Abstract
The apparent extravagance of begging displays is usually attributed to selection for features, such as loud calls, that make the signal costly and hence reliable. An alternative explanation, however, is that these design features are needed for effective signal transmission and reception. Here, we test the latter hypothesis by examining how the begging calls of tree swallow (Tachycineta bicolor) nestlings and the response to these calls by parents are affected by ambient noise. In a field study, we found that call length, amplitude and frequency range all increased with increasing noise levels at nests. In the laboratory, however, only call amplitude increased in response to the playback of noise to nestlings. In field playbacks to parents, similar levels of noise abolished parental preferences for higher call rates, but the preference was restored when call amplitude was increased to the level that nestlings had used in the laboratory study. Our results show that nestling birds, like other acoustic signallers, consistently increase call amplitude in response to ambient noise and this response appears to enhance discrimination by receivers. Thus, selection for signal efficacy may explain some of the seemingly extravagant features of begging displays.
Keywords: noise, signals, begging, signal design, signal transmission, tree swallows
1. Introduction
Explanations for the evolution of extravagant animal signals fall mostly into two categories. The first, based on handicap theory (Grafen 1990), proposes that selection by receivers for costly (typically, vigorous and intense), and therefore honest, signals could produce extravagant displays. The second, based on signal efficacy (Dawkins & Guilford 1997), suggests that signals must be intense to effectively convey information to receivers in the face of transmission and reception noise.
Young animals produce a vigorous display called begging when soliciting care from their parents. In altricial birds, where the behaviour has been most studied, the display includes visual elements, such as gaping, and vocal elements, such as loud calling. The display has been considered extravagant because it appears unnecessarily vigorous, complex and loud for transmitting information the few centimetres between parents and young (Johnstone & Godfray 2002). Indeed, because obstacles to effective signal transmission and reception seem trivial over such short distances, much of the research on the design of begging signals has focused on honest signalling explanations.
The obstacles to transmission and reception of these signals may have been underestimated, however. For example, low light levels may obscure visual signals (Heeb et al. 2003), nest walls may attenuate or distort calls and competing signals may mask individual calls (Horn & Leonard 2002); all may hinder reception by parents. The potential for errors by parents in receiving these signals could select for more intense begging displays than predicted on distance alone. If so, then selection for effective signal transmission and reception may explain much of the intensity and complexity of begging signals.
One of the most conspicuous elements of the begging display is the loud calling that accompanies gaping and posturing. Calls are typically given at high rates and at sound intensities that can reach 70–80 dB (Dearborn 1999; Leonard & Horn 2001a), an intensity equivalent to that of chorusing frogs (Wollerman 1999). The calls encode information on offspring need (Horn & Leonard 2002) and may account for a potential cost of the display, attracting predators to the nest (Haskell 2002). Despite the assumption that begging is more intense than necessary for transmitting information the short distance between parents and young, the transmission and reception of this conspicuous aspect of the display has not been examined.
A main impediment to the reception of vocal signals in general is interference from ambient noise. Such background noise comes from biotic sources, such as conspecifics, and abiotic sources, such as wind and rain (Klump 1996; Lengagne et al. 1999; Wollerman 1999). Some species that signal over long distances overcome this interference by increasing the output of their calls, through increases in amplitude, duration or rate (Lengagne et al. 1999; Brumm & Todt 2002; Pytte et al. 2003; Brumm 2004). If nestling birds showed similar responses to ambient noise, then some of the observed intensity of the begging calls might be explained by selection for signal efficacy.
The goal of our study was to determine if nestlings adjust their begging calls to ambient noise levels in ways that improve reception, and thus to help explain the intensity of this widespread signal. Specifically, we conducted three studies, using tree swallow (Tachycineta bicolor) nestlings, to determine: (i) if the structure and delivery of begging calls varies across broods in relation to ambient noise levels around the nest, (ii) if nestlings adjust their calls in response to the playback of noise in the laboratory and (iii) if the discrimination of calls by parents is hindered by noise and improved by the adjustments to noise observed in the laboratory study.
2. Methods
We conducted these studies in the 2003 (studies a and b) and 2004 (study c) breeding seasons in the Gaspereau Valley of Nova Scotia, Canada, on a population of box-nesting tree swallows (see Leonard & Horn 1996 for details).
(a) Begging calls and noise at the nest
To examine the relationship between nestling begging calls and ambient noise levels around the nest, we suspended a Genexxa 33-3003 microphone 10 cm over the nest cup at each of 29 nests of three to seven nestlings when the young were 9 or 10 days old. The microphone was attached to a Sony D6 tape recorder, set to a recording level that we had calibrated using a Radio Shack 33-2005 sound level meter. We removed all but one randomly selected nestling, and placed them in a heated container for 30 min, during which time parents fed the nestling in the nestbox, but nestlings in the container were not fed. After 30 min, the nestlings were returned to the box and their begging calls recorded for 45 min.
Ambient noise spectra were measured by digitizing one 20 s segment of tape every 2 min during the 45 min recording period (n=20 segments per nest). Segments were omitted from the analysis if a nestling or parent called within the 20 s. Sounds were digitized at 44 kHz and 16 bits using Canary 1.2 software (Charif et al. 1995) and their source was identified. For each segment, we calculated the amplitude (i.e. sound pressure level in dB; ref. 0.02 μPa) of the noise between 2 and 10 kHz (the frequency range of nestling calls; unpublished data) from the spectrum produced by Canary (Hamming window 699 Hz bandwidth and 50% overlap; for details see Charif et al. 1995).
Begging calls were digitized as reported above. From spectrographs (analysis bandwidth 699 Hz, display resolution 22 Hz×3 ms), we measured: call length (ms), call amplitude (minus background amplitude, in dB), peak frequency (the frequency with the highest amplitude, in kHz) and frequency range (highest minus lowest frequency, in kHz) for individual calls. These call features were selected for analysis because they contained information on nestling hunger or thermal state (Horn & Leonard 2002) or because they varied with ambient noise levels in other species (see §4).
Ambient noise levels were related to nestling call features using linear regressions between the mean amplitude at the nest, averaged across all 20 s periods, and the mean of each call feature, averaged across all recorded calls. Thus, each nest contributed one datum to each analysis. We also conducted regressions between call features and mean brood weight, brood size and hatch date, features that could confound the relationship between calls and noise. For all regressions, the distributions of residuals were approximately normal (Shapiro–Wilk W-tests p>0.20), so we did not transform the variables.
(b) Begging calls and the playback of noise
To determine whether nestlings adjust their calls in response to playback of white noise, on the day after the field study the two nestlings closest in weight from each of 10 broods were removed, and each one was placed alone in an artificial nest inside one of two identical nestboxes located in separate rooms in the laboratory. A 2 W speaker amplifier (Koss hdm 111BK, response±3 dB from 100 to 15 kHz), for playback of parental calls that stimulate begging, was placed on a platform at the opening of each nestbox and another, for playback of white noise and control sounds, at an open side of the nestbox on the same level as the nestling. Both parental calls and noise were played from Sony D-E351 compact disc (CD) players.
Before the trial began, nestlings were fed moistened Hartz egg biscuits for birds until they no longer begged to parental calls. They were then assigned to one of two treatments: Noise, computer-synthesized white noise that included the frequency range of nestling calls (16 bits, 0–22 kHz) played at an overall amplitude (C weighting; i.e. evenly weighting all frequencies) of 65 dB, near the high end of the amplitude range measured in the field; or Quiet, a blank CD, which, combined with ambient noise in the laboratory, yielded an amplitude of 50 dB. Treatments were balanced across rooms, began as soon as nestlings were placed in the nestbox and ran continuously throughout the trial. Twenty minutes after the feeding and every 10 min thereafter for 1 h (i.e. a total of seven test periods) without food, we stimulated nestlings to beg by playing a set of six pairs of parental contact calls. Their responses were videotaped with a Panasonic PV-900-K VHS video camera placed at the open side of each box, and calls were recorded as above, using a Sony DM-100 digital audio tape recorder.
Call measurements were identical to those measured in the field, with the addition of call rate (i.e. the total number of calls given to each set of parental contact calls, converted to calls per minute), which could be measured over a standardized period of time in the laboratory. We also videotaped postural begging intensity in the laboratory to determine if nestlings increased the intensity of the visual elements of the display when the transmission of the vocal elements was hindered in the Noise treatment. We scored maximum postural begging intensity each time a nestling begged using a scale from 0 to 5 that represented increasing intensity (see Leonard et al. 2003 for details). Scores were averaged across each set of contact calls to produce a mean postural intensity for each of the seven test periods.
The effect of noise on calls was tested by performing a mixed model ANOVA for each call variable, with the noise treatment as a fixed effect, brood as a random effect, and the call measurement, averaged across all test periods, as the dependent variable. In initial analyses, we included test period as a covariate in the model, but, because no significant treatment by test period interaction was found, responses were averaged across test periods. The ANOVA results are based on the restricted maximum likelihood routine in JMP 5.1 (SAS Institute 1989–2003). All residuals were checked for normality as above, and no transformations were needed.
(c) Parental discrimination and noise
To determine how noise, and adjustments of calls to noise, by nestlings affect parental discrimination of begging calls, the nestbox at 25 nests was replaced with an experimental box when young were 6 or 7 days old. Experimental nestboxes had a 10×10 cm2 opening in the wall opposite the nest opening and two pairs of Sony 8n8 series earbud speakers midway along the length of the box, with a speaker from each pair on the left and right sides. The speakers were level with the top of the nesting material and oriented toward the nest opening.
The next day, each pair of speakers was attached to a 1 m cable that was connected to a Sony D-E351 CD player. A Canon ES970 video camera was also placed on a tripod pointed through the opening at the back of the box, covered with a plastic bag. The resident nestlings were then removed and placed in a heated container. The two nestlings closest in weight were fed as described above. These non-begging nestlings, which served as targets for parental feeding attempts, were then placed on each side of the box next to the speakers, with their heads oriented toward the nestbox opening.
Every time a parent landed on the nestbox, 60 dB of white noise (synthesized and measured as in the previous experiment) was played continuously from the speakers closest to the nest opening and nestling begging calls were played simultaneously and continuously from the other pair of speakers. The begging calls were played at a high call rate (160 calls min−1) from one side of the box and at a low rate (80 calls min−1) from the other side. Parent tree swallows discriminate between calls given at these rates and show a preference for the higher call rate (Leonard & Horn 2001b). The begging calls of twenty-five 7-day-old tree swallow nestlings were used to create the stimulus tapes. The calls were digitized at 44 kHz and 16 bits using Canary 1.2 software and were made into continuous loops of 6 s, with the inter-call intervals decreased or increased to produce the high and low call rates described above.
The calls were presented to parents in trials that consisted of two amplitude treatments: Soft, high and low rate calls delivered at 55 dB; or Loud, high and low rate calls delivered at 65 dB. The amplitude of the white noise and the calls was chosen to create conditions in which the calls would be masked more (i.e. Soft treatment) or less (i.e. Loud treatment) by the white noise. The signal-to-noise ratio in the Loud treatment was also chosen to mimic that of the results of the Noise treatment in the previous experiment. The first treatment continued until a parent made a feeding visit to the nest or 30 min had passed, at which point all nestlings were returned to the box for an hour before the second treatment was presented. Across trials, which speaker played each call rate was alternated and, across every two trials, which treatment was played first.
An observer blind to the treatments watched the videotapes and noted the nestling to which a parent directed its first feeding attempt, the most reliable indicator of parental preference (Leonard & Horn 2001b). We did not distinguish between male and female parents because our previous experiment showed that parents did not vary in their response to the begging calls (Leonard & Horn 2001b).
In 20 of the 25 trials, one of the two playback treatments failed either because parents did not visit the nest, target nestlings moved away from the speakers or equipment malfunctioned. We therefore could not use the planned paired design to analyse the data. Instead, we used the successful treatment from the above 20 trials, and for the remaining five paired trials we separated the treatments and treated each as an independent point, yielding n=15 for each treatment. Omitting or retaining the five paired trials did not change the results, so we opted to include these trials in our analyses.
Log-likelihood tests were used to determine whether the proportion of first feeds directed to the high call rate nestling/speaker was greater in the Loud treatment than in the Soft treatment. Score confidence intervals were also calculated (SAS Institute 1989–2003) for each treatment to examine whether the proportion of first feeds directed toward higher call rates differed significantly from 0.50.
3. Results
(a) Begging calls and noise at the nest
The average ambient noise level at the study sites in the frequency range overlapping nestling calls was 49±1.4 dB and ranged from 41 to 67 dB across nests. The main sources of this noise were birds, wind, vehicles and a river, all of which contributed energy in this frequency range.
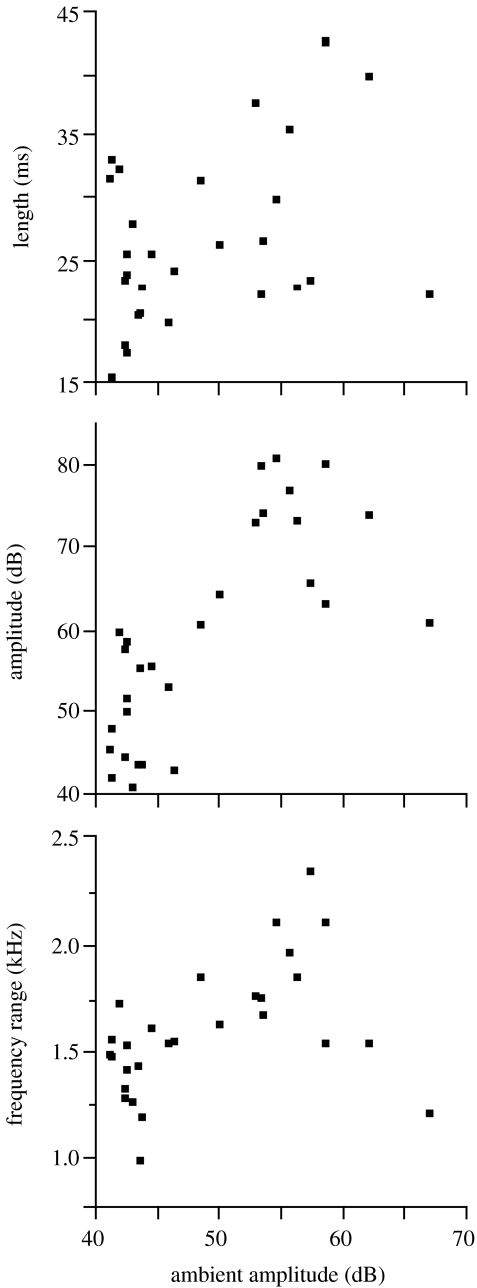
Call length, amplitude and frequency range all increased significantly with increases in ambient noise level at the nest (length: F1,27=6.03, p=0.021; amplitude: F1,27=29.86, p<0.0001; frequency range: F1,27=6.63, p=0.016; figure 1). The other call features did not vary significantly with noise level (all p>0.50) nor did call features vary significantly with brood weight, brood size or hatch date (all p>0.12).
Figure 1.
Length (ms), amplitude (dB) and frequency range (kHz) of tree swallow begging calls in relation to the amplitude of ambient noise (dB) at the nest.
(b) Begging calls and the playback of noise
Nestlings in the Noise treatment called at significantly higher amplitudes than nestlings in the Quiet treatment (table 1). Other call features and postural begging intensity did not differ significantly between the two treatments (table 1).
Table 1.
Mean (±s.e.) call features and postural begging intensity for tree swallows in Noise and Quiet playback treatments. (Noise treatment was 65 dB white noise; Quiet was 50 dB ambient laboratory noise. F, d.f. and p values for treatment effect from mixed model ANOVA.)
| variable | noise | quiet | F | d.f. | p |
|---|---|---|---|---|---|
| length (ms) | 33.0±3.61 | 32.5±9.94 | 0.62 | 1, 5 | 0.62 |
| amplitude (dB) | 67.1±1.36 | 53.2±1.89 | 26.64 | 1, 5 | 0.0002 |
| peak frequency (kHz) | 5.63±0.66 | 5.24±0.59 | 1.39 | 1, 5 | 0.26 |
| frequency range (kHz) | 1.67±0.15 | 1.81±0.19 | 0.42 | 1, 5 | 0.55 |
| rate (calls min−1) | 112.0±9.80 | 99.0±22.60 | 0.41 | 1, 5 | 0.55 |
| postural intensity | 2.06±0.05 | 2.20±0.35 | 0.24 | 1, 6 | 0.64 |
(c) Parental discrimination and noise
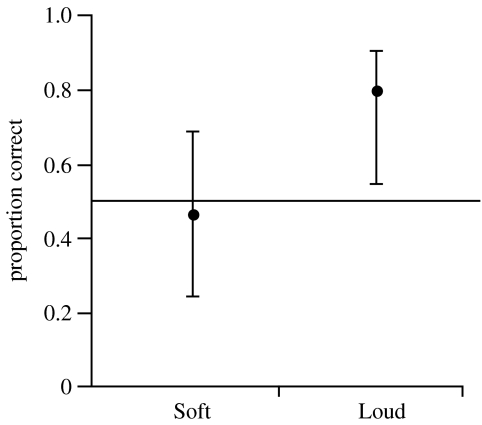
The proportion of first feeds directed toward the higher call rate was greater in the Loud treatment than in the Soft treatment (, p=0.027). Parents directed significantly more feedings toward higher call rates in the Loud treatment than would be expected by chance, but choices did not differ from chance in the Soft treatment (figure 2).
Figure 2.
Proportion of first feeding attempts directed to the playback of higher call rates in Soft and Loud treatments (n=15 in each). Error bars show 95% confidence intervals, horizontal line shows proportion=0.50.
4. Discussion
Our results are the first to show that ambient noise affects the production and reception of begging signals. We found that begging call length, amplitude and frequency range increased with an increase in ambient noise level around the nest and that call amplitude increased when nestlings were presented with playback of noise in the laboratory. A similar increase in call amplitude also enhanced the likelihood that, in the presence of noise, parents would discriminate between calls given at different rates. Below, each of these results is discussed in turn.
The detection and discrimination of acoustic signals can be greatly impaired by the masking effects of background noise (Klump 1996), particularly if the noise energy is in the spectral region of the signal (Lohr et al. 2003). Not surprisingly, then, acoustic signallers show a variety of short-term adaptations to improve signal transmission in noise. The adjustment that is most widespread phylogenetically is to increase the amplitude of the signal relative to the noise. This response to increased ambient noise has now been reported for four mammal species, including three primates (Wiggins et al. 2001; Brumm et al. 2004), four bird species (Pytte et al. 2003; Brumm 2004) and a frog (Lopez et al. 1988). The positive relationship found here between ambient noise levels and call amplitude is consistent with these results.
Of the three call features that correlated with ambient noise in the field, only amplitude changed in response to playback of noise in the laboratory. Two reasons for this discrepancy seem particularly likely. First, because the field study was correlational, call length and frequency range may be correlated to an intervening variable, rather than to ambient noise levels per se. Although potential confounding variables were accounted for, such as hunger and brood size, some other variable might have been missed that could explain the results. Second, nestlings may respond differently to the temporally and spectrally variable noise they experience in the field, than to the uniform white noise that was played back in the laboratory. Changes in the length and frequency range of calls may be more effective in the former case than in the latter. While amplitude increases are the most widespread responses to increased noise, temporal and spectral adjustments do occur in some species (Miller et al. 2000; Slabbekoorn & Peet 2003; Brumm et al. 2004). It is also worth noting that the magnitude of the amplitude effect might have been greater in the field than in the laboratory; if so, this suggests an interaction between sibling competition and ambient noise in the former situation. Clearly, the differences in the results of the two studies will require more experimentation to resolve.
The increase in call amplitude in response to ambient noise shown in both the field and laboratory results raises the question of why nestlings do not call at these higher amplitudes all the time. The most obvious explanation is that calling at high amplitudes increases the energetic and predation costs of begging. The evidence for an energetic cost of begging is weak, however (Chappell & Bachman 2002), particularly for tree swallows (Leonard et al. 2003). More probably, calling at high amplitudes might increase the risk of nest predation (Leech & Leonard 1997; but see Haskell 2002), especially if the noise does not interfere with the reception of begging calls by predators. Certainly, the fact that calls are not consistently given at the highest amplitude suggests some sort of cost to sustained, loud calling.
These studies were conducted over relatively short periods of time (i.e. field: 45 min; laboratory: 60 min), so it is not clear whether nestlings would show the same responses if exposed to high ambient noise levels over longer periods of time. If increasing call amplitude is costly, then nestlings exposed to chronic noise may adjust their calls in ways that reduce those costs, for instance by shifting the frequency region of peak call energy away from that of the noise (Lohr et al. 2003). Alternatively, given that begging has elements in several sensory modalities, nestlings might put more emphasis on the visual component of the display, although this response was not detected in the current study. Finally, nestlings living in noisier environments, such as ever-expanding urban areas, might have no choice but to adjust the amplitude of their calls over the longer term. As a consequence, however, they may show decreased fitness compared with nestlings in quieter environments. We are currently testing whether longer term exposure to elevated ambient noise might have any such fitness consequences.
In a previous paired-choice test it was shown that parent tree swallows preferentially feed nestlings calling at higher rates (Leonard & Horn 2001b), presumably because higher rates signal a greater need for food (Horn & Leonard 2002). The results of the current experiment showed that this preference was lost when the amplitude of the begging calls was low relative to the ambient noise levels, but preserved when the signal-to-noise ratio was increased by a magnitude shown by nestlings in the laboratory experiment. Further work is needed to test whether the amplitude increase made it easier for parents to perceive the difference between the call rates, or whether they were more motivated to respond in the Loud treatment. Either way, these results support the interpretation that increases in call amplitude in response to noise serve to enhance discrimination by parents.
Taken together, the present studies suggest that at least part of the intensity of the begging display, specifically the loudness of begging calls, functions in overcoming interference from ambient noise. In turn, this suggests that selection for signal efficacy may help to explain some of the apparent extravagance of this display (Dawkins & Guilford 1997).
Acknowledgments
The authors thank Alia Mukhida, Dave Crowell, Anna Dorey and Danielle Murray for help in the field and the Coldwell, Hynes and Minor families for allowing the use of their land. This study was supported by an NSERC Discovery Grant awarded to M.L.L.
References
- Brumm H. The impact of environmental noise on song amplitude in a territorial bird. J. Anim. Ecol. 2004;73:434–440. [Google Scholar]
- Brumm H, Todt D. Noise-dependent song amplitude regulation in a territorial songbird. Anim. Behav. 2002;63:891–897. [Google Scholar]
- Brumm H, Voss K, Köllmer I, Todt D. Acoustic communication in noise: regulation of call characteristics in a New World monkey. J. Exp. Biol. 2004;207:443–448. doi: 10.1242/jeb.00768. [DOI] [PubMed] [Google Scholar]
- Chappell M.A, Bachman G.C. Energetic costs of begging behaviour. In: Wright J, Leonard M.L, editors. The evolution of nestling begging: competition, cooperation and communication. Kluwer Academic Press; Dordrecht: 2002. pp. 143–162. [Google Scholar]
- Charif R.A, Mitchell S, Clark C.W. Cornell Laboratory of Ornithology; Ithaca, NY: 1995. Canary 1.2 user's manual. [Google Scholar]
- Dawkins M.S, Guilford T. Conspicuousness and diversity in animal signals. Perspect. Ethol. 1997;12:55–72. [Google Scholar]
- Dearborn D.C. Brown-headed cowbird nestling vocalizations and the risk of nest predation. Auk. 1999;116:448–457. [Google Scholar]
- Grafen A. Biological signals as handicaps. J. Theor. Biol. 1990;144:517–546. doi: 10.1016/s0022-5193(05)80088-8. [DOI] [PubMed] [Google Scholar]
- Haskell D.G. Begging behaviour and nest predation. In: Wright J, Leonard M.L, editors. The evolution of nestling begging: competition, cooperation and communication. Kluwer Academic Press; Dordrecht: 2002. pp. 163–172. [Google Scholar]
- Heeb P, Schwander T, Faoro S. Nestling detectability affects parental feeding preferences in a cavity-nesting bird. Anim. Behav. 2003;66:637–642. [Google Scholar]
- Horn A.G, Leonard M.L. Efficacy and the design of begging signals. In: Wright J, Leonard M.L, editors. The evolution of nestling begging: competition, cooperation and communication. Kluwer Academic Press; Dordrecht: 2002. pp. 127–141. [Google Scholar]
- Johnstone R.A, Godfray H.C.J. Models of begging as a signal of need. In: Wright J, Leonard M.L, editors. The evolution of nestling begging: competition, cooperation and communication. Kluwer Academic Press; Dordrecht: 2002. pp. 1–20. [Google Scholar]
- Klump G.M. Bird communication in the noisy world. In: Kroodsma D.E, Miller E.H, editors. Ecology and evolution of acoustic communication in birds. Cornell University Press; Ithaca, NY: 1996. pp. 321–338. [Google Scholar]
- Leech S.M, Leonard M.L. Begging and the risk of predation in nestling birds. Behav. Ecol. 1997;8:644–646. [Google Scholar]
- Lengagne T, Aubin T, Lauga J, Jouventin P. How do king penguins (Aptenodytes patagonicus) apply the mathematical theory of information to communicate in windy conditions. Proc. R. Soc. Lond. B. 1999;266:1623–1628. [Google Scholar]
- Leonard M.L, Horn A.G. Provisioning rules in tree swallows. Behav. Ecol. Sociobiol. 1996;38:341–347. [Google Scholar]
- Leonard M.L, Horn A.G. Dynamics of calling by tree swallow (Tachycineta bicolor) nestmates. Behav. Ecol. Sociobiol. 2001a;50:430–435. [Google Scholar]
- Leonard M.L, Horn A.G. Begging calls and parental feeding decisions in tree swallows (Tachycineta bicolor) Behav. Ecol. Sociobiol. 2001b;49:170–175. [Google Scholar]
- Leonard M.L, Horn A.G, Porter J. Does begging affect growth in nestling tree swallows, Tachycineta bicolor? Behav. Ecol. Sociobiol. 2003;54:573–577. [Google Scholar]
- Lohr B, Wright T.F, Dooling R.J. Detection and discrimination of natural calls in masking noise by birds: estimating the active space of a signal. Anim. Behav. 2003;65:763–777. [Google Scholar]
- Lopez P.T, Nairns P.M, Lewis E.R, Moore S.W. Acoustically induced call modification in the white-lipped frog, Leptodactylus albilabris. Anim. Behav. 1988;36:1295–1308. [Google Scholar]
- Miller P.J.O, Biassoni N, Samuels A, Tyack P.L. Whale songs lengthen in response to sonar. Nature. 2000;405:903. doi: 10.1038/35016148. [DOI] [PubMed] [Google Scholar]
- Pytte C.L, Rusch K.M, Ficken M.S. Regulation of vocal amplitude by the blue-throated hummingbird, Lampornis clemenciae. Anim. Behav. 2003;66:703–710. [Google Scholar]
- SAS Institute, Inc. JMP statistics and graphics guide, version 5.1 Cary, North Carolina: SAS Institute.
- Slabbekoorn H, Peet M. Birds sing at a higher pitch in urban noise. Nature. 2003;424:267–268. doi: 10.1038/424267a. [DOI] [PubMed] [Google Scholar]
- Wiggins S.M, Oleson E.M, Hildebrand J.A. Blue whale call intensity varies with ambient noise level. J. Acoust. Soc. Am. 2001;110:2771. [Google Scholar]
- Wollerman L. Acoustic interference limits call detection in a Neotropical frog Hyla ebraccata. Anim. Behav. 1999;57:529–536. doi: 10.1006/anbe.1998.1013. [DOI] [PubMed] [Google Scholar]