Abstract
Among rodents, the lineage from Progonomys hispanicus to Stephanomys documents a case of increasing size and dental specialization during an approximately 9 Myr time-interval. On the contrary, some contemporaneous generalist lineages like Apodemus show a limited morphological evolution. Dental shape can be related to diet and can be used to assess the ecological changes along the lineages. Consequently, size and shape of the first upper molar were measured in order to quantify the patterns of morphological evolution along both lineages and compare them to environmental trends. Climatic changes do not have a direct influence on evolution, but they open new ecological opportunities by changing vegetation and allow the evolution of a specialist like Stephanomys. On the other hand, environmental changes are not dramatic enough to destroy the habitat of a long-term generalist like Apodemus. Hence, our results exemplify a case of an influence of climate on the evolution of specialist species, although a generalist species may persist without change.
Keywords: Murinae, evolution, morphometrics, Fourier, shape, climate
1. Introduction
Competing hypotheses have been proposed to explain long-term evolutionary trends in the fossil record including mammals involving the climate (Stenseth & Maynard Smith 1984) or biotic interactions (Van Valen 1973). Alroy et al. (2000) concluded that there is little evidence of climate-forcing on mammalian evolution. Jernvall & Fortelius (2002) provided a different view, with evidence for a link between the drying climate of Europe during the Neogene and evolution of hypsodonty in mammals. A first prerequisite to address this question is a well-preserved fossil group with well-assessed ecology and phylogeny and a time period with major climatic variations. The well-documented evolution of large mammal faunas during the Neogene has consequently been discussed in the context of climate change (Thenius 1951; Crusafont Pairo 1965; Tobien 1967). More recently, similar interpretations have been made for small mammals (van der Meulen & Daams 1992; Reumer 1995). A second prerequisite is to avoid any circular reasoning using environmental data extracted from the same fauna to interpret change in long-term trends in a lineage. We will investigate the relationship between the major climatic changes of the Late Neogene with the evolution of a group of small mammals, the murine rodents (Murinae, Rodentia). The evolutionary patterns observed will be compared with estimates of the major climatic changes based on paleoenvironmental records independent of the mammals.
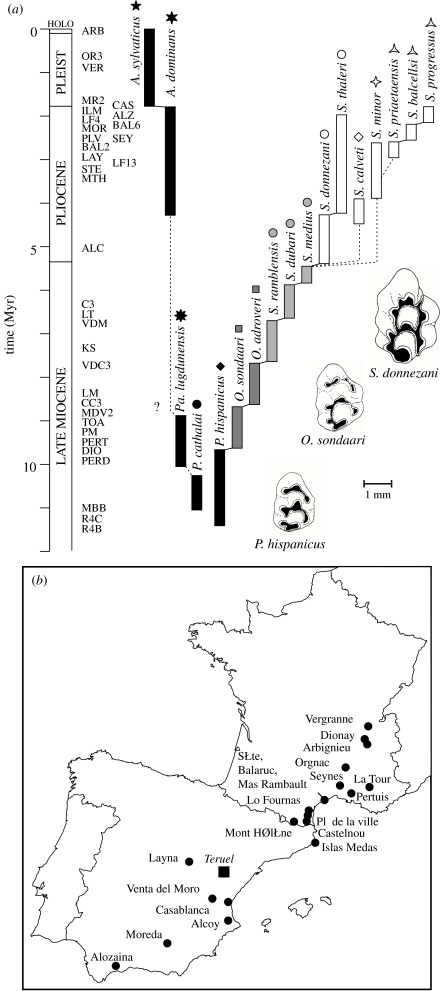
Murine rodents diversified in Europe around 11.5 Myr ago from primitive forms, Progonomys cathalai and Progonomys hispanicus (Renaud et al. 1999). The former evolved into lineages related to Apodemus still living in Europe today (Michaux et al. 1997). P. hispanicus founded a lineage which developed a peculiar dental specialization termed stephanodonty (Schaub 1938). Ancestral murine rodents as well as modern omnivorous–granivorous taxa share a basic dental pattern characterized by three longitudinal rows of cusps linked by crests to form three transversal chevrons (transverse row of cusps) on the upper molars (P. hispanicus in figure 1). Occitanomys (O. sondaari in figure 1) constitutes an intermediate stage of evolution. The derived Stephanomys (S. donnezani in figure 1) display more developed longitudinal crests connecting the transverse chevrons on the upper molars. Accompanying these changes in the dental pattern, crowns become larger and higher because of the swelling of the cusps, leading to an occlusal surface with more pronounced ridges and gutters. This specialization has been interpreted as an adaptation to a more abrasive diet, probably grass, by comparison with extant African murines such as Oenomys and Aethomys (Dieterlen 1967; Michaux 1977; Denys 1994).
Figure 1.
(a) Phylogeny of the lineages P. hispanicus–Stephanomys and P. cathalai–Apodemus sylvaticus. Codes for the deposits in table 1. Full line: agreed ancestor–descendent relationship; dashed line: proposed relationship. Schematic occlusal patterns of upper first molars (inner/lingual side to the right) shown for different stages of the Stephanomys lineage. (b) Map of the fossil deposits.
This lineage was especially abundant in southwestern European faunas and has been described as an example of phyletic gradualism on the basis of progressive size increase and change in the dental pattern (Gmelig Meyling & Michaux 1973; van de Weerd 1976; Cordy 1978; Bachelet & Castillo Ruiz 1990; van Dam 1996, 1997). The material derives from France and Spain and documents the evolution of the lineage from the ancestral P. hispanicus (∼11.5 Myr ago) to the latest Stephanomys before its extinction close to the Plio-Pleistocene boundary (table 1). To compare the evolution of dental specialization in Stephanomys with a lineage where the primitive pattern was conserved, material documenting the evolution from P. cathalai to the modern Apodemus has also been analysed. A synthetic phylogeny of the lineages (figure 1) can be proposed based on the literature (Cordy 1978; Bachelet & Castillo Ruiz 1990; Aguilar et al. 1993; Aguilar & Michaux 1996; Martín-Suárez & Mein 1998).
Table 1.
Deposits with corresponding abbreviation (code), ages in Myr (AgeC) based on the correlation proposed by Aguilar et al. (2004) and interpolated ages (AgeI) based on this calibration and the succession order, number of first upper molars measured (M1).
(In brackets, country: FR, France; SP, Spain. A star indicates a deposit from the Teruel Basin. Below line, modern rodents with country of trapping. Period: MIO, Miocene; PLIO, Pliocene; PLEI, Pleistocene; HOL, Holocene; MOD, modern.)
| deposit | period | code | ageC | ageI | genus | species | M1 |
|---|---|---|---|---|---|---|---|
| La Roma 4B (SP*) | MIO | R4B | 11.4 | Progonomys | hispanicus | 4 | |
| La Roma 4C (SP*) | MIO | R4C | 11.3 | hispanicus | 8 | ||
| Masia del Barbo 2B (SP*) | MIO | MBB | 11 | 11 | hispanicus | 20 | |
| cathalai | 6 | ||||||
| Peralejos D (SP*) | MIO | PERD | 9.7 | hispanicus | 20 | ||
| Dionay (FR) | MIO | DIO | 9.6 | hispanicus | 15 | ||
| Parapodemus | lugdunensis | 24 | |||||
| Pertuis (FR) | MIO | PERT | 9.5 | 9.5 | Occitanomys | sondaari | 5 |
| Puente Minero (SP*) | MIO | PM | 9.2 | sondaari | 20 | ||
| Tortajada A (SP*) | MIO | TOA | 9.1 | sondaari | 20 | ||
| Masada del Valle 2 (SP*) | MIO | MDV2 | 9 | 9 | adroveri | 20 | |
| Concud 3 (SP*) | MIO | CC3 | 8.7 | adroveri | 20 | ||
| Los Mansuetos (SP*) | MIO | LM | 8.5 | 8.5 | adroveri | 19 | |
| Valdecebro 3 (SP*) | PLIO | VDC3 | 8/7.5 | 7.7 | Stephanomys | ramblensis | 21 |
| Las Casiones (SP*) | PLIO | KS | 7.5/7 | 7.3 | ramblensis | 20 | |
| Venta del Moro (SP) | PLIO | VDM | 7/6.5 | 6.7 | ramblensis | 14 | |
| La Tour (FR) | PLIO | LT | 6.5/6 | 6.5 | dubari | 5 | |
| Castelnou 3 (FR) | PLIO | C3 | 6.5/6 | 6.4 | dubari | 15 | |
| Alcoy (SP) | PLIO | ALC | 5 | medius | 2 | ||
| Mont Hélène (FR) | PLIO | MTH | 3.3 | 3.3 | donnezani | 30 | |
| Apodemus | dominans | 40 | |||||
| Sète (FR) | PLIO | STE | 3.0 | 3.1 | Stephanomys | donnezani | 30 |
| Apodemus | dominans | 39 | |||||
| Lo Fournas 13 (FR) | PLIO | LF13 | 3.0 | 3 | Stephanomys | donnezani | 30 |
| Layna (SP) | PLIO | LAY | 2.9 | donnezani | 30 | ||
| Balaruc 2 (FR) | PLIO | BAL2 | 2.7 | 2.7 | calveti | 30 | |
| Apodemus | dominans | 43 | |||||
| Plà de la Ville (FR) | PLIO | PLV | 2.5/2.4 | 2.5 | Stephanomys | calveti | 30 |
| Apodemus | dominans | 10 | |||||
| Seynes (FR) | PLIO | SEY | 2.5/2.4 | 2.5 | Stephanomys | thaleri | 30 |
| Apodemus | dominans | 20 | |||||
| Moreda 1B (SP) | PLIO | MOR | 2.5/2.3 | 2.4 | Stephanomys | minor | 30 |
| 2.4 | thaleri | 3 | |||||
| Balaruc 6 (FR) | PLIO | BAL6 | 2.3 | 2.3 | thaleri | 30 | |
| Lo Fournas 4 (FR) | PLIO | LF4 | 2 | thaleri | 30 | ||
| Alozaina (SP) | PLIO | ALZ | 1.9 | minor | 14 | ||
| 1.9 | prietaensis | 30 | |||||
| Islas Medas (SP) | PLIO | ILM | 1.85 | balcellsi | 30 | ||
| Casablanca 1 (SP) | PLIO | CAS | 1.8 | 1.8 | progressus | 30 | |
| Mas Rambault 2 (FR) | PLIO | MR2 | 1.7 | thaleri | 2 | ||
| Vergranne (FR) | PLEI | VER | 0.4–0.5 | 0.45 | Apodemus | sylvaticus | 13 |
| Orgnac 3 (FR) | PLEI | OR3 | 0.35 | 0.35 | Apodemus | sylvaticus | 8 |
| Arbignieu (FR) | HOL | ARB | 0.01 | Apodemus | sylvaticus | 15 | |
| RCA | MOD | AETHH | Aethomys | hindei | 5 | ||
| Germany | MOD | AS-GER | Apodemus | sylvaticus | 54 | ||
| Ivory Coast, Senegal | MOD | ARVIC | Arvicanthis | sp. | 2 | ||
| Ivory Coast | MOD | OENO-CIV | Oenomys | ornatus | 5 | ||
| Ghana | MOD | OENO-GHA | 4 | ||||
| Guinea | MOD | OENO-GUI | 23 | ||||
| Equatorial Guinea | MOD | PRAOT | Praomys | tullbergi | 6 | ||
| Angola, Somalia | MOD | THAL | Thallomys | sp. | 2 |
2. Patterns of morphological differentiation
(a) Material
The present study includes data from 875 first upper molars (M1) attributed to three genera and 13 species along the lineage of Stephanomys and three genera and four species in the lineage related to Apodemus (table 1). Several alluvial, lacustrian and karstic deposits in Spain and France delivered the material measured in this study (figure 1). Order of succession of the deposits has been established using biochronology and magnetostratigraphy (van Dam 1997; Aguilar et al. 2004). A calibration to an absolute geological time-scale is still debated (Aguilar et al. 2004), but provides indicative numerical ages.
Teeth from a set of modern rodents (Museum d'Histoire Naturelle, Paris) of known diet were measured for comparison: the African rodents Praomys (omnivorous), Aethomys, Arvicanthis and Thallomys (herbivorous), Oenomys (herbivorous, with specialized stephanodont teeth). Molars of the modern Apodemus sylvaticus (omnivorous–granivorous) were measured on a sample of animals from Germany (Institut für Haustierkunde, Kiel).
(b) Outline analysis
The outline of the tooth registers the differences in the relative position and importance of the main cusps and hence, is appropriate to describe the overall morphology of the first upper molars in murine rodents. Outline analysis has been used successfully to analyse parts of the investigated lineage (Renaud et al. 1996, 1999; Renaud & van Dam 2002).
The outline corresponds to the two-dimensional projection of the tooth viewed from the occlusal surface (top view), with focus at the base of the crown. The starting point was defined as the maximum of curvature at the top of the molar. A radial Fourier transform (RFT; Renaud & Michaux 2003) was performed on the x, y coordinates of 64 equidistant points along the outline. The distance from each point to the centre of gravity of the outline (i.e. radius) was calculated. The variation of the radius along the outline can be approximated by a finite sum of trigonometric functions of decreasing wavelength (the harmonics). The trigonometric function corresponding to each harmonic is characterized by two Fourier coefficients An and Bn. The zeroth harmonic A0 is proportional to the size of the specimen. All other Fourier coefficients are standardized by the zeroth harmonic, in order to eliminate isometric size effects and to concentrate on shape information only. A study on related rodents (Renaud et al. 1999) showed that using the first nine harmonics for the first upper molar offers a good compromise between measurement error, information content, and number of variables to be considered.
(c) Statistical analysis
For each first upper molar, a set of 18 variables (two coefficients per nine harmonics) was obtained. Multivariate analyses of variance (MANOVA) were performed on this dataset, in order to evaluate the importance of the among-group differentiation relatively to within-group variation. The grouping factor was the species per locality. A test for significance of among-group differences (Wilks' Lambda test) is provided. Associated with the MANOVA, canonical axes are estimated, which can be considered as synthetic shape axes (Manly 1994).
(d) Morphological differentiation of the first upper molars
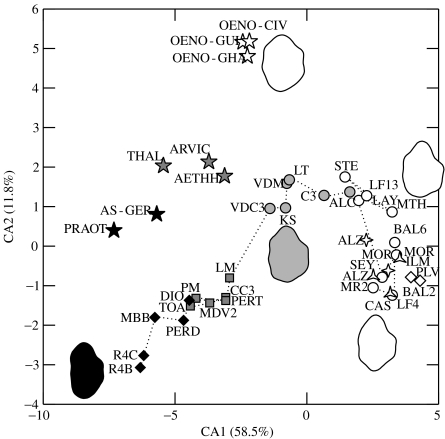
A first analysis included modern and fossil taxa. The grouping factor in the MANOVA was the species per locality for fossil deposits and the species (and country for Oenomys) for modern animals. Morphological differentiation of the first upper molars is significant among the different samples (p<0.001). The first two canonical axes explain most of the variation (70% of the among-group variance) and are the only axes explaining more than 10% of variance. The first axis (CA1), by far the most important (58.5%), displays a trend corresponding to the temporal ordination of the Stephanomys fossil lineage (figure 2). Primitive samples plot toward negative values and are characterized by a slender and asymmetrical outline. Modern taxa also segregate along this axis according to their diet. Omnivorous–granivorous rodents, exemplified by Praomys and Apodemus, fall within the range of the primitive Progonomys along CA1. The more herbivorous Aethomys, Arvicanthis and Thallomys are shifted toward more positive values corresponding to the range of Occitanomys. Oenomys, characterized by a herbivorous diet and a stephanodont dental pattern, is further shifted toward positive values and ranges with the oldest Stephanomys along CA1. This trend corresponds to broader and more symmetrical molars. The broadening of the outline is owing to a swelling of the cusps, increasing the surface of contact between upper and lower cheek teeth and hence increasing the grinding efficiency. The second axis (CA2, 11.8% of variance) displays an offset between the fossil and the modern taxa. It further expresses a late diversification within the Pliocene Stephanomys. It seems to correspond to a more pointed posterior part of the tooth, expressing higher crown with tilted cusps that appear as prominent on the outline.
Figure 2.
Morphological differentiation of the upper molar along the Stephanomys lineage and comparison with modern taxa of known diet. Dots are group means of the different species/deposits on the first canonical plane. The average morphology of some samples is visualized by reconstructed outlines. Symbols for fossil deposits as on figure 1. Modern taxa are shown by stars, black represents omnivorous, grey a trend toward herbivory and open stars herbivorous with an achieved stephanodont dental pattern.
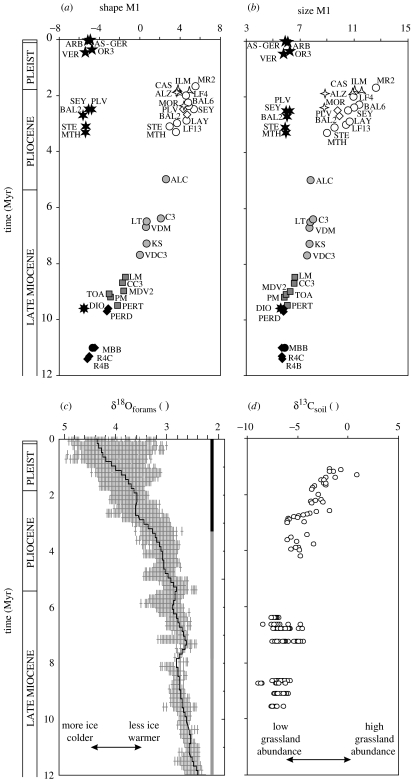
Shape evolution (figure 3a) has been evaluated by a second MANOVA focused on the lineages leading to Stephanomys on the one hand, and to Apodemus on the other hand. Shape differentiation is still highly significant (p<0.0001). Evolution along the Stephanomys lineage is characterized by brief periods of accelerated change, although the general picture is of a gradual change towards broad and symmetrical upper molars with swollen cusps. A first trend can be recognized from the oldest P. hispanicus (∼11.5 Myr) to Occitanomys adroveri (∼8.5 Myr). An important morphological step marks the appearing of Stephanomys (∼8 Myr). By 5 Myr, Stephanomys has reached extremely broad molars associated with achieved stephanodonty. The subsequent shape evolution associated with the Late Pliocene diversification of Stephanomys involves characters independent of those expressed along CA1 (cf. figure 2). On the other hand, the lineage related to Apodemus, leading from P. cathalai or a P. cathalai-like ancestor to the modern Apodemus, remained stable regarding to molar shape over a time span of 11 Myr.
Figure 3.
Size and shape evolution compared with the environmental record for the last 12 Myr. (a) Shape of the first upper molar, estimated by mean scores on the first canonical axis (69.9% of the among-group variance) of a MANOVA on the Fourier coefficients, based on an outline analysis of molars of the lineages leading to Stephanomys on the one hand and to Apodemus on the other hand. (b) Size of the first upper molar, estimated by A0. (c) Palaeotemperature proxy: global deep-sea oxygen isotope record (after Zachos et al. 2001). The grey crosses represent raw data and the full line the general trend (running average). The ice coverage is represented by the vertical bars, in black the Arctic, and in grey the Antarctic. (d) Proxy for abundance of C4 grasslands: carbon isotope record of palaeosols (after Fox & Koch 2003).
Molar size increases along the Stephanomys lineage (figure 3b). This increase occurs stepwise, with a first, slight increase in size from P. hispanicus to Occitanomys. A more important size increase is related to the apparition of the first Stephanomys. Occitanomys is decreasing in size at this time owing to interspecific competition (Renaud et al. 1999). This genus persists thereafter until its extinction during the Late Pliocene (Agustí & Julia 1990; Michaux et al. 1997). Stephanomys survives Occitanomys until the Plio-Pleistocene boundary and during the Late Miocene, experiences a diversification leading to several species of different size, for example, the small S. minor and the intermediate S. calveti. S. donnezani experiences an important intraspecific size increase from the first (MTH, ∼3.3 Myr) to the last representative (LAY, ∼2.9 Myr). Stephanomys thaleri reaches the largest size documented in the Stephanomys lineage shortly before its extinction (MR2, ∼1.7 Myr). Once again, the lineage related to Apodemus did not experience notable changes in size over its evolution.
3. Temporal trends in rodent evolution
The radiation of the murine rodents occurred in a relatively stable, humid and warm environment. During the Late Miocene, the climate changed stepwise (figure 3c,d) towards drier and more seasonal conditions (An et al. 2001). The climate during the Pliocene became generally cooler, drier, and more seasonal (deMenocal 2004). By 3.5 Myr ago, an intensification of the monsoon led to a double seasonality (combination of temperature and precipitation) in the Mediterranean region (Suc et al. 1995; An et al. 2001). This aridification continued in steps at 2.8, 1.7 and 1.0 Myr ago (deMenocal 2004). Since 2.5 Myr ago, modern vegetation seems to have been established in mid-latitude regions (Dupont & Leroy 1999). The Pleistocene climate is dominated by pronounced glacial–interglacial cycles, expressed by the enhanced variability of δ18O values of benthic foraminifers (figure 3c; Zachos et al. 2001). The cold phases were more arid than the warm ones (Dupont & Leroy 1995). The climate change had an important impact on the vegetation leading to a general expansion of open environments, namely grasslands, dominated in many regions by the expanding C4 plants (figure 3d; Cerling et al. 1993, 1998; Pagani et al. 1999; Fox & Koch 2003).
Modern species like Apodemus, a member of the Palaearctic fauna, can be found over a large range of latitude, for example, from North Africa to Scandinavia in the western part of its distribution (Wilson and Reeder 1993). It can, therefore, tolerate wide ranges of temperature. Consequently, a direct influence of climate on rodent evolution seems unlikely for such a species, but a climatic influence could be driven by changes in the vegetation. Apodemus is today associated with a forest cover (mainly deciduous forest) producing the seeds composing the major part of its diet (Montgomery & Montgomery 1990). Despite the fact that vegetation evolved significantly over the last 12 Myr, the forest habitat persisted throughout (Fauquette et al. 1999). The mosaic landscape provided habitat for omnivorous species like Apodemus. Hence, selective forces favouring morphological evolution should have been reduced in the Apodemus-related lineage. This is expressed by the relative morphological stability of this lineage (figure 3a,b).
To further test the possible link between environment and morphological evolution, size and shape values of the upper molar were compared with the marine isotopic record. Because of much more limited precision in dating the continental record compared with the marine one, mammalian data were compared with smoothed δ18O variations (running average, full line on figure 3c). Neither upper molar shape (CA1/δ18O values R2=0.000, p=0.993) nor molar size (ln(A0)/δ18O values R2=0.333, p=0.063) were significantly correlated to the palaeotemperature proxy, although the probability for size is close to the threshold value, suggesting an increase in molar size for decreasing temperature. Temperature is known to cause size variations within species of mammals according to Bergmann's rule (Dayan et al. 1991). Minor size variations in Apodemus could be related to this process (Michaux & Pasquier 1974; Michaux 1983).
Very environmentally tolerant species like Apodemus remained stable in molar shape and size over time despite climate changes. Nevertheless, the gradual trend towards drier and colder conditions caused changes in the vegetation cover, which in turn influenced the rodent evolution. The expansion of the grassland opened environmental opportunities. The exploitation of these new resources made specific adaptations necessary because grasses are very abrasive owing to silica in their vegetative parts as well as dust and grit adhering to low-lying plants in open habitats (Janis et al. 2002).
The appearance of the highly specialized Stephanomys coincides with the onset of the general expansion of grasslands. Contrary to the murine species with an ‘Apodemus-like’ dental pattern, Stephanomys is characterized by broad molars with swollen cusps and pronounced ridges sliding in corresponding gutters. These characters contribute to an increase in the surface of contact between upper and lower teeth rows and hence the grinding efficiency. An increase in the molar height ameliorates the resistance to wear throughout the life of the animal. All these characters can be interpreted as adaptation to consumption of grasses and an exploitation of newly developed habitats. The further evolution of Stephanomys can also be related to different steps of environmental changes. Vegetation in the western Mediterranean area was modified ∼3.5 Myr ago because of the establishment of a double seasonality of temperature and precipitation (Suc et al. 1995). Large size should allow a better energetic control (Damuth 1993) and may be favoured in more variable and harsh environments. Hence the large size increase observed in Stephanomys could be interpreted as a response to the increasing seasonal variability and summer drought. An increasing aridity and a vegetation shift to modern conditions (∼2.5 Myr ago) led to the diversification within Stephanomys. The environmental influence on the evolution of Stephanomys is supported by tight correlation between palaeotemperature and upper molar morphology, including shape (CA1/δ18O values R2=0.686, p<0.001) and size (ln(A0)/δ18O values R2=0.759, p<0.001).
Stephanomys did not survive the additional climatic extremes corresponding to the arid glacial and humid interglacial cycles developing at the Plio-Pleistocene boundary. The climatic variability may have been larger than the ecological range of Stephanomys and the geographical boundaries of Stephanomys in southern France and the Iberian peninsula did not allow the species to escape stress by habitat tracking.
Our results show that there is no predictive pattern of climate-forcing on morphological evolution since climate change had a different impact according to the lineage. The climatic effect is probably not direct, but relayed by the vegetation and dependent on the ecological preferences of the animals. This could explain the different patterns observed in the generalist species Apodemus versus the specialist Stephanomys. Despite the important environmental change, the habitat of Apodemus persisted and this taxon displayed a relative morphological stability over time. In contrast, Stephanomys exploited new ecological opportunities provided by the drying climate and expanding grasses in Western Europe since the Late Miocene. The same mechanism has been suggested to drive the evolution of hypsodont mammals during the Neogene in Europe (Jernvall & Fortelius 2002). The increasing specialization would have forced Stephanomys to react repeatedly to the ongoing climatic changes. Despite the opening of new ecological opportunities, the climate change apparently did not increase the number of niches available since the diversity of the murine community decreased from the beginning of the Pliocene (Aguilar et al. 1999). Owing to the broad climatic variability, an adaptation to a narrow range of conditions would have been unfavourable. In contrast, species able to exploit and sustain a wide range of environmental conditions should have been favoured. This includes a long-term generalist like Apodemus and a few specialists adapted to an increasingly common habitat, for example, open habitats and grasslands for Stephanomys.
4. Conclusions
Climate influenced the morphological evolution of Stephanomys but this result can not be generalized for all related murine rodents. These climatic influences on Stephanomys evolution would not have been direct, but rather indirect through a change in vegetation and hence diet of the rodents. The expansion of grasses, owing to increasing aridity, probably created new ecological opportunities. This would have favoured the evolution of a specialist rodent able to feed on abrasive vegetal matter by a dental adaptation, in that case stephanodonty. The expansion of the ecological resources available is therefore related to the evolution of some specialist species but does not automatically imply an increasing diversity of the community, because increasing environmental variability during the Pliocene may have favoured a broader ecological tolerance. Both a long-term generalist like Apodemus and a specialist adapted to abundant resources would have been able to cope with the deteriorating environmental conditions. The same environmental changes can therefore favour opposite patterns of morphological evolution: stability in Apodemus and an adaptive increasing specialization in Stephanomys.
Acknowledgments
We sincerely thank C. Denys for useful discussion, L. Hopper for English corrections, J. Cuisin and A. Bens for easy access to material from the Museum National d'Histoire Naturelle (Paris), as well as D. Kruska for access to the collection of the Institut für Hausetierkunde (Kiel, Germany) and R. Lücht for her assistance during work at the Institut. A portion of the measurements have been made by L. Deschamps during a practical work at the University of Lyon.
S.R. carried out this work at the Service Commun de Morphométrie, ISE-M (Université Montpellier II) and at the Pôle Morphométrie, UMR 5125 (Lyon 1). This study benefited from the support of the GDR 2474 CNRS and the Région Rhône-Alpes (grant Emergence). DNS was founded by the German Science Foundation. Contribution ISE-M (UMR 5554) no. 2004-066.
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
References
- Aguilar J.-P, Michaux J. The beginning of the age of Muridae (Mammalia: Rodentia) in southern France. Acta Zool. Cracov. 1996;39:35–45. [Google Scholar]
- Aguilar J.-P, Michaux J, Delannoy J.J, Guendon J.L. A Late Pliocene rodent from Alozaina (Malaga, Spain) Scr. Geol. 1993;103:1–22. [Google Scholar]
- Aguilar J.-P, Legendre S, Michaux J, Montuire S. Pliocene mammals and climatic reconstruction in the Western Mediterranean area. In: Wrenn J.H, Suc J.-P, Leroy S.A.G, editors. The Pliocene: time of change. American Association of Stratigraphic Palynologists Foundation; Houston, TX: 1999. pp. 109–120. [Google Scholar]
- Aguilar J.-P, Berggren W.A, Aubry M.-P, Kent D.V, Clauzon G, Benammi M, Michaux J. Mid Neogene Mediterranean marine–continental correlations: an alternative interpretation. Palaeogeogr. Palaeoclimat. Palaeoecol. 2004;204:165–186. [Google Scholar]
- Agustí J, Julia R. Paleoclimatic inferences from the Plio-Pleistocene continental sequence of the Guadix-Baza Basin (Spain) In: Suc J.-P, editor. Evolution climatique dans le domaine méditerranéen au Néogène. vol. XVII. Montpellier: Interim-Colloquium du R.C.M.N.S., Colloque associé du C.N.R.S.; Montpellier-Barcelone: 1990. pp. 269–279. [Google Scholar]
- Alroy J, Koch P.B, Zachos J. Global climate change and North American mammalian evolution. In: Erwin D.H, Wing S.L, editors. Deep time. Paleobiology's perspective. Paleobiology, Supplement to Volume 26(4) Allen Press; Lawrence, KS: 2000. pp. 259–288. [Google Scholar]
- An Z, Kutzbach J.E, Prell W.L, Porter S.C. Evolution of Asian monsoons and phased uplift of the Himalaya-Tibetan plateau since Late Miocene times. Nature. 2001;211:62–66. doi: 10.1038/35075035. [DOI] [PubMed] [Google Scholar]
- Bachelet B, Castillo Ruiz C. Radiation évolutive et lignées chez les Stephanomys (Rodentia, Mammalia), muridés dominants du Pliocène d'Europe sud-occidentale. C. R. Acad. Sci., Sér. II. 1990;311:493–499. [Google Scholar]
- Cerling T.E, Wang Y, Quade J. Expansion of C4 ecosystems as an indicator of global ecological change in the Late Miocene. Nature. 1993;361:344–345. [Google Scholar]
- Cerling T.E, Ehleringer J.R, Harris J.M. Carbon dioxide starvation, the development of C4 ecosystems, and mammalian evolution. Phil. Trans. R. Soc. B. 1998;353:159–171. doi: 10.1098/rstb.1998.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordy J.-M. Caractéristiques générales de la microévolution du genre Stephanomys (Rodentia, Muridae) Bull. Soc. Géol. France. 1978;XX:815–819. [Google Scholar]
- Crusafont Pairo M. Observations à un travail de M. Freudenthal et P.Y. Sondaar sur les nouveaux gisements à Hipparion d'Espagne. Kon. Nederl. Akad. Wet. Proc. (B) 1965;68:121–131. [Google Scholar]
- Damuth J. Cope's rule, the island rule and the scaling of mammalian population density. Nature. 1993;365:748–750. doi: 10.1038/365748a0. [DOI] [PubMed] [Google Scholar]
- Dayan T, Simberloff D, Tchernov E, Yom-Tov Y. Calibrating the paleothermometer: climate, communities, and the evolution of size. Paleobiology. 1991;17:189–199. [Google Scholar]
- deMenocal P.B. African climate and faunal evolution during the Pliocene–Pleistocene. Earth Planet. Sci. Lett. 2004;220:3–24. [Google Scholar]
- Denys C. Diet and dental morphology of two coexisting Aethomys species (Rodentia, Mammalia) in Mozambique. Implications for diet reconstruction in related extinct species from South Africa. Acta Theriol. 1994;39:357–364. [Google Scholar]
- Dieterlen F. Ökologische Populationsstudien an Muriden des Kivugebietes (Congo). Teil I. Zool. Jahrb. Abt. Syst. Ökologie Geogr. Tiere. 1967;94:369–426. [Google Scholar]
- Dupont L.M, Leroy S.A.G. Steps towards drier climatic conditions in northwestern Africa during the upper Pliocene. In: Vrba E.S, Denton G.H, Partridge T.C, Burckle L.H, editors. Paleoclimate and evolution with emphasis on human origins. Yale University Press; New Haven CT: 1995. pp. 289–298. [Google Scholar]
- Dupont L.M, Leroy S.A.G. Climatic changes in the Late Pliocene of NW Africa from a pollen record on an astronomically tuned timescale. In: Wrenn J.H, Suc J.-P, Leroy S.A.G, editors. The Pliocene: time of change. American Association of Stratigraphic Palynologists Foundation; Houston, TX: 1999. pp. 145–161. [Google Scholar]
- Fauquette S, Suc J.-P, Guiot J, Diniz F, Feddi N, Zheng Z, Bessais E, Drivaliari A. Climate and biomes in the West Mediterranean area during the Pliocene. Palaeogeogr. Palaeoclimat. Palaeoecol. 1999;152:15–36. [Google Scholar]
- Fox D.L, Koch P.L. Tertiary history of C4 biomass in the Great Plains, USA. Geology. 2003;31:809–812. [Google Scholar]
- Gmelig Meyling C, Michaux J. Le genre Stephanomys Schaub 1938 (Rodentia, Mammalia); son évolution au Pliocène supérieur. C. R. Acad. Sci. Sér. D. 1973;277:1441–1444. [Google Scholar]
- Janis C.M, Damuth J, Theodor J.M. The origins and evolution of the North American grassland biome: the story from the hoofed mammals. Palaeogeogr. Palaeoclimat. Palaeoecol. 2002;177:183–198. [Google Scholar]
- Jernvall J, Fortelius M. Common mammals drive the evolutionary increase of hypsodonty in the Neogene. Nature. 2002;417:538–540. doi: 10.1038/417538a. [DOI] [PubMed] [Google Scholar]
- Manly B.F.J. 2nd edn. Chapman & Hall/CRC; London: 1994. Multivariate statistical methods. A primer. [Google Scholar]
- Martín-Suárez E, Mein P. Revision of the genera Parapodemus, Apodemus, Rhagamys and Rhagapodemus (Rodentia, Mammalia) Geobios. 1998;31:87–97. [Google Scholar]
- Michaux J. Les muridés actuels et fossiles. In: Bons J, editor. Aspects modernes des recherches sur l'évolution. vol. 4. École Pratique des Hautes Études; Montpellier: 1977. pp. 133–143. [Google Scholar]
- Michaux J. Colloque International du CNRS, n°330, Modalite´s et Rythmes de l'Évolution Biologique. Dijon; France: 1983. Aspects de l'évolution des Murinés (Rodentia, Mammalia) en Europe sud-occidentale; pp. 195–199. [Google Scholar]
- Michaux J, Pasquier L. Dynamique des populations de mulots (Rodentia, Apodemus) en Europe durant le Quaternaire. Premières données. Bull. Soc. Géol. France. 1974;XVI:431–439. [Google Scholar]
- Michaux J, Aguilar J.-P, Montuire S, Wolff A, Legendre S. Les Murinae (Rodentia, Mammalia) néogènes du Sud de la France: évolution et paléoenvironnements. Geobios. Mém. Spéc. 1997;20:379–385. [Google Scholar]
- Montgomery S.S.J, Montgomery W.I. Intrapopulation variation in the diet of the wood mouse Apodemus sylvaticus. J. Zool. Lond. 1990;222:641–651. [Google Scholar]
- Pagani M, Freeman K.H, Arthur M.A. Late Miocene atmospheric CO2 concentrations and expansion of C4 grasses. Science. 1999;285:876–879. doi: 10.1126/science.285.5429.876. [DOI] [PubMed] [Google Scholar]
- Renaud S, Michaux J.R. Adaptive latitudinal trends in the mandible shape of Apodemus wood mice. J. Biogeogr. 2003;30:1617–1628. [Google Scholar]
- Renaud S, van Dam J. Influence of biotic and abiotic environment on dental size and shape evolution in a Late Miocene lineage of murine rodents (Teruel Basin, Spain) Palaeogeogr. Palaeoclimat. Palaeoecol. 2002;184:161–173. [Google Scholar]
- Renaud S, Michaux J, Jaeger J.-J, Auffray J.-C. Fourier analysis applied to Stephanomys (Rodentia, Muridae) molars: nonprogressive evolutionary pattern in a gradual lineage. Paleobiology. 1996;22:255–265. [Google Scholar]
- Renaud S, Michaux J, Mein P, Aguilar J.-P, Auffray J.-C. Patterns of size and shape differentiation during the evolutionary radiation of the European Miocene murine rodents. Lethaia. 1999;32:61–71. [Google Scholar]
- Reumer J.W.F. The effect of paleoclimate on the evolution of the Soricidae (Mammalia, Insectivora) In: Vrba E, Denton G, Partridge T, Burckle L, editors. Paleoclimate and evolution with emphasis on human origins. Yale University Press; New Haven, CT and London: 1995. pp. 135–137. [Google Scholar]
- Schaub S. Tertiäre und Quartäre Murinae. Abh. Schweiz. Paleontol. Ges. Basel. 1938;61:1–39. [Google Scholar]
- Stenseth N.C, Maynard Smith J. Coevolution in ecosystems: Red Queen evolution or stasis? Evolution. 1984;38:870–880. doi: 10.1111/j.1558-5646.1984.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Suc J.-P, Bertini A, Combourieu Nebout N, Diniz F, Leroy S, Russo-Ermolli E, Zheng Z, Bessais E, Ferrier J. Structure of West Mediterranean vegetation and climate since 5.3 ma. Acta Zool. Cracov. 1995;38:3–16. [Google Scholar]
- Thenius E. Die jungtertiäre Säugetierfauna des Wiener Beckens in ihrer Beziehung zu Stratigraphie und Ökologie. Erdölzeitung. 1951;5:52–54. [Google Scholar]
- Tobien H. Subdivision of pontian mammalian faunas. Committee Mediterranean Neogene Stratigraphy, Proc. IV Session, Bologna. G. Geol. 2. 1967;XXXV:1–5. [Google Scholar]
- van Dam J. Stephanodonty in fossil murids. In: Marcus L.F, Corti M, Loy A, Naylor G.J.P, Slice D.E, editors. Advances in morphometrics. vol. 84. Plenum Press; New York: 1996. pp. 449–461. [Google Scholar]
- van Dam J.A. The small mammals from the upper Miocene of the Teruel-Alfambra region (Spain): paleobiology and paleoclimatic reconstructions. Geol. Ultraiect. 1997;156:1–204. [Google Scholar]
- van de Weerd A. Rodent faunas of the Mio-Pliocene continental deposits of the Teruel-Alfambra region, Spain. Utrecht Micropaleontol. Bull. Spec. Publ. 1976;2:1–216. [Google Scholar]
- van der Meulen A.J, Daams R. Evolution of Early-Middle Miocene rodent faunas in relation to long-term palaeoenvironmental changes. Palaeogeogr. Palaeoclimat. Palaeoecol. 1992;93:227–253. [Google Scholar]
- Van Valen L. Body size and numbers of plants and animals. Evolution. 1973;27:27–35. doi: 10.1111/j.1558-5646.1973.tb05914.x. [DOI] [PubMed] [Google Scholar]
- Wilson D.E, Reeder D.M. Smithonian Institution Press; Washington: 1993. Mammals species of the world. A taxonomic and geographic reference. [Google Scholar]
- Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]