Abstract
Teleost fishes have three distinct oestrogen receptor (ER) subtypes: ER-α, ER-βa (or ER-γ) and ER-βb. ER-βa and ER-βb arose from a duplication of an ancestral ER-β gene early in the teleost lineage. Here, we describe the distribution of the three ER mRNAs in the hypothalamus and cerebellum of the Atlantic croaker to address two issues: the specific functions of multiple ERs in the neuroendocrine system and the evolution and fate of duplicated genes. ER-α was detected in nuclei of the preoptic area (POA) and hypothalamus previously shown to possess ER-αs in teleosts. AcER-βb, but not ER-βa, labelling was detected in the magnocellular neurons of the POA, nucleus posterior tuberis, the nucleus recessus posterior and cerebellum. By contrast, acER-βa, but not ER-βb, was detected in the dorsal anterior parvocellular POA and suprachiasmatic nucleus. Both ER-βs were found in posterior parvocellular and ventral anterior POA nuclei, the ventral hypothalamus, and periventricular dorsal hypothalamus. The differences we observed in ER subtype mRNA distribution within well-characterized brain nuclei suggest that ER-βa and ER-βb have distinct functions in the neuroendocrine control of reproduction and behaviour, and provide evidence that the teleost ER-β paralogues have partitioned functions of the ancestral ER-β gene they shared with tetrapods.
Keywords: oestrogen receptor, gene duplication, teleost fishes, neuroendocrine regulation, hypothalamus, brain
1. Introduction
Steroid hormone receptors are members of a large family of ligand-activated nuclear transcription factors that are critical to the reproduction, differentiation and development of vertebrates. All steroid receptors, including androgen, progesterone, glucocorticoid and mineralcorticoid receptors, are derived from an ancient oestrogen receptor (ER) that duplicated multiple times during early vertebrate evolution (Thornton 2001).
The primary physiological ligand for ERs is the sex steroid oestradiol-17β (oestradiol, E2). Oestradiol, synthesized in the gonads or locally in the brain by aromatization of testosterone, plays an important role in the neuroendocrine control of reproduction and reproductive behaviour in vertebrates (Knobil & Neill 1994). Oestradiol acts in the pituitary and the brain to influence the secretion of gonadotropin-releasing hormone (GnRH) and other neuropeptides that control reproduction and behaviour (Gore 2002; Shupnik 2002). In addition to nuclear ERs, there is evidence for membrane localization of ERs and novel ERs in brain tissues (Blaustein 2004; Thomas et al. 2004; Toran-Allerand 2004).
Despite intense focus on oestrogen actions, the presence of more than one ER subtype escaped notice for decades. The discovery of a second ER subtype, (ER-β Kuiper et al.1996), in addition to the initially described ER-α, has expanded the potential mechanisms by which oestrogens can elicit their effects. Teleost fishes have two types of ER-β: ER-βa (formerly ER-γ; see Hawkins et al. 2000; Hawkins & Thomas 2004) and ER-βb (Menuet et al. 2002) as well as an ER-α. The two ER-β subtypes arose from the duplication of an ancestral ER-β gene early in the teleost lineage after the split of tetrapods and fishes (Hawkins et al. 2000).
The fate of genes after a duplication event is of fundamental importance in evolutionary biology because it is believed that this process is the predominant mechanism through which novel proteins evolve and new species arise (Ohno 1970; Amores et al. 1998). After a duplication event, genes are subject to mutations that result in three possible outcomes: gene silencing of one member of the pair (Mighell et al. 2000), the acquisition of a novel function by one copy (neofunctionalization; Ohno 1970) or the partitioning of the original functions between the two copies (subfunctionalization; Force et al. 1999). The two ER-β subtypes of teleosts provide an excellent system to investigate the fate of duplicated genes in vertebrate genomes, because ERs are such critical and extensively studied proteins (Postlethwait et al. 2004). Our sequence and phylogenetic analyses showed that after the duplication event, ER-βa underwent rapid change followed by strict conservation of certain amino acids (Hawkins et al. 2000). This suggested the acquisition of novel functions, or neofunctionalization, after the duplication event. In addition, ER-βa has unusual binding affinities for the endogenous steroid oestriol and other oestrogenic compounds, further suggesting novel functions for this teleost-specific subtype (Hawkins & Thomas 2004).
In this study, we describe the distribution of ER-α, ER-βa and ER-βb mRNA in the hypothalamus and cerebellum of the Atlantic croaker. This is an important first step toward characterizing the role of ER subtypes in the neuroendocrine regulation of reproduction in this well-established model species (Khan & Thomas 1999, 2001; Khan et al. 1999). In addition, comparing the distribution of ER-βa and ER-βb in teleosts to that of ER-β in tetrapods could provide insights into the functional evolution of duplicated genes.
In mammals and birds, the distribution of ER-β partially overlaps that of ER-α in the brain, but some nuclei express ER-β exclusively. These include the paraventricular nuclei of the preoptic area (POA), the suprachiasmatic nucleus, the tuberal hypothalamic nuclei and the cerebellum (Shughrue et al. 1997; Bernard et al. 1999; Foidart et al. 1999; Hileman et al. 1999; Mitra et al. 2003; Mercantheler et al. 2004). By contrast, ER-α mRNA is found exclusively in the ventromedial hypothalamus and the subfornical organ (Shughrue et al. 1997).
Little information is currently available on the distribution of the three ER subtypes in the teleost brain. ER-α protein and mRNA distribution have been mapped in the forebrain of rainbow trout (Oncorhynchus mykiss; Kah et al. 1997; Menuet et al. 2003), but the neuroanatomical distribution of a possible ER-βa or ER-βb has not been investigated in this species. Our preliminary studies showed that ER-α, ER-βa and ER-βb mRNA expression patterns differ in the suprachiasmatic nucleus of the POA of Atlantic croaker Micropogonias undulatus (Hawkins et al. 2000). Similarly, all three subtypes show partially overlapping distributions in the anterior preoptic area, the ventral hypothalamus and the posterior tuberculum of the zebrafish Danio rerio (Menuet et al. 2002). These differences in distribution suggest that the three ER subtypes have distinct roles in the reproductive physiology and behaviour of fishes. It also raises the possibility that the functions of ER-βa and ER-βb have diverged from their ancestral ER-β gene. A more detailed description of the distribution of the three subtypes will begin to address their specific functions in the neuroendocrine system as well as the more general issue of the evolution and fate of duplicated genes.
2. Material and methods
(a) Animal and tissue collection
Adult female Atlantic croakers were collected from the waters surrounding Port Aransas, TX, USA, and maintained in tanks at the University of Texas Marine Science Institute under natural cycles of light and salinity. Six fishes with regressing gonads were injected intraperitoneally with E2 at a concentration (1 mg kg−1) that results in high physiological levels of E2 and upregulation of ER in fishes (Hawkins et al. 2000). Fishes were deeply anaesthetized 48 h later in a seawater : phenoxy ethanol bath (1 : 2000) and quickly killed by severing the spinal cord. Brains were removed and frozen in dry ice-chilled isopentane within 2 min. Brains were stored at −70 °C until they were sectioned.
(b) Creation of sequence-specific ER probes in Atlantic croaker
The three ER constructs used as probes were designed and prepared in the same manner as described previously (Hawkins et al. 2000). The Atlantic croaker ER-α, ER-βa and ER-βb probes encompassed amino acids 177–293, 266–406 and 443–552, respectively. [35S]CTP-labelled antisense riboprobes (New England Nuclear) were transcribed from each ER cDNA subclone.
(c) In situ hybridization
Frozen whole brains were embedded in optimal cutting temperature (OCT) compound (Tissutek) and cryosectioned at 20 μm (Frigocut, Reichert-Jung). Consecutive sections were transferred to a series of six poly-l-lysine-treated slides to localize the ER subtypes relative to each other in the same brain area. In situ hybridizations were conducted as described previously (Young et al. 1994; Hawkins et al. 2000).
After development, slides were visualized under dark field illumination to assess labelling in specific areas. In regions of low specific labelling or high background (cerebellum, NRL, PMm), positive labelling was confirmed via analysis with the grain counting software Brain as described previously (Young et al. 1994). Areas with silver grain density greater than 3× background were considered positively labelled. Four different animals were assessed for each area with the exception of the suprachiasmatic nucleus (n=3).
(d) Nomenclature
The terminology of Braford & Northcutt is followed in the text (Braford & Northcutt 1983), except in some cases where the equivalent terms of Peter & Gill are more specific (Peter et al. 1975). The terminology and abbreviations of both research groups are given in table 1.
Table 1.
The distribution of Atlantic croaker (ac)ER-α, acER-βa and acER-βb mRNA in the brain compared with the distribution of ERs and E2-concentrating cells in other teleosts. (Terminology of Peter & Gill (Peter et al. 1975) is given in italicized parentheses. The oyster toadfish (OTF; Fine et al. 1990), paradise fish (PF; Davis et al. 1977), goldfish (GF; Kim et al. 1979) and platyfish (PLF; Kim et al. 1979) tabular autoradiography data of E2-concentrating cells were taken in part from Fine et al. (1990). Autoradiography data do not differentiate ER subtypes. The zebrafish (zf ) ER mRNA data are from Menuet et al. (2002). The existence of few cells is labelled ( ). Superscript v indicates ventral labelling; superscript d indicates dorsal labelling; superscript 1 indicates rainbow trout (RT) ER-α mRNA (Kah et al. 1997); superscript 2 is (RT) ER-α antibody (Navas et al. 1995; Linard et al. 1996); superscript a means anatomical designation is not given in the original reference, but oestrogen-concentrating cells are shown in appropriate areas (Fine et al. 1990); superscript b means that no distinction was made between parvo and magnocellular (Fine et al. 1990); superscript c means designated n. saccus vasculosus in the original reference and a question mark identifies an area not investigated.)
| AcERs | Zf ERs | RT | GF | OTF | PF | PLF | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| α | βa | βb | α | βa | βb | α | E2-concentrating cells | ||||
| preoptic area (POA) | |||||||||||
| n. preopticus parvocellularis anterior (PPa) (=n. preopticus periventricularis, NPP) | +v,d | +(v)d | +v | +v | +v,(d) | +v,d | +1,2 | + | + | + | +a |
| n. preopticus magnocellularis pars parvocellularis (PMp) (=n. preopticus parvocellularis, NPOp) | + | + | + | ? | ? | ? | (+)1 | +b | (+) | + | + |
| n. preopticus magnocellularis pars magnocellularis (PMm) (=n. preopticus magnocellularis, NPOm) | + | − | + | ? | ? | ? | (+)1,2 | +b | (+) | (+) | + |
| n. preopticus parvocellularis posterioris (PPp) (=n. anterior hypothalami periventricularis, NAPv) | + | − | − | ? | ? | ? | +1 | − | (+) | − | − |
| n. suprachiasmaticus (SCN) | + | + | − | ? | ? | ? | −2 | ? | − | ? | ? |
| ventral hypothalamus (Hv): | + | + | − | ||||||||
| (n. lateralis tuberis pars anterior, NLTa) | + | + | + | ? | ? | ? | ? | (+) | (+) | (+) | − |
| (n. lateralis tuberis pars posterior, NLTp) | + | + | + | ? | ? | ? | +1,2 | + | + | + | + |
| (n. lateralis tuberis pars inferior, NLTi) | + | + | + | ? | ? | ? | +2 | + | + | + | + |
| (n. lateralis tuberis pars lateralis, NLTl ) | + | + | + | ? | ? | ? | +1,2 | ? | ? | ? | ? |
| n. anterior tuberis (TA) | + | + | + | ? | ? | ? | +2 | − | (+) | − | − |
| n. lateralis hypothalami (LH) | + | + | + | + | + | − | ? | − | + | − | − |
| dorsal hypothalamus (Hd): | |||||||||||
| (n. posterioris periventricularis, NPPv) | + | + | + | ? | ? | ? | (+)1,2 | + | + | (+) | +a |
| (n. recessus lateralis, NRL) | + | − | (+) | ? | ? | ? | +1 | − | + | + | + |
| caudal hypothalamus (Hc): | |||||||||||
| (n. recessus posterior, NRP) | + | − | − | ? | ? | ? | (+)1,2 | − | −− | − | − |
| posterior tuberculum | |||||||||||
| n. tuberis posterior (TP) | + | − | + | + | − | + | +2c | −c | +c | −c | +c |
| valvular cerebelli (VC) | − | − | + | ? | ? | ? | ? | ? | ? | ? | ? |
| corpus cerebellum (CC) | − | − | + | ? | ? | ? | ? | ? | ? | ? | ? |
3. Results
(a) The anterior hypothalamus/preoptic area
The distribution of ER-α, ER-βa and ER-βb mRNA labelling in the croaker brain is summarized and compared with findings for other teleost species in table 1. Hybridization signals for the three forms of ER exhibited some overlap in the POA but also showed several areas with marked differences. Most rostrally in the anterior preopticus parvocellularis (PPa), ER-α showed strong labelling in both the ventral and dorsal POA regions, whereas ER-βb showed more restricted labelling and ER-βa demonstrated weaker labelling (figure 1b–d). Interestingly, ER-βb labelling was restricted to the ventral portion of the PP nucleus (PPav), while some ER-βa labelling was also observed dorsal to the ER-βb labelling (PPad; figure 1c,d). Proceeding caudally, all the ER probes labelled the parvocellular portion of the magnocellular POA (PMp; table 1). More caudal and dorsal in the magnocellular nucleus (PMm), many large neurosecretory neurons were consistently and strongly labelled with the ER-βb probe, while ER-βa only very weakly labelled (less than 3× background) a few of these cells in some sections (figure 2c,d). In contrast to the patterns for ER-βb, the ER-α probe labelling over large neurons was more diffuse and was not discernibly stronger than that in the immediately surrounding tissue (figure 2b). The PPp is the most caudal nucleus of the POA and is arranged in laminae 2–5 cells thick. The PPp was labelled with ER-α probe but not with the ER-βa or ER-βb probe. ER-α and ER-βa labelling were seen in the suprachiasmatic nucleus as reported previously (Hawkins et al. 2000).
Figure 1.
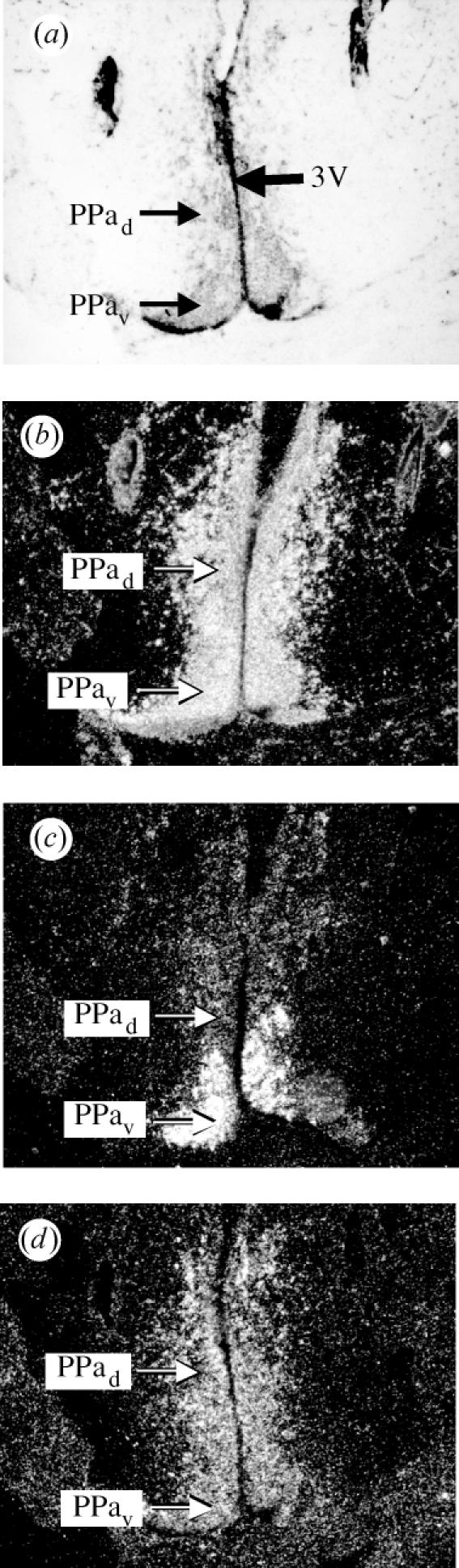
ER subtype mRNA expression patterns differ in the n. preopticus parvocellularis anterior (PPa) of the Atlantic croaker. (a) Arrows indicate the location of the dorsal PPad and ventral PPav in relation to the third ventricle (3V) in the most rostral portion of the preoptic area. (b) acER-α labelling is seen in both the PPad and PPav. (c) acER-βb labelling is detected only in the PPav. (d) acER-βa labelling is detected in the PPad, and there is some labelling in the PPav.
Figure 2.
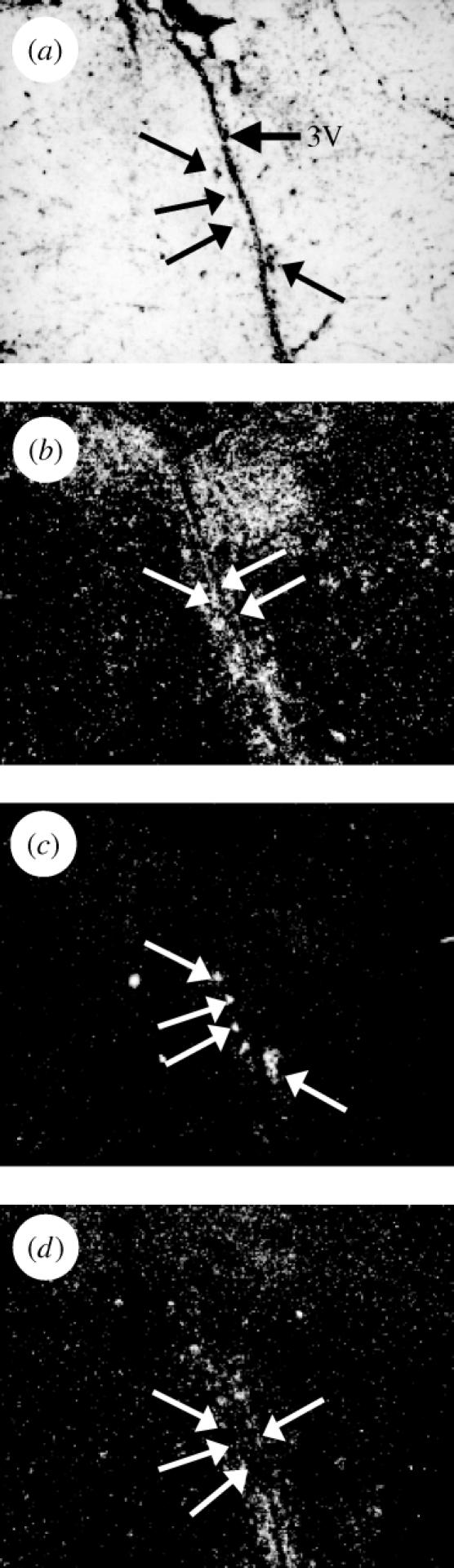
ER subtype mRNA expression patterns differ in the n. preopticus magnocellularis pars magnocellularis (PMm) of the Atlantic croaker. (a) Arrows indicate the location of large neurosecretory neurons along the third ventricle (3V). (b) acER-α labelling in the PMm is diffuse over and around large neurons. (c) acER-βb labelling is strong over magnocellular perikarya. (d) The acER-βa labelling is less than 3× background.
(b) The hypothalamus and posterior tuberculum
Most nuclei of the ventral and dorsal hypothalamus showed labelling for all three ERs (table 1). There were two notable exceptions: we found no ER-βa or ER-βb labelling in the NRP or the NRL. In the posterior tuberculum, the posterior tuberal nucleus (NPT) located dorsal to the ventricle was strongly labelled with the ER-βb probe (figure 3c). However, this nucleus was negative for ER-βa (figure 3d).
Figure 3.
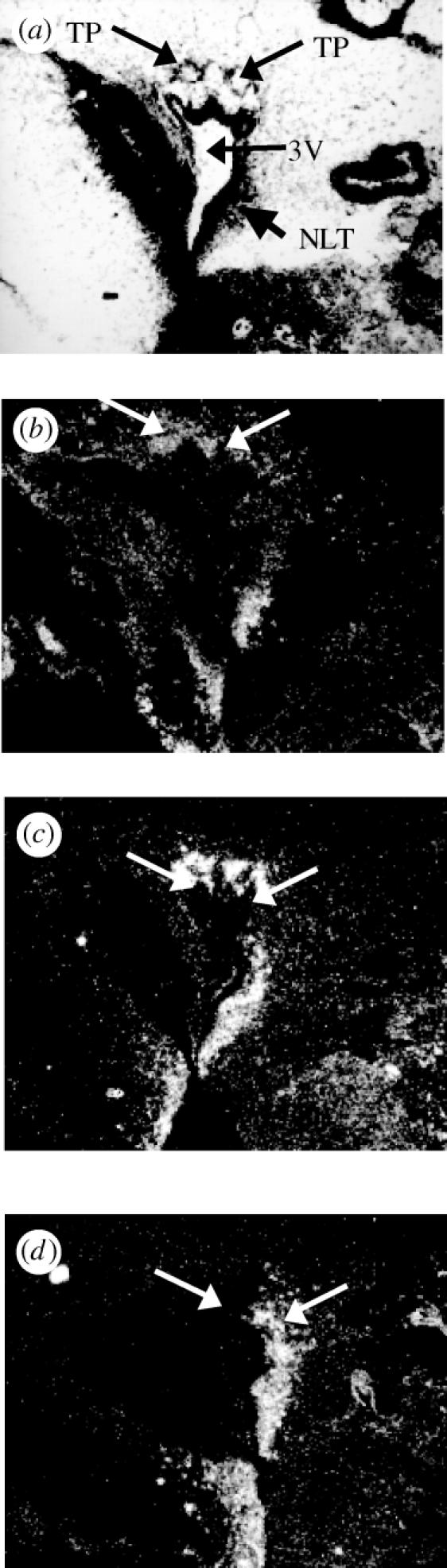
ER subtype mRNA expression patterns differ in the n. tuberis posterior (TP) of the posterior tuberculum of the Atlantic croaker. (a) Arrows indicate the location of the TP dorsal to the caudal portion of the third ventricle (3V) and the n. lateralis tuberis (NLT). (b) The acER-α probe diffusely labels the TP. (c) Strong acER-βb labelling is detected in the TP. (d) acER-βa labelling is not detected in the TP.
(c) The cerebellum
In the cerebellum, strong ER-βb labelling was observed in a bead-like arrangement in what appears to be the Purkinje cell layer (figure 4). Similar labelling was also observed in the valvular cerebelli. Neither the ER-α nor ER-βa probes labelled these regions.
Figure 4.
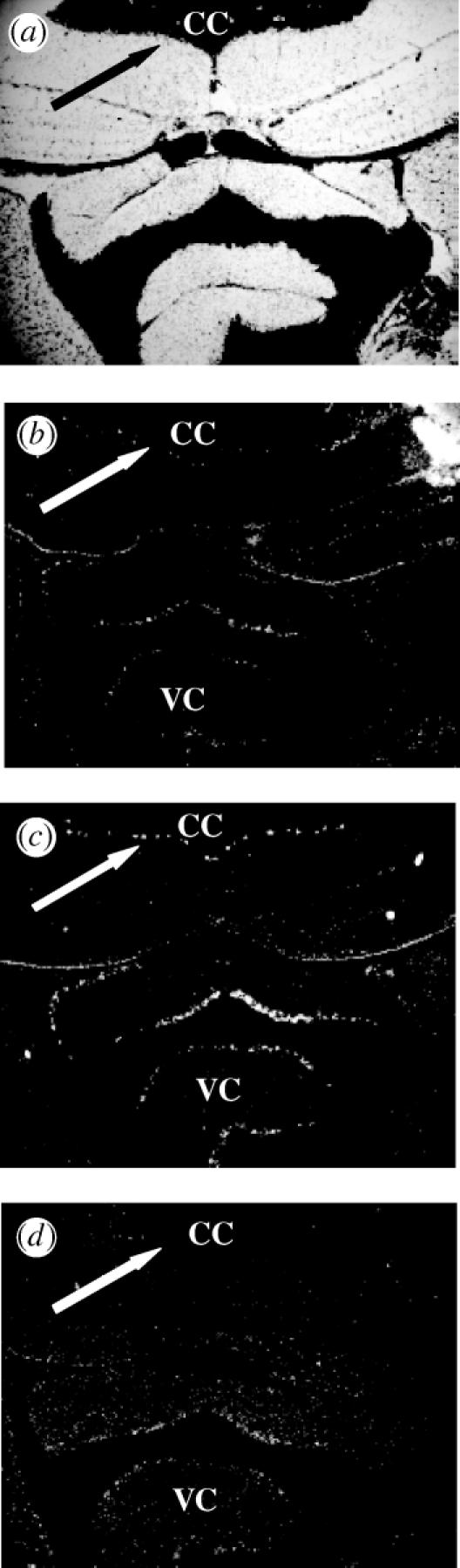
Distribution of acER mRNA in the corpus cerebellum (CC) of the Atlantic croaker. (a) Arrows indicate the location of the Purkinje cell layer. (b) acER-α labelling is less than 3× background. ‘VC’ is the valvular cerebelli. (c) The acER-βb riboprobe is specifically labelling Purkinje cells of the cerebellum. (d) acER-βa labelling is less than 3× background.
4. Discussion
Major differences were seen in the distribution of ER-βa and ER-βb subtypes in the parvo- and magnocellular preoptic areas, the suprachiasmatic nucleus, the posterior tuberis and the cerebellum of the Atlantic croaker brain. The PPa of the POA showed strong labelling with ER-βb in the ventral portion but no dorsal labelling, whereas ER-βa showed moderate labelling in both regions. There were also high levels of ER-βb expression in the PMm portion of the POA, but little or no ER-βa expression. In addition, ER-βb was expressed in the NPT and the cerebellum, while ER-βa was not detected in these areas. The only nucleus found to express ER-βa exclusively was the suprachiasmatic nucleus of the preoptic area. However, ER-βa distribution overlapped with that of ER-α and ER-βb in many areas (table 1).
The present study found that magnocellular neurons of the PM portion of the POA (PMm) express high amounts of ER-βb mRNA but little or no ER-βa or ER-α mRNA. This agrees with findings in mammals and birds, where the magnocellular region of the paraventricular nucleus (PVN) expresses ER-β, but not ER-α (Shughrue et al. 1997; Bernard et al. 1999; Foidart et al. 1999; Hileman et al. 1999; Mitra et al. 2003; Mercantheler et al. 2004). ER-β is coexpressed in magnocellular neurons of the mammalian PVN that also produce and release arginine vasopressin (AVP) or oxytocin (OT) (Hrabovsky et al. 2004). These neurohypophyseal hormones are involved in modulating reproductive behaviours in vertebrates (Moore 1992). Oestrogen treatment alters AVP and OT mRNA levels in the PVN of mice but has no effect in ER-β knockout mice (Nomura et al. 2002; Patisaul et al. 2003). It is likely that some of the cells expressing ER-βb in croaker are also AVT or OT neurons.
Some neurons of the rat POA that express ER-β produce GnRH, a neuropeptidergic hormone which is central to the control of vertebrate reproductive function and behaviour and whose release and production is modulated by E2 (Herbison & Pape 2001; Hrabovszky et al. 2001; Gore 2002). ER-α is not expressed in GnRH neurons, so until the recent discovery of ER-β in mammalian GnRH neurons (Herbison & Pape 2001), it was thought that in mammals and fishes, all of the oestrogenic effects on GnRH were indirect via interneurons that are regulated by E2 and whose neurons possess ERs (Flugge et al. 1986; Linard et al. 1996; Herbison 1998; Senthilkumaran et al. 2001). However, the recent demonstrations that GnRH neurons express ER-β in mammals suggest that E2 could have direct, as well as indirect, effects on GnRH regulation. The presence of ER-βa and ER-βb in the POA of croaker reopens the possibility that GnRH neurons in fishes may express an ER-β. This finding would suggest that ER expression in GnRH neurons is an ancient feature of vertebrate neuroendocrinology.
ER-α and ER-βb, but not ER-βa mRNA was detected in the NTP of the posterior tuberculum. These findings agree with those for zebrafish (Menuet et al. 2002). The TP as reported here appears to be at least in part the nucleus saccus vasculosus (SV) of Braford & Northcutt (1983). This nucleus is more appropriately named the TP because it does not innervate the SV organ as originally thought, but instead innervates the more rostral nucleus tuberis posterior of the posterior tuberculum. Oestradiol binding was detected here in oyster toadfish and platyfish (Kim et al. 1979; Fine et al. 1990). ER-α protein was also found in the nucleus SV of rainbow trout (Kah et al. 1997). This nucleus contains dopaminergic neurons and may be analogous to the nigrostriatal dopaminergic system of tetrapods (Rink & Wullimann 2001). In mammals, these dopaminergic neurons are responsive to oestrogen, and E2 protects them from degeneration induced by the neurotoxins 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; Arvin et al. 2000; Murray et al. 2003).
High levels of ER-βb mRNA were detected in the Purkinje cells of the cerebellum, while no ER-α or ER-βa labelling was observed. ER-βb was also detected in the goldfish and bird cerebellum by RT/PCR (Bernard et al. 1999; Foidart et al. 1999; Ma et al. 2000), and ER-β, but not ER-α, mRNA is present in Purkinje cells of the rat and mouse cerebellum (Price & Handa 2000; Mitra et al. 2003). In addition, ER-β may be involved in the E2-induced changes in transcription of GABAA receptors in rats (Barami et al. 1993). The presence of an ER-β in the cerebellum of species in three different vertebrate classes suggests an ancient function for this subtype in motor learning and muscle coordination. GnRH and 5-HT fibres were also detected in the cerebellum of the Atlantic croaker, but their function here is unknown (Khan & Thomas 1993).
The distribution of E2-concentrating cells in the brain is extremely conserved across species (Pfaff et al. 1988). In mammals, this conservation basically extends to ER-α and ER-β distribution (Shughrue et al. 1997; Hileman et al. 1999; Mitra et al. 2003; Mercantheler et al. 2004). In addition to our results presented here for croaker, there is one other study investigating the three ER subtypes' distribution in the brain of zebrafish (Menuet et al. 2002). Our results now provide a framework for comparing ER subtype distribution across fish species as well as across other vertebrate taxa. For example, in the PPa, we found ER-α and ER-βa in both the dorsal and ventral regions of the nucleus, while ER-βb was restricted to the ventral portion (table 1). In zebrafish, there is also a distinct, yet overlapping distribution of the three subtypes in the PPa. However, in this species ER-β1 (ER-βb) and ER-β2 (ER-βa) are found in both regions, whereas ER-α is restricted to the ventral region (Menuet et al. 2002). In the Hv we found all three subtypes in the nuclei investigated. However, zebrafish do not express ER-βb in the Hv. In the NPT, croaker and zebrafish have similar distributions of ERs. They show a more diffuse labelling with ER-α and very restricted labelling with ER-βb, while this region is negative for ER-βa in both species. Thus, while both species differentially express ER subtypes in many brain regions, the specific ER subtypes expressed in these areas are not conserved. A comparison incorporating more species is needed to assess the general conservation of ER subtype distributions among teleosts.
Since the ER subtypes were often found together in the same brain nuclei, it is possible that they colocalize to the same cells. If the ER subtypes are coexpressed, they could have either the same functions in these areas, or they could have different roles in the E2-controlled regulation of physiology and behaviour. There is evidence that there are regulatory differences between ER-α and ER-β in mammals and fishes (Paech et al. 1997; Menuet et al. 2002; Schultz et al. 2002). Evidence from rats and humans indicate that ERs may form heterodimers, which may result in distinct interactions with DNA and transcriptional cofactors, and cause alternative gene expression patterns (Pace et al. 1997; Pettersson et al. 1997; Ogawa et al. 1998). The three croaker ERs have different affinities for E2 and oestriol, which could allow for different levels of receptor activation in a given cell (Hawkins & Thomas 2004). Our recent findings have shown that the three ERs have distinct binding profiles for phytoestrogens and xenoestrogens (Hawkins & Thomas 2004, unpublished observations). Given the differential distribution of these receptor subtypes within the CNS, environmental exposure to oestrogenic substances may have profoundly different effects on neuroendocrine function.
ER-βa has the most restricted distribution of the three subtypes, suggesting that has very specific functions within the brain. ER-βa (as well as ER-βb) appears to have arisen in a duplication of the ancestral ER-β early in the teleost lineage (Hawkins et al. 2000). Structural comparisons and phylogenetic analyses among teleost ER-βs indicate that ER-βa quickly diverged from ER-βb after the duplication, suggesting the acquisition of novel functions by ER-βa. The exclusive presence of ER-βa in a region lacking ER-βs in other species would lend further support to the hypothesis that neofunctionalization occurred after the duplication event. Alternatively, ER-βa and ER-βb may have partitioned the ancestral ER-β functions within tissues (subfunctionalization). In croaker, the only brain areas investigated that express ER-βa and not ER-βb are the SCN (table 1; Hawkins et al. 2000) and the dorsal PPa. Conversely, ER-βb, but not ER-βa, was found in the ventral PPa, the magnocellular neurons of the POA and the Purkinje cells of the cerebellum. In rats and mice, both the SCN and the ventral and dorsal portions of the anterior preoptic area (PPa) express ER-β (Shughrue et al. 1997; Mitra et al. 2003; Merchenthaler et al. 2004). Other regions that express ER-β in mammals and birds include the magnocellular region of the PVN (Shughrue et al. 1997; Foidart et al. 1999; Hileman et al. 1999; Mitra et al. 2003; Mercantheler et al. 2004) and the cerebellum (Shughrue et al. 1997; Bernard et al. 1999; Foidart et al. 1999; Mitra et al. 2003). Thus ER-βa and ER-βb are found in different regions that express ER-β in tetrapods, suggesting that each ER-β subtype has lost ancestral ER-β functions that their duplicated counterpart (paralogue) has retained. Therefore, our data indicate that after the gene duplication event, subfunctionalization may have occurred because the distributions of ER-βa and ER-βb in croaker appear to reflect a partitioning of the functions of the ancestral ER-β gene. It is important to note that homologies for brain nuclei both within the fishes and across vertebrates are somewhat uncertain, so these regions may not be comparable. In addition, the oestrogen treatments given to upregulate ER levels in this study may have differential effects on ER subtype expression patterns (Menuet et al. 2004). Alternatively, it is also possible that ER-βb and ER-βa are expressed in different subsets of neurons within brain nuclei and have acquired novel neuron-specific functions that have resulted in neofunctionalization.
We conclude that the striking differences in the distributions of the three subtypes within brain nuclei of the POA and hypothalamus in the Atlantic croaker indicate that ER-α, ER-βa and ER-βb have distinct functions in the neuroendocrine control of reproduction and behaviour. The differential expression of the ER subtypes in other brain regions, such as the cerebellum and the posterior tuberculum, indicate that ER multiplicity is important for additional neural pathways. The combined neuroanatomical distribution of ER-βa and ER-βb follows the distribution pattern of mammalian ER-β. This conserved distribution pattern reflects the origin of ER-βa and ER-βb as a duplication of ER-β early in the teleost lineage and suggests that ancestral ER-β functions were subfunctionalized between teleost ER-βa and ER-βb after the duplication event. However, our results indicate that the largely conserved distribution patterns seen between tetrapod ER-α and ER-β may not hold true for euteleost ER-α, ER-βa and ER-βb. Additional studies of ER subtype distributions in fishes are needed to resolve the differential patterns of the three ER subtypes in teleosts and ultimately interpret their roles in the evolution of vertebrate neural function.
Acknowledgments
We thank Russell Borski, Brenda Brizuela and Craig Sullivan for laboratory support. M.B.H. was supported by The University of Texas Marine Science Institute Harry Page Marine Science Fellowship and the Houston Livestock Show and Rodeo Natural Sciences Fellowship. This work was supported by Texas Sea grant R/ES-92, EPA STAR grant R827399, NIEHS grant ESO4214 and ESO7672 (to P.T.); NIH grant MH58271 (to JG); and NIH grants MH41770 and MH57874 (to D.C.).
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
Present address: Department of Zoology, North Carolina State University, PO Box 7617, Raleigh, NC 27695, USA.
References
- Amores A, et al. Zebrafish hox clusters and vertebrate genome evolution. Science. 1998;282:1711–1714. doi: 10.1126/science.282.5394.1711. [DOI] [PubMed] [Google Scholar]
- Arvin M, Fedorkova L, Disshon K.A, Dluzen D.E, Leipheimer R.E. Estrogen modulates responses of striatal dopamine neurons to MPP+: evaluations using in vitro and in vivo techniques. Brain Res. 2000;872:160–171. doi: 10.1016/s0006-8993(00)02511-7. [DOI] [PubMed] [Google Scholar]
- Barami S, Amiri Z, Weizman A, Fares F, Gavish M. Long-term testosterone or diethylstilbestrol treatment affects gamma-aminobutyric-acid and central-type benzodiazepine receptors but not peripheral-type benzodiazepine receptors in the female rat-brain. Neuroendocrinology. 1993;57:1114–1118. doi: 10.1159/000126478. [DOI] [PubMed] [Google Scholar]
- Bernard D.J, Bentley G.E, Balthazart J, Turek F.W, Ball G.F. Androgen receptor, estrogen receptor alpha, and estrogen receptor beta show distinct patterns of expression in forebrain song control nuclei of European starlings. Endocrinology. 1999;140:4633–4643. doi: 10.1210/endo.140.10.7024. [DOI] [PubMed] [Google Scholar]
- Blaustein J.D. Minireview: neuronal steroid hormone receptors: they're not just for hormones anymore. Endocrinology. 2004;145:1075–1081. doi: 10.1210/en.2003-1485. [DOI] [PubMed] [Google Scholar]
- Braford M.R.J, Northcutt R.G. Organization of the diencephalon and pretectum of the ray-finned fishes. In: Davis R.E, Northcutt R.G, editors. Fish neurobiology. vol. 2. University of Michigan Press; Ann Arbor, MI: 1983. pp. 117–163. [Google Scholar]
- Davis R.E, Morrell J.I, Pfaff D.W. Autoradiographic localization of sex steroid-concentrating cells in brain of teleost Macropodus opercularis (Osteichthyes: Belontiidae) Gen. Comp. Endocrinol. 1977;33:496–505. doi: 10.1016/0016-6480(77)90108-3. [DOI] [PubMed] [Google Scholar]
- Fine M.L, Keefer D.A, Russelmergenthal H. Autoradiographic localization of estrogen-concentrating cells in the brain and pituitary of the oyster toadfish. Brain Res. 1990;536:207–219. doi: 10.1016/0006-8993(90)90027-9. [DOI] [PubMed] [Google Scholar]
- Flugge G, Oertel W.H, Wuttke W. Evidence for estrogen-receptive gabaergic neurons in the preoptic-anterior hypothalamic area of the rat-brain. Neuroendocrinology. 1986;43:1–5. doi: 10.1159/000124500. [DOI] [PubMed] [Google Scholar]
- Foidart A, Lakaye B, Grisar T, Ball G.F, Balthazart J. Estrogen receptor-β in quail: cloning, tissue expression and neuroanatomical distribution. J. Neurobiol. 1999;40:327–342. [PubMed] [Google Scholar]
- Force A, Lynch M, Pickett F.B, Amores A, Yan Y.L, Postlethwait J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151:1531–1545. doi: 10.1093/genetics/151.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore A.C. Kluwer Academic Publishers; Boston: 2002. GnRH, the master molecule of reproduction. [Google Scholar]
- Hawkins M.B, Thomas P. The unusual binding properties of the third distinct teleost estrogen receptor subtype ERβa are accompanied by highly conserved amino acid changes in the ligand binding domain. Endocrinology. 2004;145:2968–2977. doi: 10.1210/en.2003-0806. [DOI] [PubMed] [Google Scholar]
- Hawkins M.B, Thornton J.W, Crews D, Skipper J.K, Dotte A, Thomas P. Identification of a third distinct estrogen receptor and reclassification of estrogen receptors in teleosts. Proc. Natl Acad. Sci. USA. 2000;97:10 751–10 756. doi: 10.1073/pnas.97.20.10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison A.E. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr. Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- Herbison A.E, Pape J.R. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front. Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- Hileman S.M, Handa R.J, Jackson G.L. Distribution of estrogen receptor-beta messenger ribonucleic acid in the male sheep hypothalamus. Biol. Reprod. 1999;60:1279–1284. doi: 10.1095/biolreprod60.6.1279. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabas K, Shughrue P.J, Petersen S.L, Merchenthaler I, Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Kallo I, Steinhauser A, Merchenthaler I, Coen C.W, Petersen S.L, Liposits Z. Estrogen receptor-beta in oxytocin and vasopressin neurons of the rat and human hypothalamus: immunocytochemical and in situ hybridization studies. J. Comp. Neurol. 2004;473:315–333. doi: 10.1002/cne.20127. [DOI] [PubMed] [Google Scholar]
- Kah O, et al. Estrogen receptors in the brain–pituitary complex and the neuroendocrine regulation of gonadotropin release in rainbow trout. Fish Physiol. Biochem. 1997;17:53–62. [Google Scholar]
- Khan I.A, Thomas P. Immunocytochemical localization of serotonin and gonadotropin-releasing-hormone in the brain and pituitary gland of the Atlantic croaker Micropogonias undulatus. Gen. Comp. Endocrinol. 1993;91:167–180. doi: 10.1006/gcen.1993.1116. [DOI] [PubMed] [Google Scholar]
- Khan I.A, Thomas P. GABA exerts stimulatory and inhibitory influences on gonadotropin II secretion in the Atlantic croaker (Micropogonias undulatus) Neuroendocrinology. 1999;69:261–268. doi: 10.1159/000054427. [DOI] [PubMed] [Google Scholar]
- Khan I.A, Thomas P. Disruption of neuroendocrine control of luteinizing hormone secretion by Aroclor 1254 involves inhibition of hypothalamic tryptophan hydroxylase activity. Biol. Reprod. 2001;64:955–964. doi: 10.1095/biolreprod64.3.955. [DOI] [PubMed] [Google Scholar]
- Khan I.A, Hawkins M.B, Thomas P. Gonadal stage-dependent effects of gonadal steroids on gonadotropin II secretion in the Atlantic croaker (Micropogonias undulatus) Biol. Reprod. 1999;61:834–841. doi: 10.1095/biolreprod61.3.834. [DOI] [PubMed] [Google Scholar]
- Kim Y.S, Stumpf W.E, Sar M. Topographical distribution of estrogen target-cells in the forebrain of platyfish, Xiphophorus maculatus, studied by auto-radiography. Brain Res. 1979;170:43–59. doi: 10.1016/0006-8993(79)90939-9. [DOI] [PubMed] [Google Scholar]
- Knobil E, Neill J.D, editors. The physiology of reproduction. Raven Press; New York: 1994. [Google Scholar]
- Kuiper G, Enmark E, PeltoHuikko M, Nilsson S, Gustafsson J.A. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc. Natl Acad. Sci. USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme N, Nappi R.E, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ER alpha and ER beta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J. Neurobiol. 1998;36:357–378. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Linard B, Anglade I, Corio M, Navas J.M, Pakdel F, Saligaut C, Kah O. Estrogen receptors are expressed in a subset of tyrosine hydroxylase-positive neurons of the anterior preoptic region in the rainbow trout. Neuroendocrinology. 1996;63:156–165. doi: 10.1159/000126952. [DOI] [PubMed] [Google Scholar]
- Ma C.H, Dong K.W, Yu K.L. cDNA cloning and expression of a novel estrogen receptor beta-subtype in goldfish (Carassius auratus) Biochim. Biophys. Acta-Gene Struct. Expr. 2000;1490:145–152. doi: 10.1016/s0167-4781(99)00235-3. [DOI] [PubMed] [Google Scholar]
- Menuet A, Pellegrini E, Anglade I, Blaise O, Laudet V, Kah O, Pakdel F. Molecular characterization of three estrogen receptor forms in zebrafish: binding characteristics, transactivation properties, and tissue distributions. Biol. Reprod. 2002;66:1881–1892. doi: 10.1095/biolreprod66.6.1881. [DOI] [PubMed] [Google Scholar]
- Menuet A, Anglade I, Le Guevel R, Pellegrini E, Pakdel F, Kah O. Distribution of aromatase mRNA and protein in the brain and pituitary of female rainbow trout: comparison with estrogen receptor alpha. J. Comp. Neurol. 2003;462:180–193. doi: 10.1002/cne.10726. [DOI] [PubMed] [Google Scholar]
- Menuet A, Le Page Y, Torres O, Kern L, Kah O, Pakdel F. Analysis of the estrogen regulation of the zebrafish estrogen receptor (ER) reveals distinct effects of ER alpha, ER beta 1 and ER beta 2. J. Mol. Endocrinol. 2004;32:975–986. doi: 10.1677/jme.0.0320975. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M.V, Numan S, Dellovade T.L. Distribution of estrogen receptor α and β in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J. Comp. Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. http://dx.doi.org/doi:10.1002/cne.20128 [DOI] [PubMed] [Google Scholar]
- Mighell A.J, Smith N.R, Robinson P.A, Markham A.F. Vertebrate pseudogenes. FEBS Lett. 2000;468:109–114. doi: 10.1016/s0014-5793(00)01199-6. [DOI] [PubMed] [Google Scholar]
- Mitra S.W, Hoskin E, Yudkovitz J, Pear L, Wilkinson H.A, Hayashi S, Pfaff D.W, Ogawa S, Rohrer S.P, Schaeffer J.M, McEwen B.S, Alves S.E. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Moore F.L. Evolutionary precedents for behavioral actions of oxytocin and vasopressin. Ann. NY Acad. Sci. 1992;652:156–165. doi: 10.1111/j.1749-6632.1992.tb34352.x. [DOI] [PubMed] [Google Scholar]
- Murray H.E, Pillai A.V, McArthur S.R, Razvi N, Datla K.P, Dexter D.T, Gillies G.E. Dose- and sex-dependent effects of the neurotoxin 6-hydroxydopamine on the nigrostriatal dopaminergic pathway of adult rats: differential actions of estrogen in males and females. Neuroscience. 2003;116:213–222. doi: 10.1016/s0306-4522(02)00578-x. [DOI] [PubMed] [Google Scholar]
- Navas J.M, Anglade I, Bailhache T, Pakdel F, Breton B, Jego P, Kah O. Do gonadotrophin-releasing hormone neurons express estrogen receptors in the rainbow trout? A double immunohistochemical study. J. Comp. Neurol. 1995;363:461–474. doi: 10.1002/cne.903630309. [DOI] [PubMed] [Google Scholar]
- Nomura M, McKenna E, Korach K.S, Pfaff D.W, Ogawa S. Estrogen receptor-beta regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Mol. Brain Res. 2002;109:84–94. doi: 10.1016/s0169-328x(02)00525-9. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Inoue S, Watanabe T, Hiroi H, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. The complete primary structure of human estrogen receptor beta (hER beta) and its heterodimerization with ER alpha in vivo and in vitro. Biochem. Biophys. Res. Commun. 1998;243:122–126. doi: 10.1006/bbrc.1997.7893. [DOI] [PubMed] [Google Scholar]
- Ohno S. Springer-Verlag; Berlin: 1970. Evolution by gene duplication. [Google Scholar]
- Pace P, Taylor J, Suntharalingam S, Coombes R.C, Ali S. Human estrogen receptor beta binds DNA in a manner similar to and dimerizes with estrogen receptor alpha. J. Biol. Chem. 1997;272:25 832–25 838. doi: 10.1074/jbc.272.41.25832. [DOI] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper G, Nilsson S, Gustafsson J.A, Kushner P.J, Scanlan T.S. Differential ligand activation of estrogen receptors ER alpha and ER beta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Patisaul H.B, Scordalakes E.M, Young L.J, Rissman E.F. Oxytocin, but not oxytocin receptor, is regulated by oestrogen receptor beta in the female mouse hypothalamus. J. Neuroendocrinol. 2003;15:787–793. doi: 10.1046/j.1365-2826.2003.01061.x. [DOI] [PubMed] [Google Scholar]
- Peter R.E, Macey M.J, Gill V.E. Stereotaxic atlas and technique for forebrain nuclei of killifish, Fundulus heteroclitus. J. Comp. Neurol. 1975;159:103–127. doi: 10.1002/cne.901590107. [DOI] [PubMed] [Google Scholar]
- Pettersson K, Grandien K, Kuiper G, Gustafsson J.A. Mouse estrogen receptor beta forms estrogen response element-binding heterodimers with estrogen receptor alpha. Mol. Endocrinol. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- Pfaff D.W, Schwartz-Giblin S. Cellular mechanisms of female reproductive behaviors. In: Knobil E, Neil J, editors. The physiology of reproduction. vol. 2. Raven Press; New York: 1988. pp. 1487–1568. [Google Scholar]
- Postlethwait J, Amores A, Cresko W, Singer A, Yan Y.L. Subfunction partitioning, the teleost radiation and the annotation of the human genome. Trends Genet. 2004;20:481–490. doi: 10.1016/j.tig.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Price R.H, Handa R.J. Expression of estrogen receptor-beta protein and mRNA in the cerebellum of the rat. Neurosci. Lett. 2000;288:115–118. doi: 10.1016/s0304-3940(00)01221-0. [DOI] [PubMed] [Google Scholar]
- Rink E, Wullimann M.F. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum) Brain Res. 2001;889:316–330. doi: 10.1016/s0006-8993(00)03174-7. [DOI] [PubMed] [Google Scholar]
- Schultz J.R, Loven M.A, Melvin V.M.S, Edwards D.P, Nardulli A.M. Differential modulation of DNA conformation by estrogen receptors alpha and beta. J. Biol. Chem. 2002;277:8702–8707. doi: 10.1074/jbc.M108491200. [DOI] [PubMed] [Google Scholar]
- Senthilkumaran B, Okuzawa K, Gen K, Kagawa H. Effects of serotonin, GABA and neuropeptide Y on seabream gonadatropin releasing hormone release in vitro from preoptic-anterior hypothalamus and pituitary of red seabream, Pagrus major. J. Neuroendocrinol. 2001;13:395–400. doi: 10.1046/j.1365-2826.2001.00645.x. [DOI] [PubMed] [Google Scholar]
- Shughrue P.J, Lane M.V, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J. Comp. Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shupnik M.A. Oestrogen receptors, receptor variants and oestrogen actions in the hypothalamic-pituitary axis. J. Neuroendocrinol. 2002;14:85–94. doi: 10.1046/j.0007-1331.2001.00744.x. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo E.J, Dong J. Identity of an estrogen membrane receptor coupled to a G-protein in human breast cancer cells. Endocrinology. 2004;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Thornton J.W. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc. Natl Acad. Sci. USA. 2001;98:5671–5676. doi: 10.1073/pnas.091553298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand C.D. Minireview: a plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Young L.J, Lopreato G.F, Horan K, Crews D. Cloning and in-situ hybridization analysis of estrogen-receptor, progesterone-receptor, and androgen receptor expression in the brain of whiptail lizards (Cnemidophorus uniparens and C. inornatus) J. Comp. Neurol. 1994;347:288–300. doi: 10.1002/cne.903470210. [DOI] [PubMed] [Google Scholar]


