Abstract
Recurrent urinary tract infections (rUTIs) are a source of considerable morbidity in women. The infecting bacteria in both rUTIs and a de novo acute infection have been thought to originate from an extraurinary location. Here, we show in a murine model of UTI that uropathogenic Escherichia coli (UPEC) established quiescent intracellular reservoirs (QIRs) in Lamp1+ endosomes within the urinary bladder epithelium. Depending on the integrity of the urothelial barriers at the time of initial infection, these QIRs were established within terminally differentiated superficial facet cells and/or underlying transitional epithelial cells. Treatment of infected bladders harboring exclusively superficial facet cell QIRs with the cationic protein, protamine sulfate, led to epithelial exfoliation and eradication of bacteria in 100% of treated animals. However, when the bacterial QIRs were harbored in underlying transitional cells, stimulation of epithelial turnover triggered reemergence of viable organisms and recurrence of infection. Thus, our results suggest (i) that bacterial QIRs within the bladder may be a previously unappreciated source of recurrent UTIs and (ii) that inducing epithelial exfoliation may be a therapeutic avenue for treating this heretofore recalcitrant disease.
Keywords: bladder, endosomes, quiescent intracellular reservoir, recurrence, urinary tract infection
Urinary tract infections (UTIs), the majority of which (>80%) are caused by uropathogenic Escherichia coli (UPEC) (1), affect 8 million women annually in the United States (2) and exhibit a high recurrence rate: >25% of all UTIs recur within 6 months (3). Therapeutic regimens do not differentiate between recurrent UTIs and new UTI episodes, as both are commonly thought to be acquired from extraurinary locations (e.g., the vaginal mucosa or intestinal tracts). However, daily antibiotic treatment of perineal and periurethral areas has not been shown to prevent recurrent infections, suggesting that transmission from the intestine or vagina to the urinary tract may not be necessary for recurrence (4). Also, up to 68% of recurring UTIs (rUTIs) are caused by E. coli that are identical to the original strain (5) and can occur up to 1 year after the initial infection (6). Culture analysis of bladder biopsies taken from women with rUTI symptoms after antibiotic therapy revealed that 8 of 33 (24%) patients had positive bacterial cultures from the biopsies despite sterile urines (7), further implicating the bladder itself as a source for seeding new infections. UPEC have also been shown to persist and reemerge in the bladder despite antibiotic therapy and sterile urine cultures in a murine model of UTI (8, 9). If rUTIs are caused by bacteria persisting within the bladder, this would argue for new approaches to rUTI diagnosis and treatment.
The mammalian urinary bladder is lined by a pseudostratified transitional epithelium that is three to four cells thick. Normal urothelial renewal is perpetual but slow, with a 40-week turnover (10), and is fueled by the urothelial stem cell, which resides in the basal layer, proliferating and differentiating to form intermediate cells, which in turn terminally differentiate into superficial facet cells (11). Intact superficial facet cells pose a formidable barrier to bacterial colonization, and for decades UPEC was considered strictly an extracellular pathogen. However, superficial facet cells express integral membrane proteins called uroplakins (UP), which can serve as the receptors for UPEC, on their luminal surface (12). Mouse models show that UPEC invade terminally differentiated superficial facet cells upon binding of UPEC to those receptors. Bacterial entry into facet cell cytoplasms is a critical event (13, 14). By 12 h postinfection (hpi), virtually all surviving bacteria are intracellular (8, 9, 13, 15). Upon entry into the superficial facet cells, UPEC are able to rapidly replicate and form intracellular bacterial communities (IBCs), characterized by a defined differentiation program and enhanced resistance to antibiotics. Typically, no more than 500 IBCs per bladder develop from an inoculum of 107 bacteria, indicating that invasion and intracellular replication are relatively rare events. However, within hours of IBC development, the progeny of successfully invasive bacteria emerge from the facet cells, often in a filamentous state, and invade neighboring cells to start the cycle anew (14, 16), suggesting that invasion and intracellular replication affords a survival advantage for the bacteria to persist within the bladder.
The mammalian host is not inert in the face of bacterial invasion. Host defenses against the invading bacteria include exfoliation of superficial facet cells, which aids clearance of tissue-associated bacteria (13). Superficial facet cell exfoliation is followed by a renewal process characterized by proliferation of immature underlying transitional epithelial cells that eventually differentiate to restore the superficial facet cell barrier within days of initial bacterial invasion (13, 17). Although the vast majority of UPEC are cleared by host defenses within a few days, small clusters of intracellular bacteria have occasionally been observed to persist for months in an antibiotic-insensitive state (8, 16, 18). The long-term persistence in the face of antibiotic therapy suggests that these bacteria are within a protected location within bladder epithelial cells.
In addition to the superficial facet epithelial cell barrier, invading bacteria also face a chemical barrier: the complex network of proteoglycans/glycosaminoglycans (GAG layer) that is woven into the urothelium and is known to act as an antimicrobial adherence factor (18–21). Parsons et al. (19) have shown that protamine sulfate (PS), a highly cationic protein (pI ≈ 12) can lead to both exfoliation of the superficial facet cell barrier and biochemical inactivation of GAGs (19). Furthermore, PS treatment also increases urothelial ionic permeability (20) and facilitates bacterial entry (21). Thus, we reasoned that PS could be used as both a chemical exfoliant of infected superficial facet cells and as an adjuvant to facilitate bacterial entry into nonexfoliating transitional cells underlying the superficial facet cell layer.
In this report, we investigated the molecular and cellular mechanisms underlying formation, reemergence, and/or elimination of putative persistent uropathogenic bacteria from the bladder epithelium using PS.
Results
UPEC Establish Quiescent Intracellular Reservoirs (QIRs) within Lamp1+ Endosomes.
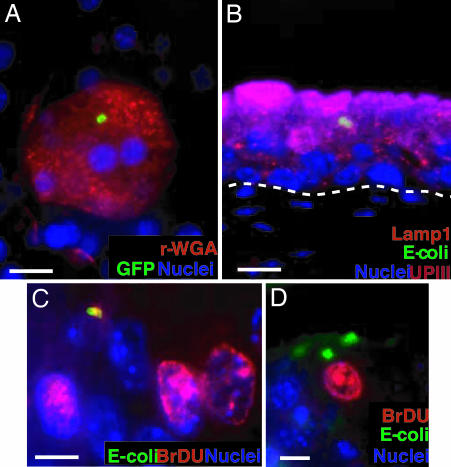
To examine the cellular features of persistent UPEC infection, bladders of adult female C57BL/6J mice were infected with UTI89, a well characterized clinical cystitis isolate (9) expressing GFP. Urine samples were then collected from all mice (n = 4–8 mice per experiment, two independent experiments) at 2 weeks after bacterial exposure and found to be sterile; samples showed no evidence of inflammatory cells, indicating that there was no active infection or extracellular UPEC colonization at this time point. Whole-mount bladder tissue analysis revealed that the urothelium had an intact superficial facet cell layer, indicating that the renewal process after acute infection was complete. Furthermore, our analysis revealed GFP-expressing bacteria persisting exclusively within the superficial facet cells (Fig. 1A). Interestingly, intracellular bacteria persisting in these cells were arranged in rosette-like clusters, suggesting that they were sequestered within a subcellular compartment (mean, eight rosettes per bladder, n = 5 mice per experiment, two experiments).
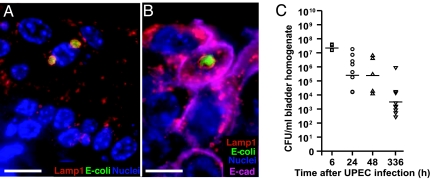
Fig. 1.
QIRs form within Lamp1+ vesicles in superficial facet cells. (A) Epifluorescence microscopy on whole-mounted bladders infected with GFP+UTI89 showing bacterial QIRs (green) persisting in differentiated facet cells stained with Alexa Fluor 94-tagged wheat germ agglutinin (r-WGA, to demarcate outlines of facet cell membranes). Epithelial nuclei stained with bis-benzimide appear blue. (Scale bar, 50 μm.) (B) Lamp1+ vesicles (orange, stained with Alexa Fluor 594-tagged rat antibody to Lamp1) containing QIRs (green, stained with Alexa Fluor 88-tagged rabbit polyclonal Abs to E. coli) are in UPIII+ terminally differentiated facet cells (purple with Alexa Fluor 47-tagged mouse mAb to UPIII) 2 weeks after infection. (Scale bar, 50 μm.) The dashed line demarcates epithelial–mesenchymal boundary. (C and D) IFA of tissue sections from BrdU-injected bladders of infected mice show a small BrdU+ (red; stained with Alexa Fluor 94-tagged goat polyclonal antibodies to BrdU) bacterial QIR (yellow) and BrdU+ transitional epithelial cells. A larger BrdU− QIR (green) is depicted in D. (Scale bar, 10 μm.)
To examine the putative UPEC subcellular niche, sections from bladders infected with UTI89-GFP for 2 weeks were probed for the presence of early endosomal (rab5a), late endosomal/early lysosomal (Lamp1), and late lysosomal (cathepsin D) markers. We found that 100% of the E. coli rosettes were enclosed within Lamp1+Rab5a−CathepsinD− vesicles (Fig. 1B). We then sought to determine whether the persisting bacteria were quiescent within their vesicular location. Mice infected with UTI89-GFP for 6 h, 24 h, 48 h, and 2 weeks were injected with a nucleotide analogue (BrdU) that labels cells in S-phase (n = 5 mice per experiment). Multilabel-immunofluorescence analysis (IFA) of tissue sections from the BrdU-labeled, infected bladders revealed that UTI89 bacteria within IBCs (6–24 hpi) were BrdU+, i.e., in a state of active replication (Fig. 7, which is published as supporting information on the PNAS web site). At 2 weeks after infection, small numbers of replicating intracellular bacteria could be visualized occasionally (Fig. 1C, a replicating bacterial doublet). However, larger bacterial clusters did not stain BrdU+ (Fig. 1D), suggesting that the bacterial rosettes had limited replicative capacity and were mostly quiescent. In addition, we found that the Lamp1+ vesicle-enclosed UPEC rosettes could survive for as long as 12 weeks in the urothelium, suggesting that UPEC can persist long term within these endosomal vesicles without eliciting a host inflammatory response or triggering exfoliation of the epithelium. Because these persistent vesicular membrane-bound quiescent bacterial rosettes could constitute a bladder epithelial reservoir that could potentially seed same-strain recurrent UTIs, we termed them QIRs.
PS Treatment Eliminates QIRs Localized to Superficial Facet Cells.
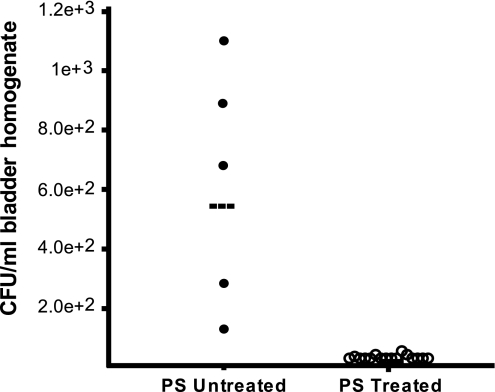
The cationic protein PS can induce superficial facet cell exfoliation. Thus, we reasoned that PS treatment should eliminate the QIRs maintained in this superficial epithelial cell layer of the bladder. To test this hypothesis, we treated mouse bladders (n = 24 mice) 2 weeks after UPEC inoculation with 10 mg/ml PS. Mice were followed daily by urinalysis that showed exfoliated facet cells, but no inflammatory cells. Seven days after PS treatment, the mice were killed, and their bladders were titrated for bacterial CFUs. CFUs in the exfoliated bladders were below detection, suggesting that no viable bacteria remained in the tissue. QIR-containing mice that had not been PS-treated continued to maintain bacterial titers of 102 to 103 CFUs/ml (P < 0.001; Fig. 2). Thus, PS treatment “cured” chronically infected mice, eliminating all persistent bacterial QIRs from the bladder.
Fig. 2.
Eradication of bacterial QIRs confined to superficial facet cells after PS treatment. CFUs in bladders infected for 2 weeks and treated with 10 mg/ml PS (n = 19 mice) were measured. Control bladders, which were infected but not treated with PS, show colonization (n = 5 mice). Horizontal lines indicate mean titer/mouse/time point.
QIRs Can Form Within Underlying Transitional Cells in PS-Pretreated Bladders.
Our findings suggest that UPEC inoculation into healthy, unperturbed mouse bladders results in bacterial persistence in the superficial facet cells. However, human bladders are routinely exposed to stimuli [e.g., cationic antimicrobial peptides, defensins (22), and PS-like low molecular weight cationic cyto-injury factors produced in human urines (23)] that may lead to both superficial facet cell exfoliation and damage of the GAG layer. A damaged superficial facet cell layer with exposed underlying transitional cells could thus constitute a physiologic condition encountered by UPEC during an ascending infection into the human urinary tract. Thus, we sought to investigate in a mouse model whether UPEC could colonize exposed underlying transitional cells of the bladder. We reasoned that if the protective cellular and chemical bladder barriers were disrupted before initial UPEC inoculation, it would allow greater access of bacteria to all layers of the bladder epithelium. Thus, we pretreated bladders with 10 mg/ml PS for 12 h and subsequently exposed them to 107 CFU of UTI89-GFP; mice were followed for 6 h, 24 h, 48 h, 72 h, and 2 weeks after bacterial exposure (n = 4–8 mice per experiment, two experiments). In bladders pretreated with PS, UTI89-GFP was able to successfully invade into the remaining nonexfoliated facet cells and establish IBCs at the early time points (6 hpi) with unusually high frequency (mean 629 IBCs per bladder vs. <500 per bladder without PS pretreatment). At those early time points, all intracellular bacteria were found in superficial facet cell cytoplasms and exhibited characteristic IBC morphology (Fig. 8, which is published as supporting information on the PNAS web site).
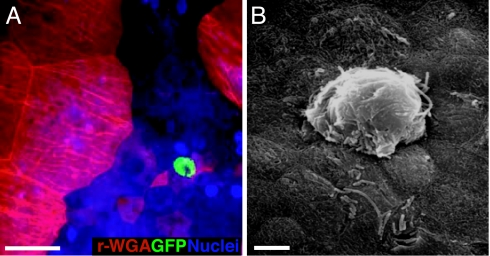
However, at later time points, we discovered IBCs where they have never been previously documented. Using whole-mount bladder tissue analysis, we observed that, at 24 hpi, bladders pretreated with PS contained cytosolic IBCs within the underlying transitional cells (mean 32 per bladder, n = 5 mice per experiment; Fig. 3A and Fig. 9, which is published as supporting information on the PNAS web site). Scanning electron microscopic analysis of these bladders confirmed that the IBCs within the immature transitional cells resulted in pod-like protrusions into the exfoliated luminal surface of the bladder with bacterial rods evident through the pod shell (Fig. 3B). Interestingly, when the entire superficial facet cell layer was chemically stripped before bacterial exposure by application of higher PS concentrations, IBCs did not form in the underlying cells, suggesting that bacteria may first need to undergo IBC development within superficial facet cells before invading underlying transitional cells.
Fig. 3.
UPEC can colonize underlying transitional cells and develop into IBCs. (A) Epifluorescence microscopy on whole-mounted bladders infected with UTI89-GFP reveal an E. coli IBC (green) in underlying transitional cells at 24 hpi. Facet cells are red (r-WGA+). (Scale bar, 50 μm.) (B) Scanning electron micrograph of an IBC at 24 hpi in an underlying transitional cell. (Scale bar, 10 μm.)
Forty-eight hpi, bladder whole mount and IFA revealed that cytoplasmic IBCs were no longer present in any cell layer (n = 5 mice per experiment, two experiments). Instead, the intracellular bacterial populations were now present as small rosette-like clusters (Fig. 4A), characteristic of the QIRs observed in non-PS-pretreated, chronically infected bladders. However, the quantity of QIRs in PS-pretreated bladders (mean 72 per bladder in PS-pretreated bladders; n = 5 mice per experiment, two experiments) was 9-fold greater than in non-PS-treated bladders. Thus, disruption of chemical and cellular host barriers exposed UPEC to epithelial locations that are not accessible in otherwise healthy bladders.
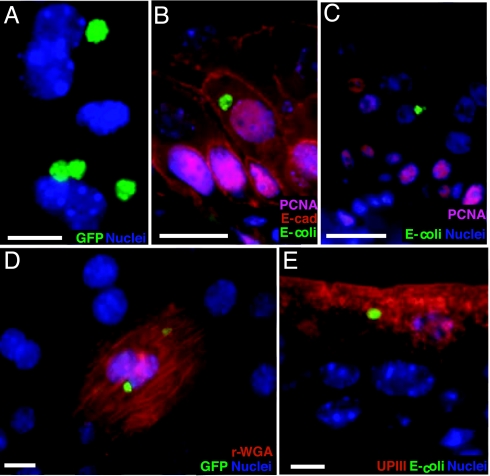
Fig. 4.
QIRs in underlying transitional cells in PS-pretreated bladders. (A) Whole-mount analysis of PS-pretreated bladders infected for 48 h with UTI89 show GFP+ bacterial QIRs (green). Nuclei appear blue. (B) Bladder tissue sections show that E. coli (green) are intracellular in proliferating PCNA+ (pink nuclei stained with Alexa Fluor 94-linked to goat polyclonal Ab to PCNA); E-cadherin+ underlying cells (red, with Alexa Fluor 94-tagged mouse mAb to E-cadherin, which labels only transitional cells and excludes superficial facet cells). (Scale bar, 10 μm.) (C) UPEC QIRs are also associated with nonproliferating cells (blue nuclei). (D) Whole-mount analysis reveals that the QIRs are GFP+ (green) 2 weeks after infection and can be found in differentiated facet cells (red stain: r-WGA). (E) IFA of bladder tissue sections shows that UPEC QIRs (green) persist in differentiated facet cells (red: UPIII+). (Scale bar, 50 μm in D and E.)
Forty-eight hours and later after infection, the underlying transitional cells are in a state of active proliferation and differentiation into mature superficial facet cells. Our data revealed that QIRs in PS-pretreated bladders were found within both proliferating [proliferating cell nuclear antigen (PCNA)+ and E-cadherin+ (Fig. 4B)] as well as nonproliferating transitional cells (Fig. 4C). Furthermore, GFP-labeled bacterial QIRs persisted stably up to 12 weeks or more after infection as revealed by surveys of whole bladders (Fig. 4D) and were present in regenerated UPIII-expressing facet cells at these later time points (Fig. 4E). Together, these data suggest that proliferation/differentiation status of the epithelial cells does not affect QIR formation.
Transitional Cell QIRs Are Also Enclosed Within Lamp1+ Vesicles.
Next, we determined whether the QIRs in underlying transitional cells were also sequestered within Lamp1+ vesicles. Sections from PS pretreated bladders infected with UTI89-GFP at the early (1.5–24 h) and late (48 h, 72 h, 2 weeks) time points were immunostained with antibodies to Lamp1 and cathepsin D, antibodies generated against whole E. coli and FimH, an E. coli type-1 pilus adhesin. We found that, at 48 hpi and later, all E. coli QIRs were enclosed within Lamp1+ vesicles in E-cadherin-labeled transitional cells (Fig. 5 A and B) and the bacteria continued to express FimH (data not shown). IFA of tissue cross-sections revealed an average of 8–10 vesicles per 5-μm section (n = 3 sections scored per mouse bladder, n = 4 mice per experiment). Each vesicle contained between 2 and 12 bacteria (n ≈ 200 Lamp1+ vesicles scored). Cathepsin D+ vesicles were found within the same cells in the same bladders, but never enclosed bacteria (data not shown), indicating that E. coli QIRs were not present in degradative lysosomes. Because QIRs were also found in non-PS-treated bladders, entry into and persistence within membrane-enclosed intracellular compartments appears to be a general feature of UPEC and is not an artifact of PS treatment. We found that the vesicle-enclosed bacterial QIRs were stable in the Lamp1+ vesicles and continued to be sequestered even as the cells that harbored them differentiated into mature superficial facet cells (2 weeks after infection). At 2 weeks after infection, abundant viable bacteria remained in the bladder tissue, although there was a 3 log reduction in CFU titers (CFU/ml = 103 at 2 weeks relative to 106 at 6 hpi, Fig. 5C).
Fig. 5.
Subcellular localization of UPEC in Lamp1+ vesicles. (A) Bacterial QIRs (green) are enclosed within Lamp1+ vesicles (red). (B) Lamp1+ vesicles (red) containing UTI89 QIRs (green) are in E-cadherin+ transitional cells (pink) at 48 hpi. (Scale bars, 10 μm.) (C) CFUs in PS-pretreated bladders at various time points after bacterial inoculation. Horizontal lines indicate mean titer/mouse/time point; n = 4–8 mice.
Bladder Epithelial Turnover Is Associated with Reemergence of UPEC from the Reservoir.
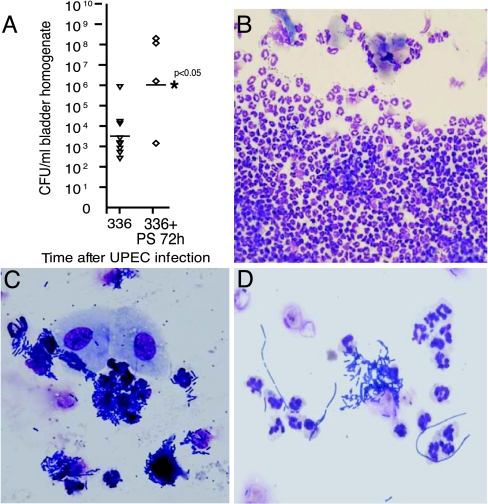
PS pretreatment of bladders exposes normally protected locations in the urothelium, presumably allowing UPEC to form QIRs within deeper cell layers. At 2 weeks after infection, a time when the mouse bladders have completed their initial renewal cycle and regenerated intact facet cell layers, the QIRs could be found throughout all cell layers, including regenerated superficial facet cells. We next determined whether the broadly distributed QIRs in PS-pretreated bladders could be cleared by triggering exfoliation, as occurred in non-PS-pretreated bladders where QIRs were confined to the superficial facet cell layer. To reinitiate regeneration in the PS-pretreated bladders, we administered a single bolus of 10 mg/ml PS at 2 weeks after initial infection (n = 30 mice). Mice were monitored daily thereafter for bacteriuria, and five mice each were killed 24, 48, and 72 h after PS retreatment. Whole-mount bladder tissue analysis with subsequent IFA of tissue sections from the same bladders revealed that, 24 h after PS treatment, there was massive exfoliation of the facet cell layer. Forty-eight hours later, the underlying cells were rapidly dividing and regenerating (data not shown). Whole-bladder tissue surveys showed no evidence of luminal bacteria or IBCs (data not shown), although IFA demonstrated the presence of isolated QIRs in the underlying cell layers, suggesting that the bacteria were protected in their intracellular locations. Surprisingly, however, 72 h after PS treatment, when the proliferating underlying transitional cells had differentiated and reformed an intact superficial facet cell layer, we found by urinalysis that four of nine mice (44%) developed bacteriuria. Bladder CFU titers in three of these mice were >106 CFU/ml (P < 0.05; Fig. 6A). Urinalysis showed a pronounced inflammatory response concomitant with epithelial exfoliation (Fig. 6 B and C) and presence of bacterial filaments (Fig. 6D). The titers and cytological findings were indistinguishable from those of a de novo acute infection (16). Therefore, our results suggested that the bacterial QIRs in underlying cells were not only viable but could be induced to reemerge from their reservoir to cause an acute recurrent UTI. Bacteria in the rUTI episode were still GFP+, confirming that they were descendents of the initial inoculum. Thus, UPEC found within membrane-bound QIRs in underlying transitional cells can serve as a seed for a new infection upon activation of transitional cell differentiation, whereas, if the bacterial QIRs are confined to the superficial facet cells, stimulation of bladder exfoliation leads to elimination of the chronic UPEC infection.
Fig. 6.
Reemergence of intracellular UPEC after initiation of epithelial turnover. (A) CFUs in bladders PS-pretreated and UPEC-infected for 2 weeks and subsequently retreated with PS for 72 h. Horizontal lines indicate mean titer/mouse/time point. (B) Urinalysis shows massive neutrophil efflux. (C) Exfoliated urothelial cells and bacteriuria indicating rUTI. (D) Bacterial filaments in rUTI urinalysis.
Discussion
Our studies demonstrate the ability of E. coli to invade bladder epithelial cells and establish quiescent intracellular reservoirs within late endosomal vesicles where they can persist for months. We found that vesicle-bound QIRs that persisted exclusively in the superficial facet cells could be eliminated in 100% of treated bladders by inducing exfoliation using PS. Presumably, PS-induced epithelial exfoliation in those bladders led to shedding of all bacteria before they were able to proliferate and establish a recurrence of infection. In contrast, when the initial bacterial inoculum was applied to bladders with damaged chemical and cellular barriers, the bacteria formed endosomal QIRs in greater abundance in all layers of the bladder. PS treatment of those bladders did not expel the bacterial reservoir, and led instead to a recurrence of acute infection in nearly half the mice examined. These results argue that, when bladder mucosal barriers are breached, E. coli can colonize underlying transitional cells and form QIRs that are resistant to both innate (e.g., exfoliation) and adaptive host defenses. Inducing exfoliation in any bladder with an intact superficial facet cell layer leads to initiation of an epithelial differentiation program. In bladders harboring bacterial QIRs in underlying transitional cells, the waves of cell differentiation and division consequent to superficial cell exfoliation could lead to activation of the QIRs as infected transitional cells differentiate into superficial facet cells, thereby seeding a recurrent infection (Fig. 10, which is published as supporting information on the PNAS web site). The superficial facet cell may provide the necessary intracellular milieu to support active bacterial replication and thus IBC formation. Previous work has demonstrated that, after exfoliation in infected bladders, TGF-β and Wnt/Ca2+ pathways are down-regulated in the bladder, promoting the differentiation of the immature underlying cells to superficial facet cells (17); thus, molecular regulation of these pathways may be important in the etiology of rUTIs.
During the course of an ascending UTI, UPEC likely encounter bladders with an intact superficial facet cell layer. Previous work has demonstrated that UPEC adhere to, invade, and establish IBCs within these facet cells (9, 16). In the present study, we found that treatment with 10 or 50 mg/ml PS led to either partial or complete stripping of the superficial facet cell layer, respectively, as determined by analyses of whole-mount bladders. Surprisingly, IBC formation in the underlying transitional cells was only supported in the bladders that were partially stripped of their superficial facet cells; IBCs were not detected in bladders where the entire facet cell layer was stripped. One explanation is that genetic cascades may be activated within the bacteria during IBC maturation in superficial facet cells that facilitate or are necessary for subsequent invasion into underlying epithelial cells. Alternatively, host signals generated during IBC formation in superficial facet cells could predispose the underlying transitional cells to bacterial invasion and IBC formation. Finally, it cannot be formally ruled out that the PS concentration necessary to strip the luminal epithelial cells may have unforeseen effects that protect the underlying transitional cells upon challenge by type 1-piliated UPEC.
Our findings suggest a potential link between disrupted epithelial barriers and formation of bacterial QIRs that could seed recurrent infection in the bladder. In humans, certain conditions result in a damaged epithelium; for example, patients with spinal cord injury often show evidence of urothelial damage similar to that caused by PS treatment and exhibit an increased incidence of bacterial cystitis (24). Even in healthy young women with presumably unperturbed epithelia, bladder GAG levels may vary during the normal menstrual cycle (25). Normal physiological changes in levels of GAGs may render bladders more vulnerable to invasion by bacteria at certain times. If UPEC inoculation were to occur in the face of a damaged epithelial layer or in hosts with inadequate GAG barriers, UPEC might colonize underlying, transitional cells and persist until subsequent exfoliating stimuli induce a recurrence. Furthermore, certain UPEC strains may contain virulence factors that allow the bacteria to penetrate into the transitional cells and form QIRs. Establishment of QIRs throughout the underlying transitional epithelium may predispose an individual to an increased likelihood of recurrence and may account for some of the frequent same-strain recurrences that are seen clinically despite appropriate antibiotic therapy.
At the cellular and mechanistic level, our data in a mouse model delineate the long-term survival of a clinical cystitis UPEC strain in a protected host niche, the nondegradative Lamp1+ endosomes. Several microorganisms that are also associated with persistent infections and evasion of host defenses (reviewed in refs. 26 and 27) are known to exist within Lamp1-containing vacuoles e.g., Helicobacter pylori (28), or Salmonella Typhimurium, which can survive and replicate in moderately acidified compartments (29). Future studies designed at investigating the bacteriologic properties of E. coli enclosed in the endosomal compartment could provide insights into mechanisms underlying their persistence in the urinary tract (30).
Our findings raise possible therapeutic avenues for the treatment of recurrent UTIs. We show that inducing epithelial exfoliation by using cationic proteins (e.g., PS) can, in some cases, expel bacteria from their intracellular locations. Therefore, protamine sulfate or other similar cationic compounds could act as potential therapeutic adjuvants in conjunction with antibiotic treatment to induce stripping of the urothelial lining containing cryptic bacterial QIRs, thus eliminating a potential source of chronic same-strain recurrent UTI episodes.
Materials and Methods
Mice.
All experiments were performed by using protocols approved by the animal studies committee of the Washington University School of Medicine. Six- to 8-week-old female C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) were used in all experiments. All mice were maintained under specified pathogen-free conditions in a barrier facility and under a strict 12-h light cycle.
Bacterial Strains.
UTI89 (9), a UPEC strain recovered from a human patient with cystitis (31), was transformed with pCOM-GFP plasmid (32) and grown for 16 h in LB as a static culture at 37°C.
Inoculations of Mice.
Mice were anesthetized and inoculated via transurethral catheterization with 50 μl of 107 CFU bacteria) in PBS as described (17). Mice were killed at the indicated times, and their bladders were aseptically removed and processed for microscopy, histology, and CFU titration.
CFU Titrations.
Bacterial titers were determined by plating serial dilutions of bladder homogenates (1 ml of PBS with 0.025% Triton X-100) on LB agar plates.
BrdU Labeling.
Some mice received an intraperitoneal injection of an aqueous solution of BrdU (120 mg/kg) and 5-fluoro-2′-deoxyuridine (12 mg/kg) (Sigma, St. Louis, MO) 90 min before death.
PS Treatment.
PS (50 μl; Sigma) was inoculated transurethrally into the bladders of adult C57BL/6J female mice at multiple doses (0.1–100 mg/ml), and the extent of facet cell exfoliation was determined at multiple time points. All analysis was performed in the urothelia of adult female C57BL/6J mice (n = 4–8 mice per experimental group for all experiments, n = 1–2 experiments). For further details, see Supporting Text, which is published as supporting information on the PNAS web site.
Histologic Analysis and IFA.
Bladders were dissected into two halves and incubated in 10% formalin overnight at 4°C. The bladder tissues were embedded in 2% agar for paraffin processing. For IFA, 4- to 5-μm serial sections were cut longitudinally, deparaffinized in fresh xylene (twice for 10 min at room temperature), rehydrated in isopropanol (three times for 5 min at room temperature), antigen retrieved by boiling for 30 min in 10 mM sodium citrate buffer, blocked in 1% BSA/0.3% Triton X-100 for 1 h at room temperature, and subsequently incubated overnight at 4°C with the following primary antibodies: (i) goat polyclonal antibodies to PCNA (1:100 final concentration in blocking buffer; Santa Cruz Biotechnology, Santa Cruz, CA), (ii) rabbit polyclonal antibodies to E. coli (1:500; US Biological, Cleveland, OH), (iii) rat anti-mouse E-cadherin (1:500; Zymed, South San Francisco, CA), (iv) mAb to uroplakin III (1:10; Research Diagnostics, Flanders, NJ), (v) rabbit polyclonal antibody to cathepsin D (1:100; Santa Cruz Biotechnology), (vi) rat mAb to LAMP1 (clone ID4B; Developmental Hybridoma Bank, National Institute of Child Health and Human Development, Bethesda, MD), (vii) goat polyclonal antibodies to BrdU (1:10,000, ref. 33), and (vii) mouse antibodies to FimH (1:100). After three PBS washes (5 min each at room temperature), antigen–antibody complexes were detected with Alexa Fluor 488-, 594-, and 647-conjugated secondary antibodies (1:500; Molecular Probes, Eugene, OR).
Scanning Electron Microscopy (SEM).
SEM was performed as described (14).
Whole-Mount Analysis.
Mice were killed, and their bladders were bisected and splayed in PBS. Splayed bladders were incubated in 4% paraformaldehyde for 1 h at room temperature, washed twice with 1× PBS, and stained for 20 min at room temperature with 10 μg/ml r-WGA to demarcate outlines of mature facet cells and 20 μg/ml biz-benzimide to visualize nuclei. All images were obtained by using a Zeiss Axioskop MOT Plus microscope equipped with an AxioCam MRM 2.0 Hi-Res digital camera for fluorescence microscopy. Axiovision software was used for image capture, processing, and documentation.
Urine Analysis.
At the indicated times, mice were induced to urinate by applying gentle pressure to the skin just below the occiput. The clean-catch urine samples were collected into sterile Eppendorf tubes, spun onto poly(l-lysine)-coated glass slides by using a CytoPro (Wescor, Logan, UT) cell spinner (6 min at 1,000 rpm), briefly heat fixed, and then stained with the Protocol Hema3 staining kit (Fisher, Pittsburgh, PA). Bacterial titers were determined by serial dilutions on Luria–Bertani (LB) agar plates.
Statistical Analysis.
Statistical analysis was performed by using Mann–Whitney U test (two-tailed). A value of P < 0.05 was considered to be significant.
Supplementary Material
Acknowledgments
We thank Jason Mills, Sheryl Justice, Joe Palermo, Tom Hannan, and Jeff Henderson for invaluable guidance and critical review of the manuscript, Diane Redmond for technical graphics assistance, and Duane Probst for help with CFU titrations. This work was supported by National Institutes of Health Grant DK51406 and Office of Research on Women's Health Specialized Center of Research Grant DK64540 (to S.J.H.) and postdoctoral support by Individual National Research Service Award F32CA108328 (to I.U.M.).
Abbreviations
- UTI
urinary tract infection
- rUTI
recurring UTI
- UPEC
unpathogenic E. coli
- UP
uroplakin
- hpi
hours postinfection
- IBC
intracellular bacterial communities
- PS
protamine sulfate
- QIR
quiescent intracellular reservoir
- IFA
immunofluorescence analysis.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ronald A. Dis Mon. 2003;49:71–82. doi: 10.1067/mda.2003.8. [DOI] [PubMed] [Google Scholar]
- 2.Foxman B. Am J Med. 2002;113(Suppl 1A):5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 3.Foxman B. Am J Public Health. 1990;80:331–333. doi: 10.2105/ajph.80.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantey JR. Am J Med. 1985;78:65–75. doi: 10.1016/0002-9343(85)90367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brauner A, Jacobson SH, Kuhn I. Clin Nephrol. 1992;38:318–323. [PubMed] [Google Scholar]
- 6.Ikaheimo R, Siitonen A, Heiskanen T, Karkkainen U, Kuosmanen P, Lipponen P, Makela PH. Clin Infect Dis. 1996;22:91–99. doi: 10.1093/clinids/22.1.91. [DOI] [PubMed] [Google Scholar]
- 7.Elliott SJ, Srinivas S, Albert MJ, Alam K, Robins-Browne RM, Gunzburg ST, Mee BJ, Chang BJ. Infect Immun. 1998;66:2040–2051. doi: 10.1128/iai.66.5.2040-2051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schilling JD, Lorenz RG, Hultgren SJ. Infect Immun. 2002;70:7042–7049. doi: 10.1128/IAI.70.12.7042-7049.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulvey MA, Schilling JD, Hultgren SJ. Infect Immun. 2001;69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jost SP. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57:27–36. doi: 10.1007/BF02899062. [DOI] [PubMed] [Google Scholar]
- 11.Hicks RM. Biol Rev Camb Philos Soc. 1975;50:215–246. doi: 10.1111/j.1469-185x.1975.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 12.Wu XR, Sun TT, Medina JJ. Proc Natl Acad Sci USA. 1996;93:9630–9635. doi: 10.1073/pnas.93.18.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 14.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 15.Duncan MJ, Li G, Shin JS, Carson JL, Abraham SN. J Biol Chem. 2004;279:18944–18951. doi: 10.1074/jbc.M400769200. [DOI] [PubMed] [Google Scholar]
- 16.Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, Hultgren SJ. Proc Natl Acad Sci USA. 2004;101:1333–1338. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mysorekar IU, Mulvey MA, Hultgren SJ, Gordon JI. J Biol Chem. 2002;277:7412–7419. doi: 10.1074/jbc.M110560200. [DOI] [PubMed] [Google Scholar]
- 18.Kerrn MB, Struve C, Blom J, Frimodt-Moller N, Krogfelt KA. J Antimicrob Chemother. 2005;55:383–386. doi: 10.1093/jac/dki002. [DOI] [PubMed] [Google Scholar]
- 19.Parsons CL, Boychuk D, Jones S, Hurst R, Callahan H. J Urol. 1990;143:139–142. doi: 10.1016/s0022-5347(17)39897-x. [DOI] [PubMed] [Google Scholar]
- 20.Tzan CJ, Berg JR, Lewis SA. Am J Physiol. 1993;265:C1637–C1647. doi: 10.1152/ajpcell.1993.265.6.C1637. [DOI] [PubMed] [Google Scholar]
- 21.Parsons CL, Stauffer CW, Schmidt JD. Infect Immun. 1988;56:1341–1343. doi: 10.1128/iai.56.5.1341-1343.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganz T. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 23.Parsons CL, Bautista SL, Stein PC, Zupkas P. J Urol. 2000;164:1381–1384. [PubMed] [Google Scholar]
- 24.Apodaca G, Kiss S, Ruiz W, Meyers S, Zeidel M, Birder L. Am J Physiol. 2003;284:F966–F976. doi: 10.1152/ajprenal.00359.2002. [DOI] [PubMed] [Google Scholar]
- 25.Maroclo MV, Pereira SD, Sampaio FJ, Cardoso LE. J Urol. 2005;173:1789–1792. doi: 10.1097/01.ju.0000154621.18695.b7. [DOI] [PubMed] [Google Scholar]
- 26.Rhen M, Eriksson S, Clements M, Bergstrom S, Normark SJ. Trends Microbiol. 2003;11:80–86. doi: 10.1016/s0966-842x(02)00038-0. [DOI] [PubMed] [Google Scholar]
- 27.Monack DM, Mueller A, Falkow S. Nat Rev Microbiol. 2004;2:747–765. doi: 10.1038/nrmicro955. [DOI] [PubMed] [Google Scholar]
- 28.Oh JD, Karam SM, Gordon JI. Proc Natl Acad Sci USA. 2005;102:5186–5191. doi: 10.1073/pnas.0407657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brumell JH, Grinstein S. Curr Opin Microbiol. 2004;7:78–84. doi: 10.1016/j.mib.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Eto DS, Sundsbak JL, Mulvey MA. Cell Microbiol. 2006;8:704–717. doi: 10.1111/j.1462-5822.2006.00691.x. [DOI] [PubMed] [Google Scholar]
- 31.Langermann S, Mollby R, Burlein JE, Palaszynski SR, Auguste CG, DeFusco A, Strouse R, Schenerman MA, Hultgren SJ, Pinkner JS, et al. J Infect Dis. 2000;181:774–778. doi: 10.1086/315258. [DOI] [PubMed] [Google Scholar]
- 32.Valdivia RH, Hromockyj AE, Monack D, Ramakrishnan L, Falkow S. Gene. 1996;173:47–52. doi: 10.1016/0378-1119(95)00706-7. [DOI] [PubMed] [Google Scholar]
- 33.Kim SH, Roth KA, Moser AR, Gordon JI. J Cell Biol. 1993;123:877–893. doi: 10.1083/jcb.123.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.