Abstract
β-Arrestins mediate internalization of plasma membrane receptors. Nephrin, a structural component of the glomerular slit diaphragm, is a single transmembrane spanning receptor and belongs to the family of adhesion molecules. Its mutation causes a hereditary nephrotic syndrome. We report the previously undescribed interaction of β-arrestin2 with the nephrin C terminus. The phosphorylation status of nephrin Y1193 regulates inversely the binding of β-arrestin2 and podocin. The Src-family member Yes, known to enhance podocin–nephrin interaction by nephrin phosphorylation, diminishes β-arrestin2–nephrin interaction. β-Arrestin2 induces nephrin endocytosis and attenuates nephrin signaling. This finding suggests that nephrin Y1193 serves as a molecular switch that determines the integrity of the slit diaphragm by functional competition between β-arrestin2 and podocin. This concept offers a molecular pathomechanism of slit diaphragm distortion and opens therapeutic avenues for glomerular diseases.
Keywords: glomerular slit diaphragm, signaling, podocin, podocyte
Mutations of the gene encoding nephrin cause severe congenital proteinuria and renal failure. This breakthrough discovery by Kestila et al. (1) initiated a new understanding of the glomerular filter and shed new light on the pathogenesis of more common proteinuric renal diseases such as diabetic nephropathy, glomerulonephritis, and hypertensive renal disease (2). It is noteworthy to say that the degree of proteinuria is of prognostic relevance. Severe proteinuria is associated with a faster loss of renal function and shorter time to end-stage renal disease (3). It is possible that altered functions of nephrin and other recently identified molecules of the glomerular slit diaphragm such as CD2AP (4), podocin (5) and the NEPH family (6) play a pathophysiological role in the above-mentioned proteinuric diseases. In line with this data, Doublier et al. (7) demonstrated a decrease of glomerular nephrin expression as an early event in diabetes. However, the mechanisms by which alterations of nephrin function are involved in proteinuria are largely unknown.
Nephrin belongs to the Ig superfamily. It is a type-1 transmembrane spanning receptor, and its extracellular domain has eight Ig-like domains and a fibronectin-like domain. Nephrin molecules from adjacent foot processes bind to each other in a homophilic manner (8, 9) and serve as a backbone for the slit diaphragm. In addition to its structural role, there is cumulating evidence that nephrin is a signaling receptor molecule. The intracellular human nephrin C terminus consists of 154 aa and has three putative tyrosine phosphorylation sites for Src family kinases, Tyr1176, Tyr1193, and Tyr1217 (10), the latter two conserved in mouse and rat nephrin. Moreover, the Src-family kinases Fyn, Src, and Yes are expressed in podocytes (11) and known to tyrosine phosphorylate the nephrin C terminus. This tyrosine phosphorylation is induced by nephrin clustering with extracellular nephrin antibodies communicating the extracellular binding status of nephrin (12). In addition, nephrin signaling is augmented by its interaction with podocin (13). Other glomerular slit diaphragm type-1 transmembrane receptors such as NEPH1 and NEPH2 also have signaling abilities (6, 13). The signal transduction properties of nephrin provoked us to search for a mechanism of receptor desensitization. So far, no such concept has been proposed or investigated.
It has been shown recently that type-1 transmembrane spanning receptors are regulated by arrestins (14–16). Chen et al. (14) revealed that β-arrestin2 binding to the TGF-β III receptor at T847 leads to receptor internalization and impairs TGFβIII-dependent signaling. Because nephrin belongs to the same group of tyrosine phosphorylated type-1 transmembrane-spanning receptors, one can speculate that arrestins also interact with nephrin. In addition, nephrin harbors several potential β-arrestin-binding sites with the consensus motif S/TX4–5S/T (17). The role of arrestins in desensitization of activated seven transmembrane-spanning G protein-coupled receptors is well documented. In addition, β-arrestins 1 and 2 are multifunctional adaptor and transducer molecules directing the recruitment, activation, and scaffolding of cytoplasmic signaling complexes (18). This mechanism regulates aspects of cell motility, chemotaxis, apoptosis, and signaling. β-Arrestins bind also to proteins of the cellular trafficking machinery and a variety of signaling proteins such as c-Src, ERK1/2, and JNK3 (18, 19)
In the present study, we hypothesized that β-arrestin2 interacts with nephrin and NEPH1 and, thereby, regulates slit diaphragm function. Our findings allow us to propose a previously undescribed concept of a dynamic equilibrium of the glomerular slit diaphragm depending on the nephrin Tyr1193 phosphorylation status. The concept described depicts the molecular interplay between nephrin and its interaction partners to understand their role in proteinuric diseases.
Results
β-Arrestins Are Expressed in Podocytes.
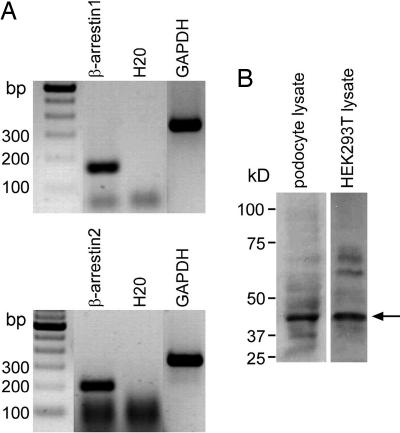
In a first step, we were able to demonstrate expression of β-arrestin1 and 2 in podocytes. β-arrestin1 and β-arrestin2 transcripts are detected within podocytes by using RT-PCR on a podocyte cell line (Fig. 1A). In Western blot analysis, the endogenous expression of β-arrestin2 was shown in the mouse podocyte cell line and HEK293T cells (Fig. 1B).
Fig. 1.
Podocytes express β-arrestin1 and β-arrestin2. (A) Cultured mouse podocytes show strong RT-PCR signals for β-arrestin1 (169 bp) (Upper) and β-arrestin2 (211 bp) (Lower). (B) In Western blot analysis, endogenous β-arrestin2 can be detected in mouse podocyte lysates and cell lysates of HEK293T cells with β-arrestin2 antiserum at 46 kDa (arrow).
Nephrin Interacts with β-Arrestin2.
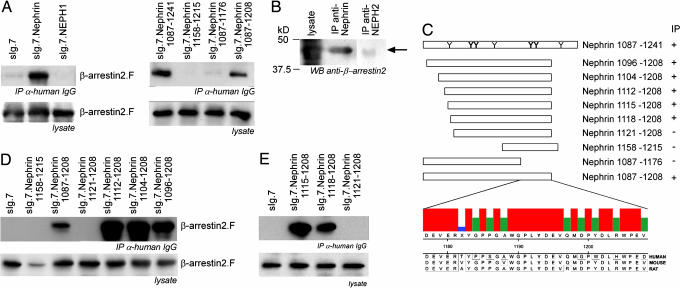
To investigate a possible interaction of β-arrestin2 with nephrin or NEPH1, we overexpressed both membrane-tagged C-terminal fusion proteins in combination with β-arrestin2 molecules in HEK293T cells. Coimmunoprecipitation with β-arrestin2 was shown for nephrin but not NEPH1, another molecular component of the slit diaphragm, pointing to a specific β-arrestin2 interaction with nephrin (Fig. 2A). Endogenous nephrin immobilizes endogenous β-arrestin2 from precleared mouse kidney lysates, whereas endogenous Neph2 could not precipitate β-arrestin2 (Fig. 2B). There is circumstantial evidence that tyrosine phosphorylation of β-arrestin-binding partners may be a necessary step for their interactions (16). Because the nephrin C terminus has seven potential tyrosine phosphorylation sites, truncation mapping was performed to identify the relevant residues (Fig. 2C). Only the nephrin truncation 1087–1208 maintained the interaction with β-arrestin2, whilst nephrin 1087–1176 does not interact with β-arrestin2 (Fig. 2A). This observation confines the regulation site of the β-arrestin2–nephrin interaction to a range of 32-aa nephrin 1177–1208 within the nephrin C terminus. The alignment of nephrin 1177–1208 sequence comparison in human, mouse, and rat revealed conserved domains including tyrosines. Further sequence analysis of those 32 nephrin amino acids by using motif scanner (http://scansite.mit.edu/motifscan_seq.phtml) and NetPhos 2.0. (www.cbs.dtu.dk/services/NetPhos) suggested the nephrin tyrosine 1193 as a likely phosphorylation target and SH2-binding site for Src-family kinases such as Yes (Fig. 2C). Surprisingly, the nephrin truncation 1158–1215 containing the identified region failed to immunoprecipitate β-arrestin2. This finding suggested that besides nephrin 1177–1208, another more N-terminal domain is required for the interaction. Therefore, we investigated the importance of the further N-terminal amino acid region 1087–1157. Being aware of the β-arrestin2-binding motif S/TX4–5S/T (17), we were then able to delineate the β-arrestin2 interaction site within nephrin to the 9-aa region 1112–1120 in additional N-terminal truncations. Thus, both nephrin domains 1177–1208 and 1112–1120 are essential for β-arrestin2 binding. Further extension of the N-terminal truncations attenuates the interaction strength (Fig. 2D). Further fine mapping pinpointed the N-terminal binding motif to the nephrin 1120–1125 TGERDT sequence. Additional fine mapping narrowed the β-arrestin2 interaction site to the nephrin residues (QWT) 1118–1120 (Fig. 2E). And indeed, N-terminal extensions of nephrin 1112 reduced the strength of β-arrestin2 binding. Taking these data together, one can state that the nephrin 1120–1125 TGERDT sequence represents the binding site for β-arrestin2, whereas nephrin 1177–1208 has a regulatory function for the interaction.
Fig. 2.
β-Arrestin2 interacts with the nephrin C terminus. (A) (Left) The C terminus of nephrin but not NEPH1 immobilizes β-arrestin2. HEK293T cells transiently overexpressing heterologous plasma membrane-bound fusion proteins (sIg.7) of the nephrin C terminus, NEPH1, or sIg.7 alone in combination with β-arrestin2 are examined. The coimmunoprecipitated β-arrestin2 is detected by Western blot analysis. To map the interaction site of β-arrestin2 within nephrin, C-terminal truncations are generated. (Right) Only the truncated sIg.nephrin 1087–1208 interacted with β-arrestin2. (B) Endogenous nephrin but not NEPH2 are able to precipitate endogenous β-arrestin2 from mouse kidney lysates. β-Arrestin2 is detected by Western blot analysis with monoclonal β-arrestin2 antisera (arrow). (C) A scheme of the nephrin C-terminal full length with the distribution of tyrosine residues that are predicted to be phosphorylated and the C-terminal nephrin truncations are displayed. Those tyrosine residues that are predicted to be most likely phosphorylated are printed in bold letters [prediction by NetPhos 2.0 (www.cbs.dtu.dk/services/NetPhos)]. The 32 nephrin residues of human, mouse, and rat regulating the β-arrestin2 interaction are aligned (ClustalW). The consensus strength is visualized by colored vertical bars. Tyrosine 1193 (bold) is embedded in a highly cross-species-conserved domain of 8 aa. To map the β-arrestin2 interaction site, the C-terminal truncation sIg.Nephrin 1087–1208 was truncated at the N terminus also. (D) β-Arrestin2 interacts with nephrin through the nephrin amino acids 1112–1120. Amino acids 1087–1111 attenuate the strength of the β-arrestin2–nephrin interaction. (E) Fine mapping delineates the β-arrestin2–nephrin interaction site to the nephrin amino acids 1118–1120 (QWT).
The β-Arrestin2 Interaction Is Regulated at Nephrin Residue Y1193 and by Its Phosphorylation Status.
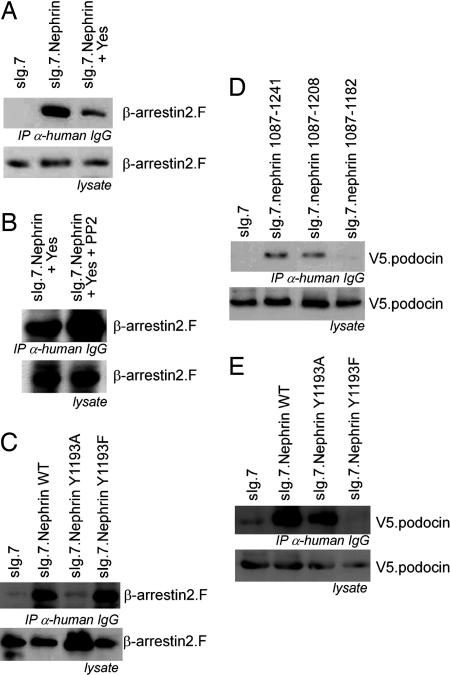
The nephrin C terminus is tyrosine phosphorylated by Src kinases when clustered (12). One can speculate that extracellular nephrin homodimer formation leads to intracellular nephrin tyrosine phosphorylation by Src kinases. Nephrin contains such a phosphorylation site at nephrin Y1193 within the 1177–1208 domain. We therefore expected that nephrin Y1193 phosphorylation ameliorates β-arrestin2 binding to nephrin to prevent endocytosis and slit diaphragm disintegration. And, indeed, coexpression of the Src kinase Yes diminished the β-arrestin2-nephrin binding supporting our assumption (Fig. 3A). This observation strengthens the importance of the tyrosine residues for the interaction between nephrin and β-arrestin2. Accordingly, PP2, a specific tyrosine kinase inhibitor, enhances the nephrin–β-arrestin2 interaction (Fig. 3B). To confirm the importance of nephrin Y1193 for the interaction, we replaced nephrin Y1193 by either alanine or phenylalanine. The point mutation to alanine leads to a dramatic decrease of the nephrin–β-arrestin2 interaction. In contrast, the nephrin point mutant Y1193F, mimicking an unphosphorylated tyrosine, maintained the nephrin-β–arrestin2 interaction (Fig. 3C). This result underscores the functional relevance of the unphosphorylated nephrin Y1193 for β-arrestin2 binding.
Fig. 3.
Nephrin Y1193 is the switch for β-arrestin2 or podocin interaction with nephrin controlled by phosphorylation. (A) Immunoprecipitation of the nephrin C terminus in the presence of Yes decreases the interaction with β-arrestin2. (B) Addition of PP2 reverses the Yes-mediated attenuation of the interaction between nephrin and β-arrestin2. (C) Point mutation of the Y1193 within the nephrin C terminus modulates the interaction with β-arrestin2. The mutation of tyrosine 1193 to alanine (Y1193A) attenuates the interaction with β-arrestin2. The nephrin mutation of tyrosine 1193 to phenylalanine (Y1193F) interacts with β-arrestin2 as strong as nephrin wild type. (D) The podocin interaction with the nephrin C terminus maps to the same nephrin region that regulates the interaction with β-arrestin2. Immunoprecipitation of the nephrin C terminus and its truncations (as labeled) delineates the podocin interaction motif to the nephrin amino acids 1177–1208, the same region that regulates the interaction with β-arrestin2. (E) In contrast to β-arrestin2, podocin interacts with nephrin Y1193A but not with nephrin Y1193F. (Overexpression experiments were done in HEK293T.)
Podocin Interacts with the Nephrin C Terminus Where β-Arrestin2 Binding Is Regulated.
We then explored a possible role for β-arrestin2 in proteinuric diseases by looking at podocin as another important molecule known to stabilize the slit diaphragm via nephrin interaction. We expected that β-arrestin2 behaves as a functional opponent of podocin. The podocin–nephrin interaction was mapped to the C-terminal nephrin residues 1183–1208, the same region in which β-arrestin2 binding to nephrin is modulated (Fig. 3D). We therefore speculated that phosphorylation of the tyrosine residue Y1193 of nephrin enhances the interaction between nephrin and podocin but attenuates binding between nephrin and β-arrestin2. And, in fact, the nephrin mutation Y1193F, which mimics an unphosphorylated tyrosine, favored the interaction with β-arrestin2 and abolished the interaction with podocin (Fig. 3E). In contrast to the β-arrestin2-binding properties, podocin bound nephrin Y1193A but not Y1193F. These data suggest that the nephrin tyrosine residue is a phosphorylation-dependent switch either promoting the podocin interaction when phosphorylated or promoting the β-arrestin2 interaction when unphosphorylated. Furthermore, this observation underlines the functional antagonism of podocin and β-arrestin2 with respect to their binding to nephrin. The podocin-binding site was mapped to the N-terminal part of nephrin 1183–1208, being independent of the nephrin Y1193 (data not shown).
β-Arrestin2 Promotes Nephrin Endocytosis and Terminates Signaling.
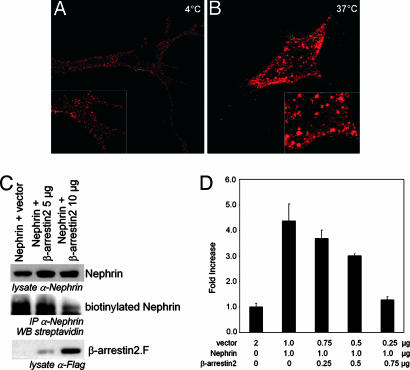
Nephrin endocytosis was visualized by fluorescent nephrin labeling of live cells at 4°C to inhibit the continuous receptor endocytosis. Confocal microscopy revealed nephrin within numerous small clusters at the cell surface (Fig. 4A). The incubation at 37°C for 20 min merged the small nephrin clusters to larger intracellular vesicles as a consequence of nephrin endocytosis (Fig. 4B). A comparable pattern change has been shown for the endocytosed TGFβIII receptor (14). According to the known functions of β-arrestin2, we expected that the β-arrestin2–nephrin interaction leads to nephrin internalization. And, indeed, this functional consequence of the β-arrestin2–nephrin interaction was investigated by nephrin biotinylation, demonstrating a decrease of cell surface nephrin with increasing amounts of β-arrestin2. The expression of full-length nephrin wild type with increasing amounts of β-arrestin2 mediated β-arrestin2-dependent cell surface disappearance of nephrin. HEK293T cells overexpressing nephrin and β-arrestin2 were biotinylated and subsequently lysed. Nephrin was immunoprecipitated by nephrin antiserum (Fig. 4C). The ability to transactivate activator protein 1 (AP-1) was taken as proof of principle for the nephrin-signaling properties (6, 13). We therefore investigated whether β-arrestin2 binding has an influence on nephrin-induced AP-1 transactivation. The coexpression of β-arrestin2 in increasing amounts with fixed amounts of nephrin lead to a dose-dependent decrease of the nephrin-mediated AP-1 transactivation within HEK293T cells (Fig. 4D). The concentration-dependent reduction of nephrin mediated AP-1 transactivation by increasing amounts of β-arrestin2 confirms the functional relevance of cell surface disappearance and nephrin endocytosis. Our data strongly suggest that tyrosine phosphorylation induced by extracellular nephrin binding and mediated by Yes protects nephrin from endocytosis and leads to AP-1 signaling. Furthermore, this mechanism must be viewed as a sensor for extracellular nephrin binding, thereby communicating the structural intactness of the slit diaphragm.
Fig. 4.
β-Arrestin2 promotes nephrin endocytosis and regulates nephrin-dependent signaling. (A) Nephrin molecules form numerous small clusters at the cell surface. Nephrin expressed at the surface of vital COS7 cells is labeled with a polyclonal nephrin antiserum followed by anti-rabbit-rhodamine red antisera. The cells are kept at 4°C. (B) After incubation at 37°C for 20 min, the small cell surface nephrin clusters merged to larger intracellular vesicles below the plasma membrane. Images were obtained with confocal microscopy. (C) Coexpression of β-arrestin2 in HEK293T cells diminishes the amount of biotinylated nephrin drastically. HEK293T cells expressing nephrin and β-arrestin2 as labeled were biotinylated. Nephrin was precipitated by nephrin antiserum from whole-cell lysates. The probes were blotted and detected by nephrin, M2-antiserum, or streptavidin, respectively. (D) β-Arrestin2 attenuates nephrin-mediated AP-1 transactivation in a dose-dependent manner. Nephrin-dependent AP-1 transactivation is attenuated by increasing amounts of β-arrestin2. Data sets represent mean values with SD of triplicates that were corrected for transfection efficiency by β-gal activity. AP-1 activation is normalized for the vector control and displayed as fold increase. (Experiments were done in HEK293T.)
Discussion
The disease relevance of nephrin and its interaction partner podocin has been demonstrated in human diseases and mouse models due to mutations within the encoding genes (1, 5, 20). Although a dual role for nephrin as a podocyte adhesion molecule and signal-transducing receptor has been suggested (21), no investigation has dealt with this issue so far. We felt that an activated receptor molecule of the glomerular slit diaphragm should undergo the same fate as other activated cell membrane receptors. The expanding capability of β-arrestins to bind and internalize also non-G protein-coupled receptors (14) with structural similarities to nephrin, let us investigate the interaction of nephrin and β-arrestin2.
β-Arrestin Interacts with the Nephrin C Terminus.
In a first step, we were able to demonstrate expression of β-arrestin1 and 2 in podocytes. To further investigate a possible interaction of β-arrestin2 with nephrin or NEPH1, we overexpressed both molecules in HEK293T cells. Coimmunoprecipitation with β-arrestin2 was shown for nephrin but not NEPH1, pointing to a specific β-arrestin2 interaction with nephrin. Binding of β-arrestin2 with nephrin, but not with NEPH2, was shown in mouse kidney lysates, underlining the in vivo relevance. Furthermore, Lahdenpera et al. (12) precipitated a ≈46-kDa tyrosine phosophorylated molecule with nephrin. We feel quite confident that this protein might be β-arrestin2. The binding characteristics of β-arrestin2 and podocin to nephrin and its point mutants of Y1193 revealed that the nephrin-binding sites for β-arrestin2 and podocin are N-terminal of nephrin Y1193 at 1118–1120 and 1183–1208, respectively. Nevertheless the phosphorylation status of nephrin Y1193 regulates the binding of both proteins in an inverse manner.
The fact that the most C-terminal known nephrin mutation R1160X is sufficient to cause the full clinical phenotype of the Nephrotic syndrome of the Finnish type (22, 23) strengthens the importance of those C-terminal amino acids that enclose the podocin- and β-arrestin2-regulatory site. In addition, as in other interacting partners of β-arrestins such as the orexin-1 receptor (24), domains adjacent to the β-arrestin binding site have been shown to have regulatory influences.
The Phosphorylation Status of Nephrin Y1193 Determines and Reflects the Slit Diaphragm Integrity.
The data presented here calls for a previously undescribed dynamic concept of the glomerular slit diaphragm. If nephrin fails to find or hold contact to its extracellular partner (nephrin or NEPH1/2) within the glomerular slit diaphragm, the intracellular association to podocin decreases in favor for the β-arrestin2–nephrin interaction. This interaction subsequently causes the endocytosis of the β-arrestin2-bound nephrin molecules, thereby reducing nephrin-dependent signaling. This dynamic model fits with the previously hypothesized function of nephrin as a structural sensor for the intactness of the slit diaphragm, implying that podocytes have to know whether nephrin is correctly positioned (i.e., when nephrin molecule has reached its position within the slit diaphragm and settled there correctly to bind to nephrin and NEPH1/NEPH2 of opposing podocytes) (12). However, if the slit diaphragm is broken and nephrin loses its normal extracellular contacts or nephrin is misplaced on the podocyte surface (i.e., within the apical cell membrane above the slit diaphragm; ref. 25), reduced tyrosine phosphorylation of the nephrin C terminus occurs (12) and β-arrestin2 binds to the extracellular unbound nephrin to initiate its endocytosis, thereby also terminating nephrin-signaling. This model allows a dynamic regulation of the dynamic equilibrium between assembly and disassembly of the slit diaphragm controlled by the activity of the Src-family kinases (Fig. 5; Movie 1, which is published as supporting information on the PNAS web site). Additional support for the in vivo relevance of our findings can be derived from previous animal studies. Mice deficient for the Src-family kinases Fyn/Yes present glomerular renal damage and proteinuria (11, 26). However, Verma et al. (11) found that nephrin phosphorylation was enhanced in glomerular fractions prepared from Yes null mice, whereas nephrin phosphorylation was decreased in Fyn null mice. The decrease in nephrin phosphorylation was not enhanced further in glomerular fractions prepared from mice lacking both Yes and Fyn. These data may suggest that Fyn is the dominant Src-family kinase that phosphorylates nephrin, which needs to be clarified in future experiments.
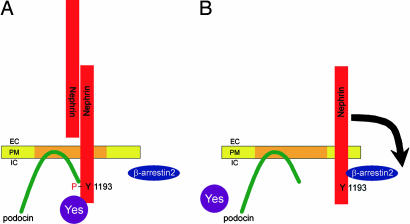
Fig. 5.
The phosphorylation status of the nephrin Y1193 determines and reflects the slit diaphragm integrity. β-Arrestin2 and podocin interact with nephrin in dependence of the nephrin Y1193 phosphorylation status. (A) Extracellular binding, e.g., homodimerization of nephrin molecules, leads to tyrosine phosphorylation of the nephrin C terminus by Src-kinases (i.e., Yes). Subsequently, podocin interacts with the phosphorylated nephrin, anchors the protein complex in detergent-resistant plasma membranes, and enhances nephrin signaling. (B) As soon as nephrin loses its extracellular-binding partner, it becomes dephosphorylated at nephrin Y1193, terminating the podocin interaction. The nephrin molecule loses its recruitment to detergent-resistant membranes and now interacts with β-arrestin2. This interaction initiates the cell surface disappearance of nephrin and terminates nephrin-mediated signaling. EC, extracellular; PM, plasma membrane; IC, intracellular.
Here we present a previously undescribed dynamically regulated molecular model of the glomerular slit diaphragm that enables nephrin to respond to the extracellular conditions and to signal the podocyte the integrity status of the glomerular slit diaphragm. Phosphorylation of nephrin Y1193 by Src-family kinases seems to be crucial for switching between the interaction of nephrin with podocin and β-arrestin2. This mechanism controls the fate of nephrin and, presumably indirectly, the integrity of the glomerular slit diaphragm. It could be of therapeutical importance to regulate the phosphorylation status of nephrin Y1193 with pharmacological agents, thereby influencing the integrity of the glomerular slit diaphragm in proteinuric diseases.
Materials and Methods
Plasmids.
The human nephrin cDNA (untagged) and mouse podocin (N-terminal V5-tagged) cDNA plasmids have been described in ref. 13 and were kindly provided by Gerd Walz (University of Freiburg, Freiburg, Germany). Point mutations were introduced according to the QuikChange Mutagenesis Protocol (Stratagene, La Jolla, CA). C-terminal Flag-tagged β-arrestin2 was a generous gift from Robert Lefkowitz (Duke University, Durham, NC). Expression plasmids of Fyn and Yes were a gift of Filippo G. Giancotti (Memorial Sloan–Kettering Cancer Center, New York, NY). The mouse podocyte cell line was provided by Peter Mundel (Albert Einstein College, New York, NY).
Membrane-bound fusion proteins of the C-terminal cytoplasmic domains of nephrin were generated by using a pCDM8 cassette that contained the leader sequence of CD5 fused to the CH2 and CH3 domains of human IgG1, followed by the transmembrane region of CD7 (6).
Antibodies.
The following antibodies were used β-arrestin2 H-9 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-M2 (Sigma, Taufkirchen, Germany), anti-human-Ig (Amersham, Freiburg, Germany), and anti-V5 (Serotec, Raleigh, NC). Nephrin 30142 extracellular polyclonal rabbit antibody and NEPH2-antibody were characterized in ref. 27. Secondary antibodies were purchased from Dako (Glostrup, Denmark).
Cell Culture.
HEK293T cells were grown in DMEM-F-12 supplemented with 10% FCS. Conditionally immortalized mouse podocytes were generated as described previously and grown at permissive temperature in the presence of 10 units of IFN-γ per ml. To induce differentiation, the cells were maintained on type I collagen at 37°C without IFN-γ for at least 14 days.
Coimmunoprecipitation.
Coimmunoprecipitations were performed as described in ref. 6. Briefly, HEK293T cells were transiently transfected by the calcium phosphate method. After incubation for 24 h, cells were washed twice and lysed in a 1% Triton X-100 lysis buffer. After centrifugation (15,000 × g for 15 min at 4°C), cell lysates containing equal amounts of total protein were incubated for 1 h at 4°C with the appropriate antibody, followed by incubation with 40 μl of protein G Sepharose for ≈3 h. The Sepharose was washed extensively with lysis buffer, and bound proteins were resolved by 10% SDS/PAGE and visualized by Western blotting. V5.podocin and β-arrestin2.F were detected by Western blotting with V5-antisera and M2-antisera, respectively.
For endogenous interaction, eight mouse kidneys were homogenized by using a glass potter, cleared by centrifugation, and solubilized in lysis buffer supplemented with 20 mM CHAPS and 3 mM ATP. Before immunoprecipitation, cellular lysates were further precleared by ultracentrifugation and absorption to protein G Sepharose. All tissues were freshly prepared and perfused in situ with ice-cold PBS before lysis. Control samples were incubated with anti-NEPH2 rabbit antiserum followed by protein A Sepharose. β-Arrestin2 is detected by Western blot analysis with monoclonal β-arrestin2 antibody.
Measurement of Cell-Surface Expression of Nephrin.
HEK293T were cultured in 10-cm dishes and then transiently cotransfected with a fixed amount (2 μg) of the nephrin cDNA and increasing amounts of β-arrestin (0–10 μg). In all cases, the total DNA per transfection was constant at 10 μg per dish by adjusting with empty parental vector pCDM8. After transfection (24 h), the cells were placed on ice and washed three times with ice-cold PBS buffer containing 0.1 mM CaCl2 and 1 mM MgCl2, pH 8.0 (PBSCM). Plasma membrane proteins were isolated by using a cell-surface biotinylation assay as described in ref. 28. Briefly, plasmalemmal proteins were indiscriminately labeled with membrane-impermeable N-hydroxysulfosuccinimydyl-SS-biotin (0.5 mg/ml; Pierce, Rockford, IL) for 30 min at 4°C. The solution was then discarded, and unreacted biotin was quenched three times with ice-cold PBSCM containing 20 mM glycine. The cells were lysed in PBS buffer containing 0.2% deoxycholic acid, 1% Triton X-100, and proteinase inhibitor mixture (Roche, Mannheim, Germany) for 30 min on ice and centrifuged at 12,000 × g for 30 min at 4°C to remove insoluble cellular debris. A portion of the resulting supernatant was removed and represents the total fraction. The remaining supernatant was incubated with anti-nephrin rabbit antiserum followed by protein A Sepharose to extract biotinylated surface-diaphragm proteins according to manufacturers’ instructions. The proteins were resolved by SDS/PAGE followed by Western blotting. The intensities of the bands were quantified by densitometry.
Endocytosis Assay.
For internalization studies, cells were plated in six wells on glass slides (Corning, Acton, MA) and transfected with the indicated constructs by the calcium phosphate precipitation method. HEK293T cells expressing nephrin wild type were incubated with rabbit anti-nephrin antibody (ec 30142), described in ref. 25, in DMEM for 1 h at 4°C. After three washes with buffer, cells were incubated with Rhodamine-Red-X-conjugated goat anti-rabbit antibody in DMEM for 1 h at 4°C. Cells then were washed and incubated at 4°C or 37°C for 20 min before finally fixing them with 4% paraformaldehyde. Images were obtained by using the Olympus (Hamburg, Germany) BX51 microscope and software equipped with fluorescence optics and Deltavision deconvolution microscopy software (Applied Precision, Issaquah, WA).
Luciferase Assay.
HEK293T cells seeded in 12-well plates were transiently transfected with a luciferase reporter construct, a β-gal expression vector (kindly provided by C. Cepko, Harvard Medical School, Boston, MA), and vectors directing the expression of the proteins as indicated. Total DNA amount was 1.5–2.0 μg per well. Cells were serum-starved for 12 h, harvested in cold PBS, and lysed in 100 μl of reporter lysis buffer (Applied Biosystems, Norwalk, CT) for 15 min at room temperature. Lysates were centrifuged at 12,000 × g for 15 min to remove insoluble material. Luciferase activity was determined by using a commercial assay system (Applied Biosystems) and normalized for β-gal activity to correct for transfection efficiency. Experiments were done as triplicates, and data are shown as mean with SD. Equal expression of proteins was ensured by Western blot analysis.
Supplementary Material
Acknowledgments
We thank Bettina Priesch for her excellent technical assistance and Karl Jakobs and Gerd Walz for careful reading and helpful criticism of the manuscript. This work was supported by University of Bochum Grant FoRUM F355-2002 (to L.S.).
Abbreviation
- AP-1
activator protein 1
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, et al. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 2.Tryggvason K, Patrakka J, Wartiovaara J. N Engl J Med. 2006;354:1387–1401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 3.Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry SG, Seifter JL. Ann Intern Med. 1995;123:754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS. Science. 1999;286:312–315. doi: 10.1126/science.286.5438.312. [DOI] [PubMed] [Google Scholar]
- 5.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 6.Sellin L, Huber TB, Gerke P, Quack I, Pavenstadt H, Walz G. FASEB J. 2003;17:115–117. doi: 10.1096/fj.02-0242fje. [DOI] [PubMed] [Google Scholar]
- 7.Doublier S, Salvidio G, Lupia E, Ruotsalainen V, Verzola D, Deferrari G, Camussi G. Diabetes. 2003;52:1023–1030. doi: 10.2337/diabetes.52.4.1023. [DOI] [PubMed] [Google Scholar]
- 8.Gerke P, Huber TB, Sellin L, Benzing T, Walz G. J Am Soc Nephrol. 2003;14:918–926. doi: 10.1097/01.asn.0000057853.05686.89. [DOI] [PubMed] [Google Scholar]
- 9.Khoshnoodi J, Sigmundsson K, Ofverstedt LG, Skoglund U, Obrink B, Wartiovaara J, Tryggvason K. Am J Pathol. 2003;163:2337–2346. doi: 10.1016/S0002-9440(10)63590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Lemay S, Aoudjit L, Kawachi H, Takano T. J Am Soc Nephrol. 2004;15:3006–3015. doi: 10.1097/01.ASN.0000146689.88078.80. [DOI] [PubMed] [Google Scholar]
- 11.Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, Wiggins RC, Killen P, Holzman LB. J Biol Chem. 2003;278:20716–20723. doi: 10.1074/jbc.M301689200. [DOI] [PubMed] [Google Scholar]
- 12.Lahdenpera J, Kilpelainen P, Liu XL, Pikkarainen T, Reponen P, Ruotsalainen V, Tryggvason K. Kidney Int. 2003;64:404–413. doi: 10.1046/j.1523-1755.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- 13.Huber TB, Kottgen M, Schilling B, Walz G, Benzing T. J Biol Chem. 2001;276:41543–41546. doi: 10.1074/jbc.C100452200. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Kirkbride KC, How T, Nelson CD, Mo J, Frederick JP, Wang XF, Lefkowitz RJ, Blobe GC. Science. 2003;301:1394–1397. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 16.Spiegel A. Science. 2003;301:1338–1339. doi: 10.1126/science.1089552. [DOI] [PubMed] [Google Scholar]
- 17.Cen B, Xiong Y, Ma L, Pei G. Mol Pharmacol. 2001;59:758–764. doi: 10.1124/mol.59.4.758. [DOI] [PubMed] [Google Scholar]
- 18.Lefkowitz RJ, Shenoy SK. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 19.Luttrell LM, Lefkowitz RJ. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 20.Roselli S, Heidet L, Sich M, Henger A, Kretzler M, Gubler MC, Antignac C. Mol Cell Biol. 2004;24:550–560. doi: 10.1128/MCB.24.2.550-560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benzing T. J Am Soc Nephrol. 2004;15:1382–1391. doi: 10.1097/01.asn.0000130167.30769.55. [DOI] [PubMed] [Google Scholar]
- 22.Beltcheva O, Martin P, Lenkkeri U, Tryggvason K. Hum Mutat. 2001;17:368–373. doi: 10.1002/humu.1111. [DOI] [PubMed] [Google Scholar]
- 23.Patrakka J, Kestila M, Wartiovaara J, Ruotsalainen V, Tissari P, Lenkkeri U, Mannikko M, Visapaa I, Holmberg C, Rapola J, et al. Kidney Int. 2000;58:972–980. doi: 10.1046/j.1523-1755.2000.00254.x. [DOI] [PubMed] [Google Scholar]
- 24.Milasta S, Evans NA, Ormiston L, Wilson S, Lefkowitz RJ, Milligan G. Biochem J. 2005;387:573–584. doi: 10.1042/BJ20041745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerjaschki D. J Clin Invest. 2001;108:1583–1587. doi: 10.1172/JCI14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein PL, Vogel H, Soriano P. Genes Dev. 1994;8:1999–2007. doi: 10.1101/gad.8.17.1999. [DOI] [PubMed] [Google Scholar]
- 27.Gerke P, Sellin L, Kretz O, Petraschka D, Zentgraf H, Benzing T, Walz G. J Am Soc Nephrol. 2005;16:1693–1702. doi: 10.1681/ASN.2004060439. [DOI] [PubMed] [Google Scholar]
- 28.Szabo EZ, Numata M, Lukashova V, Iannuzzi P, Orlowski J. Proc Natl Acad Sci USA. 2005;102:2790–2795. doi: 10.1073/pnas.0407444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.