal assessment using 10 VAS items that included known nicotine effects (e.g., Gourlay, Forbes, Marriner, Pethica, & McNeil, 1995; Pullan et al., 1994): nauseous, dizzy, lightheaded, nervous, sweaty, headache, excessive salivation, heart pounding, confused, and weak.
Digit Symbol Substitution Test. The Digit Symbol Substitution Test (DSST; McLeod, Griffiths, Bigelow, & Yingling, 1982) is a performance measure sensitive to nicotine/tobacco abstinence (Eissenberg, Griffiths, & Stitzer, 1996) and consists of randomly selected digits appearing on the center of a video screen. Participants used the numeric keypad to reproduce a geometric pattern associated with a digit, according to the digit code presented at the top of the screen, and completed as many patterns as possible during the 90-s task presentation. Data collected included the number of trials attempted and the number of correct trials completed.
Missing Data
Computer malfunction, participant comfort (e.g., bathroom breaks), and human error resulted in approximately 1% missing data points. Where possible, the missing values were replaced by the average of the single values surrounding the missing points (e.g., Eissenberg et al., 1996). When several consecutive plasma nicotine values were unavailable, as occurred for 5 participants, the analysis was carried out by excluding the participants with missing data.
Data Analysis
All data were entered into a mixed analysis of variance (ANOVA) where gender was a between-subject variable and dose (4 levels: 0, 7, 21, 42 mg) and times were within-subject variables. The number of levels for the time variable varied depending on outcome measure. For plasma nicotine and subjective and performance measures, there were 7 levels (plasma samples were analyzed for baseline and hourly post-TN samples only). For heart rate and blood pressure there were 12 levels. Also, in order to clarify the effects of nicotine dose on study outcomes, all data were analyzed with AUC analysis (as in Strain, Moody, Stoller, Walsh, & Bigelow, 2004). For all analyses, Huynh-Feldt corrections adjusted for violations of the sphericity assumption (Huynh & Feldt, 1976). Differences between means were examined using Tukey’s honestly significant difference (HSD; Keppel, 1991). Comparisons for which p < .05 are reported as significant.
Results
This study examined the ability of transdermal nicotine doses to suppress tobacco abstinence in men and women over 6 hr. Statistical analysis results are reported in Tables 2 (time course) and 3 (AUC). The study was most concerned with potential dose-related effects over time that may differ by smokers’ gender; thus, the results of greatest interest involve interactions among these variables, as high-lighted below.
Table 2.
Statistical Analysis Results for All Measures for Main and Interaction Effects
| Dose |
Time |
Dose × Time |
Dose × Gender |
Tme × Gender |
Dose × Time × Gender |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | F | p | F | p | F | p | F | p | F | p | F | p |
| Plasma nicotinea | 906.7 | <.001 | 466.1 | <.001 | 235.1 | <.001 | 1.0 | ns | 1.6 | ns | 1.3 | ns |
| Heart rateb | 114.3 | <.001 | 108.0 | <.001 | 13.6 | <.001 | 3.6 | <0.5 | 3.0 | <.05 | 1.3 | ns |
| Hughes—Hatsukamic | ||||||||||||
| Urges to smoke | 12.6 | <.001 | 65.0 | <.001 | 2.6 | <.005 | 0.3 | ns | 0.7 | ns | 1.4 | ns |
| Irrationality/frustration/anger | 3.3 | <.05 | 22.2 | <.001 | 2.2 | <.05 | 0.5 | ns | 1.2 | ns | 2.5 | <.005 |
| Anxious | 2.8 | <.05 | 26.6 | <.001 | 0.9 | ns | 0.7 | ns | 0.4 | ns | 1.0 | ns |
| Difficulty concentrating | 1.6 | ns | 5.7 | <.001 | 1.2 | ns | 1.7 | ns | 0.8 | ns | 0.5 | ns |
| Restlessness | 0.5 | ns | 3.9 | <.05 | 1.2 | ns | 0.7 | ns | 1.2 | ns | 1.2 | ns |
| Hunger | 1.4 | ns | 77.2 | <.001 | 1.7 | ns | 2.5 | ns | 3.6 | <.05 | 1.4 | ns |
| Impatient | 3.7 | <.05 | 11.7 | <.001 | 1.5 | ns | 1.1 | ns | 1.0 | ns | 1.0 | ns |
| Craving a cigarette/nicotine | 14.2 | <.001 | 54.5 | <.001 | 4.0 | <.001 | 1.1 | ns | 0.7 | ns | 1.6 | ns |
| Drowsiness | 1.6 | ns | 13.4 | <.001 | 1.4 | ns | 1.8 | ns | 0.5 | ns | 0.8 | ns |
| Depression/feeling blue | 2.0 | ns | 3.2 | <.05 | 0.9 | ns | 0.3 | ns | 1.0 | ns | 0.9 | ns |
| Desire for sweets | 1.8 | ns | 3.3 | <.05 | 0.7 | ns | 4.9 | <.005 | 1.5 | ns | 1.1 | ns |
| QSUc | ||||||||||||
| Factor 1 | 19.7 | <.001 | 56.2 | <.001 | 6.0 | <.001 | 3.2 | <.05 | 1.3 | ns | 1.7 | ns |
| Factor 2 | 11.9 | <.001 | 44.3 | <.001 | 6.0 | <.001 | 1.2 | ns | 1.3 | ns | 1.7 | ns |
| Direct effects | ||||||||||||
| Nauscousc | 22.6 | <.001 | 8.2 | <.001 | 5.8 | <.001 | 1.2 | ns | 2.2 | ns | 1.0 | ns |
| Dizzyc | 17.9 | <.001 | 5.3 | <.005 | 4.6 | <.001 | 1.2 | ns | 1.8 | ns | 2.1 | <.05 |
| Lightheadedd | 16.3 | <.001 | 6.3 | <.001 | 3.6 | <.001 | 2.6 | ns | 0.9 | ns | 1.3 | ns |
| Nervousc | 9.5 | <.001 | 4.7 | <.005 | 1.8 | ns | 2.0 | ns | 1.2 | ns | 0.8 | ns |
| Sweatyc | 13.8 | <.001 | 1.2 | ns | 3.9 | <.001 | 3.2 | <0.5 | 0.4 | ns | 0.4 | ns |
| Headachec | 3.1 | <.05 | 3.8 | <.01 | 1.6 | ns | 0.7 | ns | 0.9 | ns | 0.7 | ns |
| Salivationd | 4.8 | <.005 | 0.6 | ns | 1.2 | ns | 1.2 | ns | 0.4 | ns | 0.5 | ns |
| Heart poundingc | 8.3 | <.001 | 4.8 | <.01 | 1.4 | ns | 0.1 | ns | 1.3 | ns | 0.8 | ns |
| Confusedc | 3.8 | <.05 | 3.4 | <.05 | 1.7 | ns | 1.5 | ns | 2.1 | ns | 1.7 | ns |
| Weakc | 19.2 | <.001 | 9.6 | <.001 | 4.6 | <.001 | 1.5 | ns | 1.1 | ns | 1.3 | ns |
| DSSIe | 1.0 | ns | 4.8 | <.005 | 0.9 | ns | 0.9 | ns | 0.3 | ns | 1.7 | ns |
Note. No main effects of gender were significant, so these results have been omitted. Dose, Dose × Gender: a(3, 363) b(3, 378) c(3, 378)d(3, 357) e(3, 375); time. Time × Gender: a(6, 762), b(11, 1386), c(6, 756), d(6, 714), e(6, 750); dose × Time, Dose × Time × Gender: a(18, 2178), b(33, 4158), c(18, 2268), d(18, 2142), e(18, 2250), QSU = Questionnaire of Smoking Urges; DSST = Digit Symbol Substitution Test.
Table 3.
Statistical Analysis Results for All Measures for Area Under the Curve
| Dose |
Gender |
Dose × Gender |
||||
|---|---|---|---|---|---|---|
| Measure | F | p | F | p | F | p |
| Plasma nicotinea | 1,023.7 | <.001 | 0.5 | ns | 1.2 | ns |
| Heart maleb | 123.2 | <.001 | 3.5 | ns | 3.5 | <.05 |
| Hughes-Hatsukamib | ||||||
| Urges to smoke | 13.3 | <.001 | 0.2 | ns | 0.8 | ns |
| Irrationality/frastration/anger | 4.1 | <.05 | 0.4 | ns | 0.5 | ns |
| Irrationality/frustration/anger | 4.1 | <.05 | 0.4 | ns | 0.5 | ns |
| Anxious | 2.8 | <.05 | 0.0 | ns | 0.6 | ns |
| Difficulty concentrating | 1.8 | ns | 1.1 | ns | 2.1 | ns |
| Restlessness | 1.3 | ns | 0.9 | ns | 1.1 | ns |
| Hunger | 1.9 | ns | 0.1 | ns | 2.2 | ns |
| Impatient | 4.6 | <.01 | 0.0 | ns | 0.6 | ns |
| Craving a cigarette/nicotine | 15.0 | <.001 | 0.1 | ns | 1.7 | ns |
| Drowsiness | 1.7 | ns | 1.8 | ns | 2.0 | ns |
| Depression/feeling blue | 2.2 | ns | 0.0 | ns | 0.3 | ns |
| Desire for sweets | 2.4 | ns | 0.7 | ns | 5.0 | <.005 |
| QSUb | ||||||
| Factor 1 | 21.0 | <.001 | 0.3 | ns | 3.2 | <.05 |
| Factor 2 | 14.8 | <.001 | 0.9 | ns | 1.4 | ns |
| Direct effects | ||||||
| Nauscousa | 25.1 | <.001 | 0.9 | ns | 3.4 | ns |
| Dizzyb | 19.2 | <.001 | 1.5 | ns | 0.9 | ns |
| Lightheadedc | 19.6 | <.001 | 1.4 | ns | 3.2 | <.05 |
| Nervousb | 10.7 | <.001 | 0.8 | ns | 1.7 | ns |
| Sweatyb | 16.0 | <.001 | 0.4 | ns | 2.6 | ns |
| Headacheb | 3.6 | <.05 | 1.0 | ns | 0.4 | ns |
| Salvationc | 6.5 | <.005 | 0.6 | ns | 1.1 | ns |
| Heart poundingb | 9.4 | <.001 | 0.7 | ns | 0.0 | ns |
| Confusedb | 3.8 | <.05 | 1.6 | ns | 1.3 | ns |
| Weakb | 21.5 | <.001 | 3.5 | ns | 2.1 | ns |
| DSSTb | 2.0 | ns | 3.5 | ns | 0.4 | ns |
Note. Dose, Dose × Gender: a(3, 363) b(3, 357); gender: a(1, 121), b(1, 126), c(1, 119). QSU = Questionnaire of Smoking Urges; DSST = Digit Symbol Substitution Test.
Plasma Nicotine
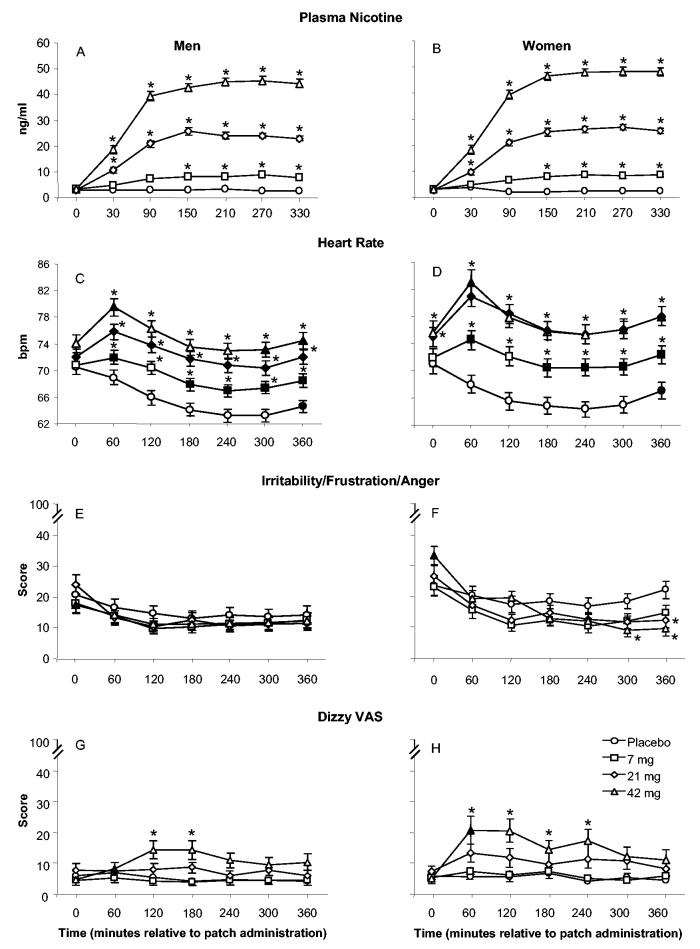
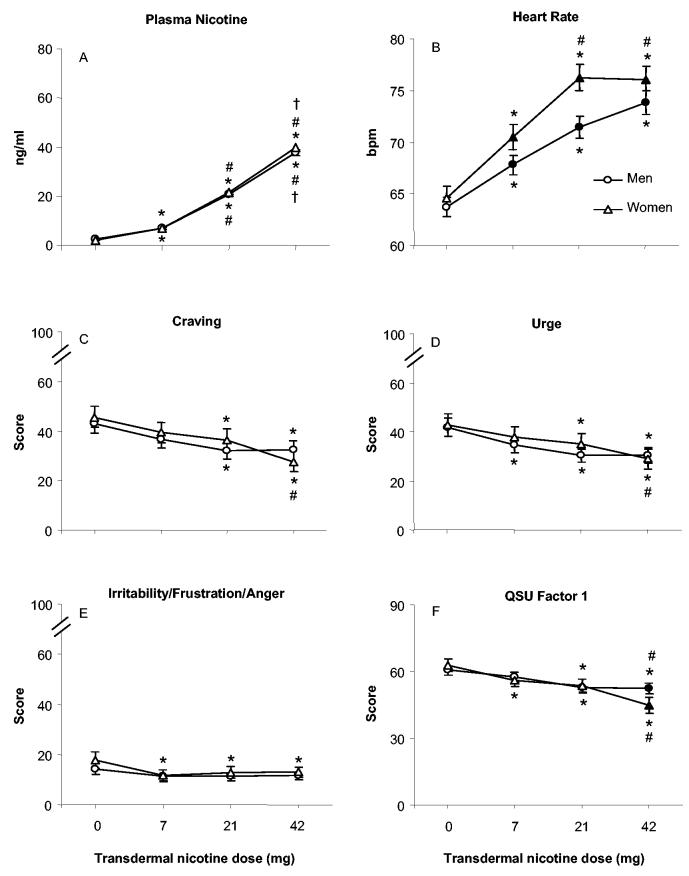
Plasma nicotine levels did not differ by gender, and Table 2 shows a significant Dose × Time interaction. Figures 1A and 1B show that, for men and women, plasma nicotine level increased significantly, relative to baseline, for all active doses by 150 min after TN administration (30 min for the 21- and 42-mg doses). Nicotine levels peaked at approximately 150 to 210 min. Plasma nicotine showed clear dose-related increases, also indicated by the significant effect of dose for the AUC data (see Table 3 and Figure 2A). With AUC data, collapsed across gender, mean plasma nicotine level was 2.4 ng/ml (SD = 1.5) for placebo, which differed significantly from the mean of 7.0 ng/ml (SD = 3.5) observed for 7 mg, the mean of 21.1 ng/ml (SD = 7.6) observed for 21 mg, and the mean of 38.5 ng/ml (SD = 11.1) observed for 42 mg (ps < .05, Tukey’s HSD). In addition, the mean plasma nicotine level observed for the 42-mg nicotine dose was significantly greater than that observed for the 21-mg dose, which was significantly greater than the mean observed for the 7-mg dose (all ps < .05, Tukey’s HSD).
Figure 1.
Mean data (±1 SEM) for plasma nicotine (Panels A and B; N = 123, with 51 women), heart rate (Panels C and D; N = 128, with 53 women), irritability/frustration/anger visual analogue score (VAS; Panels E and F; N = 128), and dizzy VAS (Panels G and H; N = 128) for men (left) and women (right) by dose and time. Filled symbols indicate a significant difference between men and women at that time within that dose, and asterisks indicate a significant difference from placebo at that time. All ps < .05, Tukey’s HSD.
Figure 2.
Mean area under the curve data (±1 SEM) for men and women by dose (0, 7, 21, or 42 mg transdermal nicotine), collapsed across time, for plasma nicotine (Panel A; N = 123, with 51 women), heart rate (Panel B; N = 128, with 53 women), craving visual analogue score (VAS; Panel C; N = 128), urge VAS (Panel D; N = 128), irritability/frustration/anger VAS (Panel E; N = 128), and Questionnaire of Smoking Urges (QSU; Tiffany & Drobes, 1991) Factor 1 (Panel F; N = 128). Filled symbols indicate a significant difference between men and women at that dose; asterisks indicate a significant difference from placebo for men and women; number signs indicate a significant difference from 7 mg for men and women; and daggers indicate a significant difference from 21 mg for men and women. All ps < .05, Tukey’s HSD.
Heart Rate
For heart rate, Table 2 shows a significant Dose × Time interaction, as well as significant Dose × Gender and Time × Gender interactions. The averaged data are displayed in Figures 1C and 1D. Heart rate for men and women decreased over time in the placebo condition: Bradycardia is a sign of nicotine/tobacco abstinence (American Psychiatric Association, 1994). Relative to placebo, all active doses produced significantly greater heart rate beginning 1 hr after TN administration. For men and women, heart rate increased significantly 60 to 90 min after 21- and 42-mg patch administration (and after 7 mg for women only; all ps < .05, Tukey’s HSD). Heart rate showed clear dose-related increases, as indicated by the significant main effect of dose for the AUC data (see Table 3 and Figure 2B); a significant Dose × Gender interaction was also observed. For women and men, significant increases in heart rate, relative to placebo, were apparent at each active dose, with greater increases for women at 7 and 21 mg (despite plasma nicotine levels that did not differ, see earlier discussion; all ps < .05, Tukey’s HSD).
Participant-Rated Tobacco Abstinence Effects and Direct Effects of Nicotine
Table 2 reveals a significant Dose × Time × Gender interaction for the VAS item assessing irritability/frustration/anger, and the averaged data are displayed in Figures 1E and 1F. As the figures show, Tukey’s HSD revealed that the baseline score for 42-mg TN for women (32.0, SD = 35.4) was significantly higher than the baseline score for 42-mg TN for men (17.5, SD = 24.7; p < .05). In addition, the postbaseline pattern of results was different for men and women on this VAS item. For men, across all active dose conditions, the mean VAS scores differed from baseline at several times (120 min after patch application for 7 mg; 60, 120, 180, 240, 300, and 360 min after patch application for 21-mg TN), and there were no significant differences between any active dose and placebo at any time. In contrast, for women, the mean scores for active TN doses at most times were significantly lower as compared with baseline. By session’s end, for women, relative to baseline, mean scores for 42 mg of nicotine decreased 70.8%, mean scores for 21 mg decreased 53.8%, and mean scores for 7 mg of TN decreased 36.5%. In addition, by the end of the session, scores in the 21- and 42-mg dose conditions were significantly lower than placebo (all ps < .05).
Table 2 reveals two other significant interactions involving gender for VAS measures of abstinence effects: a Time × Gender interaction for hunger and a Gender × Dose interaction for desire for sweets. For hunger, at the session onset men’s mean ratings were slightly higher (M = 25.0, SD = 30.6) as compared with women’s (M = 20.3, SD = 26.0), but by the end of the session, men’s mean scores were slightly lower (M = 44.2, SD = 36.3) than women’s (M = 49.7, SD = 33.2; all ns, Tukey’s HSD). For desire for sweets, a Gender × Dose interaction was observed. For men, mean ratings on this item were steady across the four dose conditions: for placebo, the mean was 17.6 (SD = 24.5), for 7 mg the mean was 18.3 (SD = 24.7), for 21 mg the mean was 17.8 (SD = 25.4), and for 42 mg the mean was 19.3 (SD = 27.3; ns, Tukey’s HSD). For women, more variability across doses was apparent: for placebo, the mean was 28.4 (SD = 29.7), for 7 mg the mean was 21.2 (SD = 25.8), for 21 mg the mean was 20.9 (SD = 24.0), and for 42 mg the mean was 17.8 (SD = 21.6; ns, Tukey’s HSD).
For AUC data, significant dose effects were observed for several measures of tobacco abstinence, including the VAS items urges to smoke, irritability/frustration/anger, craving a cigarette/nicotine, and both factors of the QSU (a significant Dose × Gender interaction was also observed for QSU Factor 1). The data for these items, for men and women, are displayed in Figure 2 (Panels C-F). Generally, the main effect of dose reflects significantly lower scores in the 21- and 42-mg conditions relative to placebo (except for irritability/frustration/anger, where significant reductions, relative to placebo, were observed for women only and across all active conditions). In addition to lower mean scores in the 21- and 42-mg conditions relative to placebo, for urges to smoke and craving, women’s mean scores for the 42-mg condition were significantly lower than those of the 7-mg condition. For Factor 1 of the QSU, mean scores in the 42-mg condition were significantly lower than in the 7-mg condition for men and women. Moreover, at the 42-mg dose, women’s mean score (44.7, SD = 26.0) was significantly lower than men’s (52.3, SD = 19.5; p < .05, Tukey’s HSD). For Factor 2 of the QSU, for which a main effect of dose was observed, mean data collapsed across gender for the 42-mg (21.4, SD = 14.8), 21-mg (23.7, SD = 15.0), and 7-mg (25.0, SD = 16.1) conditions were significantly lower than placebo (27.9, SD = 16.4), and mean of the 7-mg condition was significantly higher than the 42-mg condition (all ps < .05, Tukey’s HSD).
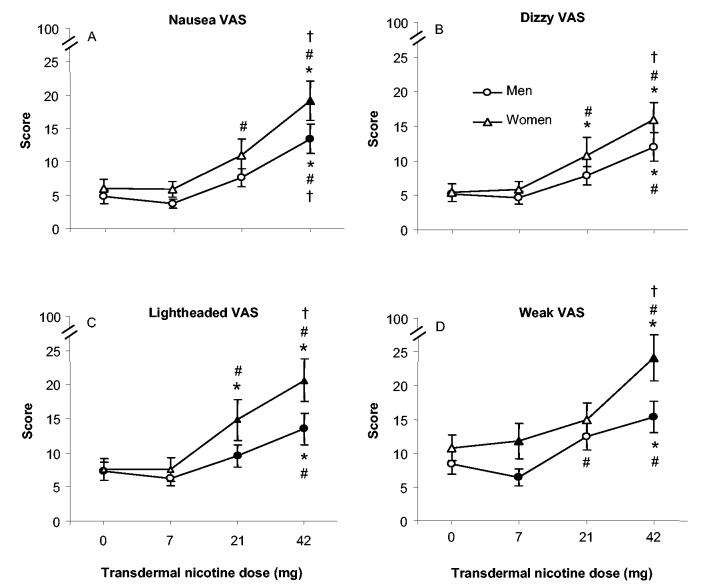
For participant-reported measures of the direct effects of nicotine, there were few significant interactions involving gender, although Table 2 reveals many significant interactions of dose and time, and Table 3 reveals many significant main effects of dose. With respect to gender, for time course data, there was a significant Dose × Time × Gender interaction for the VAS item assessing dizziness and a significant Dose × Gender interaction for sweatiness. The data for the VAS item dizzy are displayed in Figures 1G (men) and 1H (women). As the figures show, there was substantial variability, but generally, women experienced more dizziness in the 21- and 42-mg TN conditions, especially within the first hour after dosing. For example, women’s mean VAS score for the 42-mg TN at 60 min after patch application (20.5, SD = 29.9) was significantly greater than men’s (8.5, SD = 15.7; p < .05, Tukey’s HSD). Compared with placebo, self-reported ratings of dizziness were significantly higher in the 42-mg condition at 120 and 180 min after the patch application for men, and at 60, 120, 180, and 240 min after the patch application for women. Additionally, the 21-mg TN significantly increased dizziness for women at the first hour after dosing. For the VAS item sweaty, the greatest between-gender difference was observed at the 42-mg TN with women reporting greater mean scores (12.4, SD = 20.8) as compared with men (8.6, SD = 16.5; ns, Tukey’s HSD). With regard to the interactions of dose and time, the 42-mg nicotine condition produced higher ratings of nauseous, lightheaded, and weak at almost every time point from 60 to 360 min after patch administration. For example, collapsed across gender, for the 42-mg dose condition, the mean rating of nauseous was 3.9 (SD = 8.6) at baseline; this rating increased significantly to as much as 18.8 (SD = 28.1) at 3 hr after patch administration and remained significantly elevated (relative to baseline) at the end of the 6-hr after dosing evaluation period (M = 11.8, SD = 22.0; ps < .05, Tukey’s HSD).
TN’s dose-related direct effects were also apparent using the AUC data. Figure 3 shows data from the four measures with the greatest F values for the main effects of dose: nausea, dizzy, lightheaded, and weak (a Dose × Gender interaction was also observed for lightheaded). As seen in the figure, significant increases relative to placebo were observed at the 42-mg dose for all measures and, for women, at the 21-mg dose for dizzy and lightheaded. Between-gender differences were also observed for two of these measures: for lightheaded at 21 mg (men’s M = 9.6, SD = 13.8; women’s M = 14.9, SD = 20.3) and 42 mg (men’s M = 13.5, SD = 19.6; women’s M = 20.6, SD = 21.2) and for weak at 7 mg (men’s M = 6.4, SD = 10.5; women’s M = 11.8, SD = 19.3) and 42 mg (men’s M = 15.4, SD = 19.4; women’s M = 24.1, SD = 25.1; ps < .05, Tukey’s HSD).
Figure 3.
Mean area under the curve data (±1 SEM) for men and women by dose (0, 7, 21, or 42 mg transdermal nicotine), collapsed across time, for visual analogue score (VAS) items nausea (Panel A; N = 128, with 53 (visual analogue score) women), dizzy (Panel B; N = 128), lightheaded (Panel C; N = 121, with 53 women), and weak (Panel D; N = 128). Filled symbols indicate a significant difference between men and women at that dose; asterisks indicate a significant difference from placebo for men and women; number signs indicate a significant difference from 7 mg for men and women; and daggers indicate a significant difference from 21 mg for men and women. All ps < .05, Tukey’s HSD.
Digit Symbol Substitution Test
There were no significant main effects or interactions observed for the DSST, except for a significant improvement over time: Collapsed across session, mean percentage correct increased from 93.48% (SD = 12.5) at baseline to 95.62% (SD = 7.8) at 360 min after patch application (p < .005).
Discussion
Periods of tobacco abstinence result in a well-defined, quantifiable syndrome that includes observable signs, including decreased heart rate (Buchhalter et al., 2005), behavioral decrements (e.g., Eissenberg et al., 1996), and aversive symptoms (irritability, headache, hunger, restlessness, impatience; Hughes & Hatsukami, 1986). This abstinence syndrome likely contributes to difficulty in quitting (Fagerström et al., 1993; Hughes & Hatsukami, 1986; Killen, Fortmann, Kraemer, Varady, & Newman, 1992), and suppressing it is one putative mechanism of action underlying the effectiveness of NRT (Hughes, Hatsukami, Pickens, & Svikis, 1984; Hurt et al., 1998; Leischow et al., 1997; Molander et al., 2000; West & Shiffman, 2001; West et al., 1984). Some clinical trials suggest that, relative to men, abstinence rates are lower for women using TN (Bohadana, Nilsson, Rasmussen, & Martinet, 2003; Gourlay, Forbes, Marriner, Pethica, & McNeil, 1994; Nørregaard, Tonnesen, & Petersen, 1993; Swan, Jack, & Ward, 1997; Transdermal Nicotine Study Group, 1991), and this potential for decreased efficacy in women may reflect differential suppression of abstinence symptoms. Abstinence symptom suppression may also be dependent on NRT dose (e.g., Dale et al., 1995). This study was designed to examine the influence of gender and dose on TN-induced abstinence symptom suppression in an acute laboratory setting. Results revealed that tobacco abstinence produced a variety of observable signs and symptoms, some of these symptoms were suppressed by active TN, and gender had little influence on TN-induced abstinence symptom suppression. TN also produced a range of side effects, to which women were more sensitive.
Signs and Symptoms of Nicotine/Tobacco Abstinence
In this study, 8-12 hr of objectively verified tobacco abstinence was required before each of four sessions, to provide an abstinence symptom baseline that might be suppressed by TN. As expected, mean presession scores for many abstinence measures were elevated relative to scores observed in smokers who are not undergoing a period of tobacco abstinence (e.g., irritability/frustration/anger; see Buchhalter et al., 2005). Overall, the signs and symptoms observed at baseline in this study are consistent with those observed in previous research where nicotine/tobacco abstinence has been documented under controlled conditions (D. Gilbert et al., 2004; R. Gilbert & Pope, 1982; Hatsukami, Hughes, & Pickens, 1985; Hatsukami, Hughes, Pickens, & Svikis, 1984; Knapp, Bliss, & Wells, 1963; Myrsten, Elgerot, & Edgren, 1977; Persico, 1992; Teneggi et al., 2002; West & Russell, 1987). As has been reported elsewhere (Foulds et al., 1997; Hughes, Gust, Skoog, Keenan, & Fenwick, 1991; Hughes & Hatsukami, 1986; Shiffman et al., 2000; Ward, Swan, & Jack, 2001), the baseline signs and symptoms of nicotine/tobacco abstinence observed in this study generally were independent of gender.
TN-Induced Suppression of Tobacco Abstinence
TN increased plasma nicotine levels in a dose-dependent manner, and active TN suppressed nicotine/tobacco abstinence signs and symptoms, at least partially. First, plasma nicotine levels were low at baseline in all conditions (consistent with presession abstinence) and, as reported elsewhere (e.g., Fant, Henningfield, Shiffman, Strahs, & Reitberg, 2000; Henningfield & Kennan, 1993; Ingram et al., 2004; Mendelson, Sholar, Goletiani, Siegel, & Mello, 2005), increased as TN dose increased in the active conditions only (peak levels occurred approximately 3-4 hr after TN administration; see Figures 1A and 1B). These results are important, as they demonstrate (a) participants’ compliance with the required presession smoking abstinence, (b) the effectiveness of the TN preparation used in the study, and (c) the fact that, under placebo dosing conditions, participants did not receive nicotine. More generally, plasma nicotine results demonstrate that 7-, 21-, and 42-mg TN doses result in plasma nicotine levels that approximate these doses.
Second, TN suppressed, in a dose-dependent manner, the bradycardia observed under placebo conditions (see Figure 1C and 1D), as reported elsewhere with a variety of methods of nicotine delivery (e.g., Garrett & Griffiths, 2001; Jones, Garrett, & Griffiths, 1999; Krivokapich, Schneider, Child, & Jarvik, 1985). TN-induced increases in heart rate occurred within 60 min after administration, consistent with previous studies that indicate that transdermal nicotine can increase heart rate after 30 min (Rose et al., 1985).
Third, AUC data revealed that TN reliably suppressed many tobacco abstinence symptoms (see Figures 2C-2F). In many cases, this TN-induced abstinence symptom suppression was independent of dose for men and women (e.g., irritability/frustration/anger, see Figure 2E; also true for anxious VAS). However, dose-dependent symptom suppression was observed on some measures. For example, on Factor 1 of the QSU, for men and women, 42-mg TN suppressed scores relative to 7-mg nicotine (see Figure 2F). Also, AUC data for craving a cigarette/nicotine showed clear dose-related suppression when collapsed across gender. For craving a cigarette/nicotine, the mean AUC VAS scores were 37.7 (SD = 29.4) for 7-mg TN, 33.9 (SD = 28.4) for 21-mg TN, and 30.3 (SD = 28.4) for 42-mg TN (Tukey’s HSD revealed that the difference between 7 and 42 mg was significant, p < .05). A similar pattern was observed for urges to smoke. Although they are not abstinence symptoms according to the Diagnostic and Statistical Manual of Mental Disorders (4th ed.; American Psychiatric Association, 1994), craving and urge for cigarettes are commonly reported reasons for relapse (Nørregaard et al., 1993), can increase in intensity minutes after finishing a cigarette (Schuh & Stitzer, 1995), and may reach peak levels within 6-24 hr of abstinence (Maude-Griffin & Tiffany, 1996). Therefore, craving and urge are important abstinence symptoms that must be addressed during a quit attempt. Higher TN doses may be more effective at suppressing these symptoms.
The failure to observe TN dose-related suppression on some abstinence measures is inconsistent with results from some clinical trials (Dale et al., 1995; Hatsukami et al., 1995; Leischow et al., 1997) and may reflect a difference in participants (i.e., nontreatment seeking vs. actively trying to quit) and/or study duration (i.e., 6 hr vs. several days). The current results and previous reports (Dale et al., 1995; Hatsukami et al., 1995; Leischow et al., 1997) support the notion that TN suppresses many symptoms reported by abstinent smokers (i.e., craving, urges to smoke, irritability/frustration/anger, and anxiety). Thus, this study highlights one mechanism by which TN helps abstinent smokers avoid relapse. However, these results also suggest that some symptoms are not suppressed by TN (e.g., difficulty concentrating, restlessness, hunger, impatience), at least under the conditions reported here. To the extent that TN does not suppress some abstinence symptoms in a dose-related manner and does not suppress other symptoms at all, clinicians may want to consider carefully the specific TN dose that they recommend.
The Effects of Gender on TN-Induced Suppression of Nicotine/Tobacco Abstinence Symptoms
This study was designed to examine the effects of gender on TN-induced abstinence symptoms suppression. Of the 260 total F tests performed in this study (with an alpha level of .05; see Tables 2 and 3), only 12 involving a gender variable were significant (3 for physiological effects, 6 for abstinence symptoms, and 3 for direct effects). By chance, 13 significant F tests might be expected. Thus, these results provide little support for the notion that gender differences in TN-induced abstinence symptom suppression underlie differential treatment outcomes that have been reported in some clinical trials.
Some observed differences between men and women were likely not due to TN. For example, gender differences for irritability/frustration/anger involved women’s higher baseline (i.e., predosing) scores in the 42-mg TN condition. This result, recorded prior to TN administration, may reflect the observation that women are more likely than men to report subjective states of withdrawal (Franklin, 2005). This high baseline in the 42-mg condition may indicate greater TN-induced suppression of irritability/frustration/anger with the 42-mg TN dose for women, as they reported a mean 70.8% decrease from baseline to end of session scores, as opposed to a 29.9% decrease for men. Also, a significant Time × Gender interaction was observed for the hunger VAS; because no significant main effects or interactions involving the dose variable were observed for this measure, these results cannot be explained by a differential response to TN.
Other observed differences between men and women may reflect a differential effect of TN. For example, women’s heart rate was significantly higher than men’s at all active TN doses (see Figure 2B). Also, a gender difference in the pattern of response was observed on the VAS measures of desire for sweets: Mean ratings in the placebo condition were greater for women and, relative to placebo, were significantly lower in active patch conditions for women only. Gender differences were also observed for QSU Factor 1 Intention to Smoke. As shown in Figure 2F, significantly lower QSU Factor 1 scores were observed for women relative to men in the highest TN dose condition. Overall, the pattern of results for men and women observed in this study does not provide strong support for a gender difference in TN-induced abstinence symptom suppression. Thus, this study, by itself, cannot explain why NRT, the most widely used treatment strategy for smoking cessation, appears to be less effective for women in some studies (e.g., Wetter, Kenford, et al., 1999; Perkins & Scott, 2005). However, this study did not address TN’s ability to blunt the effects of a concurrently administered cigarette or the influence of smoking-related stimuli. Gender differences related to these variables may be relevant to differential outcome (e.g., Perkins et al., 2001).
TN-Induced Side Effects
TN increased reports of the direct effects of nicotine in a dose-related manner, with some gender differences, especially at higher doses (see Figures 1G and 1H and Figure 3). For example, for men and women, dose-related increases were observed for ratings of dizzy, lightheaded, and weak, such that mean ratings observed for the 42-mg dose were significantly greater than those for the 7-mg dose (ps < .05). For nausea, lightheaded, and weak, women’s mean ratings in the 42-mg condition were significantly higher than men’s. These results support the suggestion that women may be more sensitive to the direct effects of nicotine (Grunberg, Winders, & Wewers, 1991). Taken in combination with the lack of a dose effect for some abstinence symptoms, the benefits of high-dose NRT, especially for women, may not outweigh the costs (e.g., unpleasant side effects).
Limitations
The results of this study must be interpreted in the context of its potential limitations, which include sensitivity to gender differences in TN-induced abstinence symptom suppression and the restrictions of the laboratory setting. First, although the sample size was large relative to previous studies examining NRT-induced abstinence symptom suppression (e.g., Fagerström et al., 1993; Levin et al., 1994; Lunell et al., 1995; Schneider et al., 1984; Schneider & Jarvik, 1984; West et al., 1984), the failure to observe Gender × Dose interactions on measures of abstinence symptom suppression may reflect a Type II error. However, the observation of significant Gender × Dose interactions on several other measures (e.g., heart rate, self-reported lightheadedness, nausea, and weakness) suggests that the study was sensitive to small-to-medium effect sizes (Cohen’s, 1988, f for the Gender × Dose interactions for these measures ranged from 0.08 to 0.17). Thus, the failure to observe significant Gender × Dose interactions on measures of tobacco abstinence may indicate that the effect, if it exists, is of minimal clinical significance.
Second, the clinical laboratory offers precise control over many factors (e.g., smoking-related stimuli, concurrent drug administration, activity, social situation, food intake). How-ever, this level of control can also be a limitation, as factors that precipitate or contribute to tobacco abstinence in the natural environment are eliminated. For example, environmental cues, such as the sight and smell of a lit cigarette, can increase urge to smoke (Abrams, Monti, Carey, Pinto, & Jacobus, 1988; Niaura, Abrams, Pedraza, Monti, & Rohsenow, 1992; Niaura et al., 1998), but this study’s laboratory setting minimized the influence of these cues. To the extent that these and other factors controlled in this study may underlie the gender differences that have been observed in outpatient (i.e., naturalistic environment) smoking cessation trials, the study’s generalizability may be limited. However, laboratory studies of potential drug dependence pharmacotherapies often predict clinical outcome (Jasinski, Pevnick, & Griffith, 1978; Rose et al., 1985), so there is evidence that the controlled laboratory environment has real world relevance.
In addition to sensitivity and setting, the results of the study must be interpreted in the context of the fact that study participants had no interest in smoking cessation and completed a relatively brief period of presession tobacco abstinence (i.e., 8-12 hr) and subsequent assessment (i.e., approximately 6 hr). Outside of this study, most consumers of nicotine replacement treatment are interested in smoking cessation and will be interested in longer term abstinence symptom suppression.
Summary and Conclusion
This study is the first laboratory examination of TN-induced abstinence symptom suppression to use a large sample of men and women and multiple TN doses. Results clearly indicate that TN can suppress some abstinence symptoms, and that higher doses are more effective at suppressing a subset of these symptoms. Most symptom suppression was independent of smokers’ gender. Unfortunately, TN’s side effects were dose-related, and women were more sensitive to them. To the extent that tobacco abstinence symptom suppression is related to successful smoking cessation, results from this laboratory study support the continued use of TN as a pharmacotherapy. The study does not support the use of higher doses (>21 mg), except for suppression of craving and urges to smoke. Even then, the side effect profile associated with these higher doses, at least in the short term, may limit effectiveness. Study results also suggest that gender differences in some smoking cessation trials are not related to differential abstinence symptom suppression. Future research addressing this important issue may benefit from focusing on a potential interaction between gender and other effects of TN (i.e., blunting the effects of a concurrently administered cigarette) and/or on other triggers for relapse (i.e., smoking-related stimuli).
Footnotes
Portions of this work were presented at the 11th annual meeting of the Society for Research on Nicotine and Tobacco, March 20-23, 2005. This work was supported by Public Health Service Grants DA11082, DA07027, and DA018447. This article is based on dissertation research that was conducted, completed, and defended at Virginia Commonwealth University by Sarah E. Evans under the guidance of Thomas Eissenberg.
References
- Abrams DB, Monti PM, Carey JB, Pinto RP, Jacobus SI. Reactivity to smoking cues and relapse: Two studies of discriminant validity. Behaviour Research and Therapy. 1988;26:225–233. doi: 10.1016/0005-7967(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Allen SS, Hatsukami DK, Christianson D, Nelson D. Effects of transdermal nicotine on craving, withdrawal, and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine and Tobacco Research. 2000;2:231–241. doi: 10.1080/14622200050147493. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory II (BDI-II) Psychology Corporation; San Antonio, TX: 1996. [Google Scholar]
- Benowitz NL, Zevin S, Jacob P., III. Suppression of nicotine intake during ad libitum cigarette smoking by high-dose transdermal nicotine. Journal of Pharmacology and Experimental Therapeutics. 1998;287:958–962. [PubMed] [Google Scholar]
- Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Gender differences in quit rates following smoking cessation with combination nicotine therapy: Influence of baseline smoking behavior. Nicotine and Tobacco Research. 2003;5:111–116. doi: 10.1080/1462220021000060482. [DOI] [PubMed] [Google Scholar]
- Breland AB, Buchhalter A, Evans S, Eissenberg T. Evaluating acute effects of harm reduction products for smokers: Clinical laboratory methodology. Nicotine and Tobacco Research. 2002;4(S2):S131–S140. doi: 10.1080/1462220021000032780. [DOI] [PubMed] [Google Scholar]
- Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine and Tobacco Research. doi: 10.1080/14622200600789585. (in press) [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T. Tobacco abstinence symptom suppression: The role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction. 2005;100:550–559. doi: 10.1111/j.1360-0443.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Schrinel L, Eissenberg T. With-drawal suppressing effects of a novel smoking system: Comparison with own brand, not own brand, and denicotinized cigarettes. Nicotine and Tobacco Research. 2001;3:111–118. doi: 10.1080/14622200110042636. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Cigarette smoking among adults—United States, 2004. Morbidity and Mortality Weekly Report. 2005;54(44):1121–1124. [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Covey LS. Tobacco cessation among patients with depression. Primary Care. 1999;26:691–706. doi: 10.1016/s0095-4543(05)70124-x. [DOI] [PubMed] [Google Scholar]
- Dale LC, Hurt RD, Offord KP, Lawson GM, Croghan IT, Schroeder DL. High-dose nicotine patch therapy— Percentage of replacement and smoking cessation. Journal of the American Medical Association. 1995;274(17):1353–1358. [PubMed] [Google Scholar]
- Daughton DM, Fortmann SP, Glover ED, Hatsukami DK, Heatley SA, Lichtenstein E, et al. The smoking cessation efficacy of varying doses of nicotine patch delivery systems 4 to 5 years post-quit day. Preventative Medicine. 1999;28:113–118. doi: 10.1006/pmed.1998.0391. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Griffiths RR, Stitzer ML. Mecamylamine does not precipitate withdrawal in cigarette smokers. Psychopharmacology. 1996;127:328–336. doi: 10.1007/s002130050094. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Stitzer ML, Henningfield JE. Current issues in nicotine replacement. In: Seidman DF, Covey LS, editors. Helping the hard-core smoker: A clinician’s guide. Erlbaum; Mahwah, NJ: 1999. pp. 137–158. [Google Scholar]
- Fagerström KO, Schneider NG, Lunell E. Effectiveness of nicotine patch and nicotine gum as individual versus combined treatments for tobacco withdrawal symptoms. Psychopharmacology. 1993;111:271–277. doi: 10.1007/BF02244941. [DOI] [PubMed] [Google Scholar]
- Fant RV, Henningfield JE, Shiffman S, Strahs KR, Reitberg DP. A pharmacokinetic crossover study to compare the absorption characteristics of three transdermal nicotine patches. Pharmacology, Biochemistry and Behavior. 2000;67:479–482. doi: 10.1016/s0091-3057(00)00399-3. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Dorman SF, Goldstein MG, Gritz ER, et al. Treating tobacco use and dependence. Clinical practice guideline. U.S. Department of Health and Human Services, Public Health Service; Rockville, MD: 2000. [Google Scholar]
- Foulds J, Stapleton JA, Bell N, Swettenham J, Jarvis MJ, Russell MA. Mood and physiological effects of subcutaneous nicotine in smokers and never-smokers. Drug and Alcohol Dependence. 1997;44(23):105–115. doi: 10.1016/s0376-8716(96)01327-0. [DOI] [PubMed] [Google Scholar]
- Franklin TR. Menstrual cycle phase at quit date: Effect on treatment outcome, irrespective of treatment modality; 65th annual meeting of the College on Problems of Drug Dependence; Orlando, FL. 2005, June. [Google Scholar]
- Frederickson PA, Hurt RD, Lee GM, Wingender L, Croghan IT, Lauger G, et al. High dose transdermal nicotine therapy for heavy smokers: Safety, tolerability and measurement of nicotine and cotinine levels. Psychopharmacology. 1995;122:215–222. doi: 10.1007/BF02246542. [DOI] [PubMed] [Google Scholar]
- Garrett BE, Griffiths RR. Intraveneous nicotine and caffeine: Subjective and physiological effects in cocaine abusers. Journal of Pharmacology and Experimental Therapeutics. 2001;296:486–494. [PubMed] [Google Scholar]
- Gilbert D, McClernon J, Rabinovich N, Sugai C, Plath L, Asgaard G, et al. Effects of quitting smoking on EEG activation and attention last for more than 31 days and more severe with stress, dependence, DRD2 A1 allele, and depressive traits. Nicotine and Tobacco Research. 2004;6:249–267. doi: 10.1080/14622200410001676305. [DOI] [PubMed] [Google Scholar]
- Gilbert RM, Pope MA. Early effects of quitting smoking. Psychopharmacology. 1982;78:121–127. doi: 10.1007/BF00432247. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Prospective study of factors predicting outcome of transdermal nicotine treatment in smoking cessation. British Medical Journal. 1994;309:842–846. doi: 10.1136/bmj.309.6958.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Double-blind trial of repeated treatment with transdermal nicotine for relapsed smokers. British Medical Journal. 1995;311:363–366. doi: 10.1136/bmj.311.7001.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg NE, Winders SE, Wewers ME. Gender differences in tobacco use. Health Psychology. 1991;10:143–153. [PubMed] [Google Scholar]
- Hajek P, Jarvis MJ, Belcher M, Sutherland G, Feyerabend C. Effect of smoke-free cigarettes on 24 h cigarette withdrawal: A double-blind placebo-controlled study. Psychopharmacology. 1989;9:99–102. doi: 10.1007/BF00443421. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Hughes JR, Pickens R. Characterization of tobacco withdrawal: Physiological and subjective effects. NIDA Research Monograph. 1985;53:56–67. [PubMed] [Google Scholar]
- Hatsukami DK, Hughes JR, Pickens RW, Svikis D. Tobacco withdrawal symptoms: An experimental analysis. Psychopharmacology. 1984;84:231–236. doi: 10.1007/BF00427451. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Skoog K, Allen S, Bliss R. Gender and the effects of different doses of nicotine gum on tobacco withdrawal symptoms. Experimental and Clinical Psychopharmacology. 1995;3:163–173. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. Journal of Consulting and Clinical Psychology. 1993;61:743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Goldstein MG, Hurt RD, Shiffman S. Recent advances in the pharmacotherapy of smoking. Journal of the American Medical Association. 1999;281:72–76. doi: 10.1001/jama.281.1.72. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Keenan RM, Fenwick JW. Effect of dose on nicotine’s reinforcing, withdrawal-suppression, and self-reported effects. Journal of Pharmacology and Experimental Therapeutics. 1990;252:1175–1183. [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal. A replication and extension. Archives of General Psychiatry. 1991;48:52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Pickens RW, Svikis DS. Consistency of the tobacco withdrawal syndrome. Addictive Behaviors. 1984;11:459–462. doi: 10.1016/0306-4603(84)90043-1. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Hatsukami D. Effects of abstinence from tobacco: A critical review. In: Kozlowski LT, Annis HM, Cappell HD, Glaser FB, Goodstadt MS, Israel Y, et al., editors. Research advances in alcohol and drug problems. Plenum Press; New York: 1990. pp. 317–384. [Google Scholar]
- Hughes JR, Shiffman S, Callas P, Zhang J. A meta-analysis of the efficacy of over-the-counter nicotine replacement. Tobacco Control. 2003;12(1):21–27. doi: 10.1136/tc.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Croghan GA, Gomez-Dahl LC, Wolter TD, et al. Temporal effects of nicotine nasal spray and gum on nicotine withdrawal symptoms. Psychopharmacology. 1998;140:98–104. doi: 10.1007/s002130050744. [DOI] [PubMed] [Google Scholar]
- Huynh HS, Feldt LS. Estimation of the Box correction for degrees of freedom from sample data in randomized block and split-plot designs. Journal of Statistics Education. 1976;1:69–82. [Google Scholar]
- Ingram JR, Routledge P, Rhodes J, Marshall RW, Buss DC, Evans BK, et al. Nicotine enemas for treatment of ulcerative colitis: A study of the pharmacokinetics and adverse events associated with three doses of nicotine. Ailment Pharmacological Therapies. 2004;20:859–865. doi: 10.1111/j.1365-2036.2004.02199.x. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: A potential agent for treating narcotic addiction. Archives of General Psychiatry. 1978;35:501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Hapke U, Rumpf HJ, Schumann A. Nicotine dependence, quit attempts, and quitting among smokers in a regional population sample from a country with a high prevalence of tobacco smoking. Preventive Medicine. 2004;38(3):35–38. doi: 10.1016/j.ypmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Jones HE, Garrett BE, Griffiths RR. Subjective and physiological effects of intravenous nicotine and cocaine in cigarette smoking cocaine abusers. Journal of Pharmacology and Experimental Therapeutics. 1999;288:188–197. [PubMed] [Google Scholar]
- Jorenby DE, Hatsukami DK, Smith SS, Fiore MC, Allen S, Jensen J, Baker TB. Characterization of tobacco withdrawal symptoms: Transdermal nicotine reduces hunger and weight gain. Psychopharmacology. 1996;128:130–138. doi: 10.1007/s002130050118. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: A researcher’s handbook. 3rd ed. Prentice Hall; Englewood Cliffs, NJ: 1991. [Google Scholar]
- Killen JD, Fortmann SP, Kraemer HC, Varady A, Newman B. Who will relapse? Symptoms of nicotine dependence predict long-term relapse after smoking cessation. Journal of Consulting and Clinical Psychology. 1992;60:797–801. doi: 10.1037//0022-006x.60.5.797. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Newman B, Varady A. Evaluation of a treatment approach combining nicotine gum with self-guided behavioral treatments for smoking relapse prevention. Journal of Consulting and Clinical Psychology. 1990;58:85–92. doi: 10.1037//0022-006x.58.1.85. [DOI] [PubMed] [Google Scholar]
- Kinnunen T, Nordstrom BL, Utman CH, Garvey AJ. Gender differences in tobacco use and withdrawal; annual convention of the American Psychological Association; San Francisco. 1998, August. [Google Scholar]
- Knapp PH, Bliss CM, Wells H. Addictive aspects in heavy cigarette smoking. American Journal of Psychiatry. 1963;119:966–972. doi: 10.1176/ajp.119.10.966. [DOI] [PubMed] [Google Scholar]
- Krivokapich J, Schneider NG, Child JS, Jarvik ME. Cardiovascular effects of nicotine gum and cigarettes assessed b ECG and echocardiography. NIDA Research Monograph. 1985;53:42–55. [PubMed] [Google Scholar]
- Leischow SJ, Valente SN, Hill AL, Otte PS, Aickin M, Holden T, et al. Effects of nicotine dose and administration method on withdrawal symptoms and side effects during short-term smoking abstinence. Experimental and Clinical Psychopharmacology. 1997;5:54–64. doi: 10.1037//1064-1297.5.1.54. [DOI] [PubMed] [Google Scholar]
- Levin ED, Westman EC, Stein RM, Carnahan E, Sanchez M, Herman S, et al. Nicotine skin patch treatment increases abstinence, decreases withdrawal symptoms, and attenuates rewarding effects of smoking. Journal of Clinical Psychopharmacology. 1994;14:41–49. [PubMed] [Google Scholar]
- Lunell E, Molander L, Leischow SJ, Fagerström KO. Effect of nicotine vapour inhalation on the relief of tobacco withdrawal symptoms. European Journal of Clinical Pharmacology. 1995;48(3):235–240. doi: 10.1007/BF00198304. [DOI] [PubMed] [Google Scholar]
- Marks JL, Pomerleau CS, Pomerleau OF. Effects of menstrual cycle phase on reactivity to nicotine. Addictive Behaviors. 1999;24(1):127–134. doi: 10.1016/s0306-4603(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Masson CL, Gilbert DG. Cardiovascular and mood responses to quantified doses of cigarette smoke in oral contraceptive users and nonusers. Journal of Behavioral Medicine. 1999;22:589–604. doi: 10.1023/a:1018793729594. [DOI] [PubMed] [Google Scholar]
- Maude-Griffin PM, Tiffany ST. Production of smoking urges through imagery: The impact of affect and smoking abstinence. Experimental and Clinical Psychopharmacology. 1996;4:198–208. [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the Digit Symbol Substitution Test (DSST) Behavior Research Methods & Instrumentation. 1982;14:463–466. [Google Scholar]
- Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK. Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology. 2005;30:1751–1763. doi: 10.1038/sj.npp.1300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander L, Lunell E, Fagerström KO. Reduction of tobacco withdrawal symptoms with a sublingual nicotine tablet: A placebo controlled study. Nicotine and Tobacco Research. 2000;2:187–191. doi: 10.1080/713688123. [DOI] [PubMed] [Google Scholar]
- Muramoto ML, Ranger-Moore J, Leischow SJ. Efficacy of oral transmucosal nicotine lozenge for suppression of withdrawal symptoms in smoking abstinence. Nicotine and Tobacco Research. 2003;5:223–230. doi: 10.1080/1462220031000073270. [DOI] [PubMed] [Google Scholar]
- Myrsten AL, Elgerot A, Edgren B. Effects of abstinence from tobacco smoking on physiological and psychological arousal levels in habitual smokers. Psychosomatic Medicine. 1977;39(1):25–38. doi: 10.1097/00006842-197701000-00004. [DOI] [PubMed] [Google Scholar]
- Naidong W, Shou W, Chen YL, Jiang X. Novel liquid chromatographic-tandem mass spectrometric methods using silica columns and aqueous-organic mobile phases for quantitative analysis of polar ionic analytes in biological fluids. Journal of Chromatography and Biomedical Science Applications. 2001;754:387–399. doi: 10.1016/s0378-4347(01)00021-4. [DOI] [PubMed] [Google Scholar]
- Niaura R, Abrams DB, Pedraza M, Monti PM, Rohsenow DJ. Smokers’ reactions to interpersonal interaction cues and presentation of smoking cues. Addictive Behaviors. 1992;17:557–566. doi: 10.1016/0306-4603(92)90065-4. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: Effects of gender and cue type. Addictive Behaviors. 1998;23:209–224. doi: 10.1016/s0306-4603(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Nørregaard J, Tonnesen P, Petersen L. Predictors and reasons for relapse in smoking cessation with nicotine and placebo patches. Preventive Medicine. 1993;22:261–271. doi: 10.1006/pmed.1993.1021. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Individual variability in responses to nicotine. Behavioral Genetics. 1995;25(2):119–132. doi: 10.1007/BF02196922. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchinson S. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine and Tobacco Research. 2001;3:141–150. doi: 10.1080/14622200110043059. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Stiller RL, Fonte C, Goettler JE. Nasal spray nicotine replacement suppresses cigarette smoking desire and behavior. Clinical Pharmacology & Therapeutics. 1992;52:627–634. doi: 10.1038/clpt.1992.201. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Scott J. Comment on Shiffman and colleagues, “Nicotine patch and lozenge are effective for women.”. Nicotine and Tobacco Research. 2005;7:915–916. doi: 10.1080/14622200500358895. [DOI] [PubMed] [Google Scholar]
- Persico AM. Persistent decrease in heart rate after smoking cessation: A 1-year follow-up study. Psychopharmacology. 1992;106:397–400. doi: 10.1007/BF02245425. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiore MC, Baker TB. Smoking withdrawal dynamics in unaided quitters. Journal of Abnormal Psychology. 2000;109:74–86. doi: 10.1037//0021-843x.109.1.74. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Fant RV, Butschky MF, Henningfield JE. Effects of transdermal nicotine delivery on measures of acute nicotine withdrawal. Journal of Pharmacology and Experimental Therapeutics. 1996;279:450–456. [PubMed] [Google Scholar]
- Pomerleau CS, Mehringer A, Marks JL, Downey K, Pomerleau OF. Effects of menstrual phase smoking abstinence in smokers with and without a history of major depressive disorder. Addictive Behaviors. 2000;25:483–497. doi: 10.1016/s0306-4603(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Tate JC, Lumley MA, Pomerleau OF. Gender differences in prospectively versus retrospectively assessed smoking withdrawal symptoms. Journal of Substance Abuse. 1994;6:433–440. doi: 10.1016/s0899-3289(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Pullan RD, Rhodes J, Ganesh S, Mani V, Morris JS, Williams GT, et al. Transdermal nicotine for active ulcerative colitis. New England Journal of Medicine. 1994;330:811–815. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- Rose JE, Herskovic JE, Trilling Y, Jarvik ME. Transdermal nicotine reduces cigarette craving and nicotine preference. Clinical Pharmacology & Therapeutics. 1985;38:450–456. doi: 10.1038/clpt.1985.203. [DOI] [PubMed] [Google Scholar]
- Sachs DPL, Sawe U, Leischow SJ. Effectiveness of a 16-hour transdermal nicotine patch in a medical practice setting, without intensive group counseling. Archives of Internal Medicine. 1993;153:1881–1890. [PubMed] [Google Scholar]
- Schneider NG, Jarvik ME. Time course of smoking withdrawal symptoms as a function of nicotine replacement. Psychopharmacology. 1984;82:143–144. doi: 10.1007/BF00426399. [DOI] [PubMed] [Google Scholar]
- Schneider NG, Jarvik ME, Forsythe AB. Nicotine vs. placebo gum in the alleviation of withdrawal during smoking cessation. Addictive Behaviors. 1984;9:149–156. doi: 10.1016/0306-4603(84)90052-2. [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Stitzer ML. Desire to smoke during spaced smoking intervals. Psychopharmacology. 1995;120:289–295. doi: 10.1007/BF02311176. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Fant RV, Buchhalter AR, Gitchell JG, Henningfield JE. Nicotine delivery systems. Expert Opinion on Drug Delivery. 2005;2:563–577. doi: 10.1517/17425247.2.3.563. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis MH, Shadel WG. Reduction of abstinence-induced withdrawal and craving using high dose nicotine replacement therapy. Psychopharmacology. 2005;1:1–8. doi: 10.1007/s00213-005-0184-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Khayrallah M, Nowak R. Efficacy of the nicotine patch for relief of craving and withdrawal 7-10 weeks after cessation. Nicotine and Tobacco Research. 2000;2:371–378. doi: 10.1080/713688158. [DOI] [PubMed] [Google Scholar]
- Silagy C, Mant D, Fowler G, Lodgy M. Meta-analysis on efficacy of nicotine replacement therapies in smoking cessation. The Lancet. 1994;343(8890):139–142. doi: 10.1016/s0140-6736(94)90933-4. [DOI] [PubMed] [Google Scholar]
- Strain EC, Moody DE, Stoller KB, Walsh SL, Bigelow GW. Relative bioavailability of different buprenorphine formulations under chronic dosing conditions. Drug and Alcohol Dependence. 2004;74:37–43. doi: 10.1016/j.drugalcdep.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Ward MM. Subgroups of smokers with different success rates after use of transdermal nicotine. Addiction. 1997;92:207–218. [PubMed] [Google Scholar]
- Teneggi V, Tiffany ST, Squassante L, Milleri S, Ziviani L, Bye A. Smokers deprived of cigarettes for 72 h: Effect of nicotine patches on craving and withdrawal. Psychopharmacology. 2002;164:177–187. doi: 10.1007/s00213-002-1176-1. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Tønnesen P, Mikkelsen KL. Smoking cessation with four nicotine replacement regimes in a lung clinic. European Respiratory Journal. 2000;16:717–722. doi: 10.1034/j.1399-3003.2000.16d25.x. [DOI] [PubMed] [Google Scholar]
- Tønnesen P, Paoletti P, Gustavsson G, Russell MA, Saracci R, Gulsivik A, et al. Higher dosage nicotine patches increase one-year smoking cessation rates: Results from the European CEASE trial. European Respiratory Journal. 1999;13:238–246. doi: 10.1034/j.1399-3003.1999.13b04.x. [DOI] [PubMed] [Google Scholar]
- Transdermal Nicotine Study Group Transdermal nicotine for smoking cessation. Journal of the American Medical Association. 1991;266:3133–3138. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . The health consequences of smoking: Nicotine addiction—A report of the Surgeon General. U.S. Department of Health and Human Services, Public Health Service; Rockville, MD: 1988. (DHHS Publication No. CDC 88-8406) [Google Scholar]
- Ward MM, Swan GE, Jack LM. Self-reported abstinence effects in the first month after smoking cessation. Addictive Behaviors. 2001;26:311–327. doi: 10.1016/s0306-4603(00)00107-6. [DOI] [PubMed] [Google Scholar]
- West RJ, Jarvis MJ, Russell MAH, Carruthers ME, Feyerabend C. Effect of nicotine replacement on the cigarette withdrawal syndrome. British Journal of Addiction. 1984;79:215–219. doi: 10.1111/j.1360-0443.1984.tb00265.x. [DOI] [PubMed] [Google Scholar]
- West RJ, Russell MAH. Cardiovascular and subjective effects of smoking before and after 24 h of abstinence from cigarettes. Psychopharmacology. 1987;92:118–121. doi: 10.1007/BF00215491. [DOI] [PubMed] [Google Scholar]
- West R, Shiffman S. Effect of oral nicotine dosing forms on cigarette withdrawal symptoms and craving: A systematic review. Psychopharmacology. 2001;155:115–122. doi: 10.1007/s002130100712. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Fiore MC, Young TB, McClure JB, deMoor CA. Gender differences in response to nicotine replacement therapy: Objective and subjective indexes of tobacco withdrawal. Experimental and Clinical Psychopharmacology. 1999;7:135–144. doi: 10.1037//1064-1297.7.2.135. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. Journal of Consulting and Clinical Psychology. 1999;67:555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- Winchell C, Wright C, Burke L, Kramer ED, Longmire AW, Scheinbaum ML. Long-term abstinence in users of OTC nicotine replacement. Clinical Pharmacology & Therapeutics. 1997;61:191. [Google Scholar]