Abstract
Ecosystems and populations are known to be influenced not only by long-term climatic trends, but also by other short-term climatic modes, such as interannual and decadal-scale variabilities. Because interactions between climatic forcing, biotic and abiotic components of ecosystems are subtle and complex, analysis of long-term series of both biological and physical factors is essential to understanding these interactions. Here, we apply a wavelet analysis simultaneously to long-term datasets on the environment and on the populations and breeding success of three Antarctic seabirds (southern fulmar, snow petrel, emperor penguin) breeding in Terre Adélie, to study the effects of climate fluctuations on Antarctic marine ecosystems. We show that over the past 40 years, populations and demographic parameters of the three species fluctuate with a periodicity of 3–5 years that was also detected in sea-ice extent and the Southern Oscillation Index. Although the major periodicity of these interannual fluctuations is not common to different species and environmental variables, their cyclic characteristics reveal a significant change since 1980. Moreover, sliding-correlation analysis highlighted the relationships between environmental variables and the demography of the three species, with important change of correlation occurring between the end of the 1970s and the beginning of the 1980s. These results suggest that a regime shift has probably occurred during this period, significantly affecting the Antarctic ecosystem, but with contrasted effects on the three species.
Keywords: climate change, cyclic variability, regime shift, Antarctic marine predators, time-series analysis
1. Introduction
Climate records indicate that the Earth is experiencing dramatic changes (Hughes 2000), with an overall trend of increasing temperatures for the past 100 years. Long-term monitoring studies suggest that these recent climate changes probably already affect species abundance, distribution or phenology (Walther et al. 2002). However, superimposed to long-term warming trends, other short-term climatic changes, such as interannual and decadal variabilities or stepwise changes associated with system shifts, affect ecosystems (Hare & Mantua 2000; Stenseth et al. 2002).
In ecological studies, population cycles have been the focus of interest for a long time. The possible causes of population cycles are linked to intrinsic biological processes, such as a combination of direct and delayed effects of density dependence, and to extrinsic environmental factors (Berryman 2002). There is evidence that several ecological and population processes are affected by climatic oscillations. In particular, the North Atlantic Oscillation and the El Ñino Southern Oscillation (ENSO) have been shown to affect a variety of ecosystems and populations (Stenseth et al. 2002). Moreover, recent studies have also shown that ecosystems can switch between stable states, or regimes, and that these shifts can be triggered by small environmental changes (Hare & Mantua 2000; Scheffer & Carpenter 2003).
Antarctica and the surrounding Southern Ocean play a critical role in global climate changes, and constitute a particularly interesting place to study the impact of climatic trends, oscillations and anomalies on ecosystems. In the Southern Ocean, interannual anomalies of atmospheric and oceanic parameters like sea-surface temperature (SST) and sea-ice extent (SIE) occur in close relation to tropical ENSO events (Park et al. 2004). In addition, a noticeable change in climate or a regime shift in the Southern Ocean has been suggested during the late 1970s (Masson-Delmotte et al. 2003; Weimerskirch et al. 2003) affecting the entire ecosystem (Reid & Croxall 2001; Weimerskirch et al. 2003). The consequences of this complex set of environmental variabilities on the biological patterns and processes are poorly understood, especially in waters close to Antarctica where little information is available on the different components of the ecosystem.
Marine mammals and seabirds are upper trophic-level predators in marine ecosystems, and are sensitive indicators of changes in oceanic environments. Changes in the foraging habitats, distribution and abundance of prey species of marine top predators may affect their population dynamics and distribution. Since their demographic parameters can easily be measured on breeding sites, seabirds can provide an integrative view of the ecological consequences of environmental variability (Croxall et al. 2002), and even amplify the effects of climatic forcing on lower marine trophic levels.
Population size or demographic parameter changes of several seabird species have already been related to environmental fluctuations occurring worldwide in marine ecosystems (Croxall 1992), and more specifically to abiotic components such as sea ice, SST or air temperature (Wilson et al. 2001; Barbraud & Weimerskirch 2001a,b; Jenouvrier et al. 2003; Weimerskirch et al. 2003). Such long-term datasets are essential to understand these interactions and provide a unique opportunity to observe how changes in the physical environment can affect marine ecosystems on long-term scales (Hare & Mantua 2000; Croxall et al. 2002; Stenseth et al. 2002). However, very few decadal-scale studies of seabirds are available to assess the impacts of oscillations and regime shift in climate. Here, we analyse the changes in population size and a key demographic parameter (breeding success) of three Antarctic seabird species breeding in sympatry in Adélie land, in conjunction with a series of regional and global environmental parameters over a 40 year time-span. We focus particularly on the cyclicity of demographic and environmental time-series, to examine the periodicity changes over time and the possibility that a regime shift occurred during the 1980s.
Because interactions between climatic forcing, biotic and abiotic components of ecosystems are subtle and complex, analysis of long-term series of biophysical parameters requires adequate methodologies (Croxall et al. 2002; Stenseth et al. 2003). The detection of temporal oscillation in time-series is greatly complicated by both non-stationary temporal variations, and unavoidable noise components. Here, we used improved methodologies for time-series analysis, namely wavelet analysis, to describe the non-stationarity of the demographic parameters, and of environmental fluctuations (e.g. Klvana et al. 2004).
2. Material and methods
(a) Species and environmental variables
We used population abundance data of southern fulmars (Fulmar glacialoides), snow petrels (Pagodroma nivea) and emperor penguins (Aptenodyptes forsteri) breeding on Ile des Pétrels, Pointe Géologie Archipelago (66°40′S, 140°01′E), Terre Adélie, Antarctica. Breeding population monitoring was carried out every year from 1963 to 2002 for the southern fulmar and snow petrel, and from 1962 to 2001 for the emperor penguin (see Jenouvrier et al. 2003, Chastel et al. 1993 and Barbraud & Weimerskirch 2001a, respectively, for details on monitoring methodology). Breeding success for each species was estimated as the proportion of eggs that fledged a chick.
To investigate the relationship between environmental fluctuations and population dynamics, we analysed time-series of regional and global environmental parameters. We used SIE during Austral winter (April–November), because winter is a critical biological period for krill (Loeb et al. 1997) and Antarctic seabirds (Barbraud et al. 2000; Barbraud & Weimerskirch 2001a; Jenouvrier et al. 2003). It was computed for a sector around Dumont d'Urville station between the longitudes 130–150° E using the sea-ice data available from the Antarctic CRC and Australian Antarctic Division Climate Datasets (http://www.antcrc.utas.edu.au/~jacka/seaice_C_html). We used the Southern Oscillation Index (SOI) as a proxy of overall climate condition over the Southern Ocean (Liu et al. 2002; Kwok & Comiso 2002; Stenseth et al. 2003; Park et al. 2004). SOI is available from the Climatic Research Unit, University of East Anglia, Norwich, UK (http://www.cru.uea.ac.uk/cru/data/soi.htm).
(b) Wavelet analysis
Because our time-series showed marked changes in cycle period through time, we applied a wavelet analysis to take into account the non-stationarity in population dynamics. By decomposing a time-series into time and frequency domains, the wavelet analysis can determine both the dominant modes of variability, and how those modes vary in time (Torrence & Compo 1998; Klvana et al. 2004). Owing to the maximum length of time-series of 40 years, low-frequency components having periods greater than 8 years (corresponding to one-fifth of the total length of time-series) may not be well resolved. Therefore, before applying the method, we removed low frequencies in our time-series using an 8 year high-pass Gaussian filter (Park & Gambéroni 1995) and focused on periods between 2 and 8 years.
We used the Morlet wavelet function ψ0 that is essentially a damped complex exponential function, which can quantify local cyclic fluctuations in the time-series. The frequency–time range over which it does this is set by a scale parameter, s, relating to the conventional Fourier period of oscillations, and a translation parameter n. The Morlet wavelet function is , where ω0 is the non-dimensional frequency and η=n/s (Torrence & Compo 1998).
With ω0=6, the wavelet scale s is inversely proportional to the central frequency of the wavelet, and the frequency is equal to 1/s, or the period p is equal to s. The continuous wavelet transformation (CWT), Wn(s), of the time-series Xn is calculated as the convolution of Xn with ψ0. Local matching of wavelet function for a scale s at a time point n with the signal Xn leads to a high value of Wn(s). Inversely, with no matching, one obtains a low value for Wn(s). The wavelet transform coefficients Wn(s) represent the contribution of the scale s to the signal at different time position n. By taking into account a range of s and n values, one can explore and identify the structures relating to time and frequency.
The wavelet power spectrum (WPS), at time point n and scale s, is then given by |Wn(s)|2. The WPS gives a measure of the variance distribution of the time-series at time point n and scale s. To compare WPS with classical spectral methods, global wavelet spectrum is computed as the time-average of the WPS for each frequency component. It has been shown that the global wavelet spectrum provides an unbiased and consistent estimation of the Fourier spectrum (Percival 1995).
As with classical Fourier analysis using fast Fourier transform, the data were padded with zero up to the next-highest power of two (Torrence & Compo 1998). The ‘cone of influence’ is a reflection of a consequent loss in statistical power near the start and the end of the series. This area dashed in the WPS figure and delimited by a white line (see § 3) should be interpreted cautiously. Nevertheless, the zero padding, owing to numerous introduced zeros, mainly induces reduction in the CWT and in the associated quantities.
The 5% and 10% significance levels were determined with bootstrapped simulations. We consider the null hypothesis as the observed time-series being different from that expected through chance alone. We constructed 1000 surrogate datasets, and calculated for each surrogate the CWT. Based on these surrogate series, one constructs the distribution under the null hypothesis of WPS and global WPS. These distributions are then used to establish the 5% and 10% significance levels for the WPS and global WPS.
Algorithms and notations used here were based on a practical guide to meteorological wavelet analysis (Torrence & Compo 1998). Nevertheless, the results presented in this work have been computed on the basis of a new MatLab package, which has the advantage of incorporating both cross analyses and adapted statistical procedures (B. Cazelles, M. Chavez, D. Berteaux, F. Menard, J. O. Vik, S. Jenouvrier & N. Chr. Stenseth, unpublished data).
(c) Relationships between environmental variables and demographic parameters
There are several reasons to believe that the strongest relationship between an environmental variable and population size does not necessarily occur in phase but could occur with a lag through an effect on recruitment (e.g. Guinet et al. 1998; Thompson & Ollason 2001). We estimated lagged Pearson correlation coefficients of the breeding population size of each seabird in response to the assumed ‘external forcing’ of SOI and SIE, within a lag range of 0–10 years. For breeding success, we focused on Pearson correlations without lag, because breeding success depends mainly on the availability of resources during the incubation and rearing period in seabirds (e.g. Barbraud & Chastel 1999). We used bootstrap methods to calculate the significance levels. We considered the null hypothesis as the observed time-series being different from that expected through chance alone. We constructed 1000 surrogate datasets, calculated for each surrogate Pearson correlation coefficient, and established distribution of Pearson correlation coefficient under the null hypothesis.
We also conducted a 7 year sliding window correlation incremented by 1 year of the number of breeding pairs, breeding success and environmental variables to highlight change in correlation pattern over time. We specified the mean confidence intervals calculated with bootstrap methods, based on the Pearson correlation coefficient distributions calculated for each species with a times-series of the same length as the window. We focused on correlation coefficient sign change. We tested the possibility that the observed correlations sign change was developed by chance alone by generating 1000 random sequences of the demographic times-series. The significant level of this change was denoted pc (see the Electronic Appendix).
3. Results
(a) Species and environmental variables
(i) General description of time-series
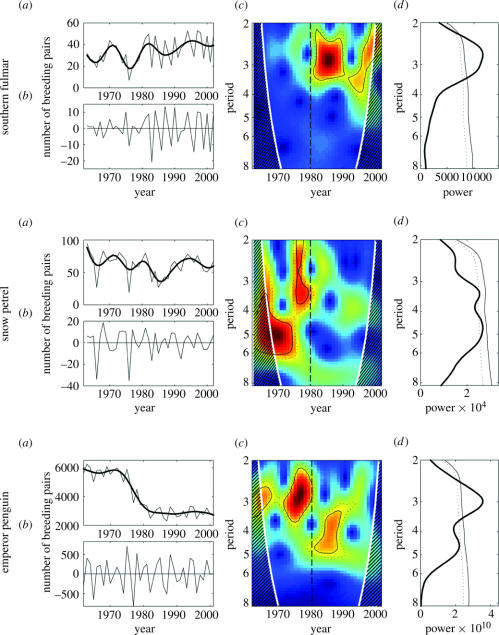
The number of breeding pairs of southern fulmars and snow petrels fluctuated strongly, averaging 33±11 and 62±17 pairs, respectively (figure 1a). It was particularly low in 1976 for the southern fulmar, and in 1966, 1976 and 1985 for the snow petrel. These two petrel species show a similar trend, with minima during the mid-1970s and 1980s. From 1962 to 1975, the colony of emperor penguins numbered 5709±371 pairs, but the population decreased dramatically between 1975 and 1980, but stabilized since 1983 to 2853±320 breeding pairs.
Figure 1.
(a) Annual variation of the number of breeding pairs for southern fulmars, snow petrels between 1963 and 2002, and emperor penguins between 1962 and 2002, at Dumont d'Urville, Terre Adélie, Antarctica (thin line). The thick line represents the low-pass filtered times-series (see § 2). (b) Data used for the analyses (difference between the unfiltered and low-pass filtered times-series of figure (a)). (c) Local wavelet power spectrum of breeding population sizes. The y-axis was in log 2 scale. The thick black contour is the 5% significance level and the dotted black contour is the 10% significance level calculated with a bootstrap method (see § 2). The white contour indicates the cone of influence, and the dashed area stands for the region where zero padding has reduced the variance. The local wavelet power spectrum gives a measure of the variance distribution of the time-series according to time and for each periodicity; high variability is represented by red colour, whereas blue colour indicates a weak variability. The dark dashed lines at 1980 were a visual help. (d ) Global wavelet power spectrum of interannual variability (2–8 years) of breeding pairs of the three species. The dotted and dashed black lines are, respectively, the 5% and 10% significance levels.
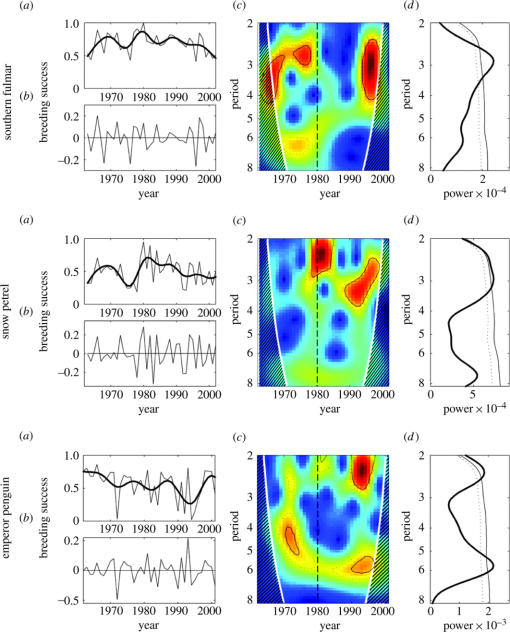
The breeding success of the three species showed high interannual variability (figure 2a). The mean breeding successes were, respectively, 0.70±0.14, 0.50±0.19 and 0.56±0.23 for the southern fulmar, snow petrel and emperor penguin. The breeding success was particularly low in 1964, 1976, 1996 and 2001 for the southern fulmar, and in 1963–1964, 1975–1978 and 1992–1993 for the snow petrel. These two petrel species show a somewhat similar trend, with a minimum in the late 1970s, followed by an abrupt increase peaking in 1980. For both species, there is a gradual decreasing trend since that time. The breeding success of the emperor penguin reveals a different trend, without any noticeable drop in the late 1970s, but decreasing continually from a maximum in the early 1960s to a minimum in the mid-1990s. On two occasions (1972, 1994), there was a near-complete failure (greater than 95%) of breeding. The emperor penguin breeding performance returned to relatively high values in the late 1990s.
Figure 2.
Same as figure 1, but for the breeding success of southern fulmars, snow petrels and emperor penguins between 1962 and 2002.
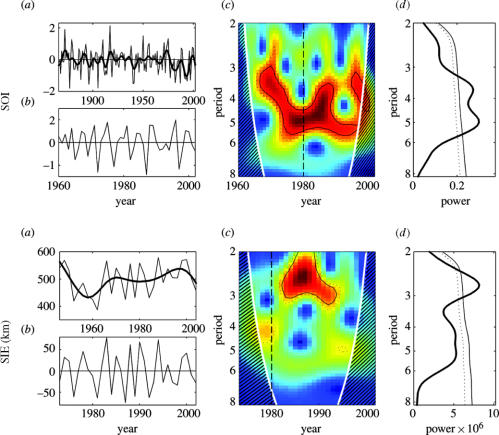
Winter SIE and SOI showed strong year-to-year variations (figure 3a). An abrupt sea-ice retreat occurred between 1976 and 1981, corresponding to a similar drop in SOI values. Indeed, the 13 lowest SOI values from the low-pass filtered times-series since 1866 (figure 3a) fall within the 1979–1984 and 1990–1996 periods (mean: −0.60±0.10 and −0.70±0.17, respectively), whereas the four highest values occurred in the 1972–1975 period (mean: −0.47±0.06).
Figure 3.
Same as figure 1, but for environmental variables: winter SIE for a sector of 130–150°E off Dumont d'Urville since 1973, and SOI for two time-scales ((a)1866–2002 and (b) 1960–2002).
(ii) Wavelet analysis
The population size and breeding success of all three species (figures 1 and 2b) fluctuated with a main cyclic periodicity of 3–5 years (figures 1 and 2d), which was also detected in SIE and SOI (figure 3d). However, these periodicities were not constant over time, nor were they common to different species and environment factors, but they revealed a significant change in their cyclic characteristics since 1980 (figures 1–3c).
The fulmar population showed pronounced quasi-regular fluctuations with a 3 year periodicity only after 1980 (figure 1b), especially during 1980–1990. Conversely, the snow petrel population revealed a significant periodicity only before 1980, with a 5 year periodicity before 1975, and a 2–4 year periodicity from 1975 to 1980. The emperor penguin population was also characterized by a marked change in periodicity around 1980. A significant 3 year periodicity appeared before 1980, especially in 1972–1980, which was followed by a significant change to a longer period of 4 years, especially during 1980–1990.
The breeding success of southern fulmars revealed a significant 3 year periodicity before 1980 and around 1995 (figure 2b). Outside these time-intervals, there was no significant variability. Conversely, the snow petrel breeding success showed pronounced quasi-regular fluctuations with a dominant period of 2–3 years only after the 1980s, but with a changing periodicity around 3–4 years after the 1988. The emperor penguin breeding success was characterized by two marked periodicities of 2–3 years and 6 years after 1990, and a single periodicity of 4–5 years between 1975 and 1978.
Wavelet power spectra revealed a clear shift in the SIE periodicity, from a weak periodicity of 4 years before 1980 to a strong one of 2–3 years between 1980 and 1995 (figure 3b). On the other hand, the periodicity of SOI increased from 3 to 5 years during the early 1970s, and decreased from 5 to 3 years at the end of the 1980s and 1990s. A dominant periodicity of 5 years appeared in the mid-1970s and remained steady until the end of the study.
(b) Relationships between demographic parameters and environmental variables
(i) Number of breeding pairs
For a given environmental variable, there were contrasted correlations for different species (table 1), and change in correlation pattern over time (figure 4).
Table 1.
Lagged Pearson correlations coefficient between environmental variables (SOI, SIE) and demographic parameters of three seabirds at Terre Adélie, Antarctica. ( Values within parentheses represent lags in year for the first significant coefficient correlation during the overall period. Significant correlations at a 0.10 (*), 0.05 level (**) and 0.01 level (***) calculated with bootstrap methods are indicated.)
| SIE | SOI | |||
|---|---|---|---|---|
| southern fulmar | number of breeding pairs | no lag | −0.10 | −0.21 |
| lag | 0.30* (4) | 0.33** (9) | ||
| breeding success | no lag | 0.06 | −0.41*** | |
| snow petrel | number of breeding pairs | no lag | −0.06 | 0.03 |
| lag | 0.34** (7) | 0.26* (6) | ||
| breeding success | no lag | 0.02 | −0.14 | |
| emperor penguin | number of breeding pairs | no lag | 0.24* | 0.39*** |
| lag | 0.35 (6)** | 0.38** (5) | ||
| breeding success | no lag | −0.08 | 0.30** | |
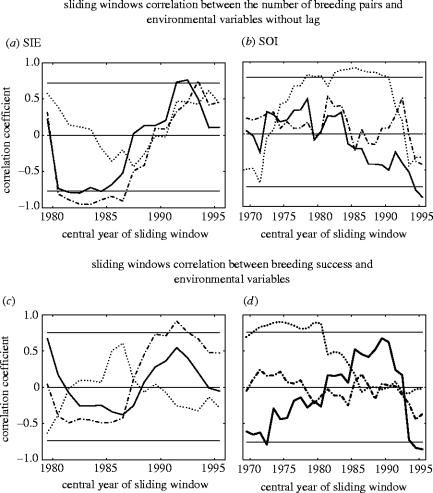
Figure 4.
A 7 year sliding window correlation between the demographic data (figures 1b and 2b) and the environmental data (figure 3b). Sliding windows correlation between the number of breeding pairs of the three species and (a) SIE and (b) SOI without lag. Sliding windows correlation between the breeding success of the three species and (c) SIE and (b) SOI. The symbols represent the three species: southern fulmar (solid line), snow petrel (dashed line) and emperor penguin (dotted line). The Pearson's product–moment correlations were conducted for overlapping 7 year intervals incremented by 1 year and the correlation value of each window was affected to the central year of the window. The hairline represents the limit of the mean confidence intervals based on the Pearson correlation coefficient distributions calculated by bootstrap for each species with times-series of the same length as the window. More details on the signification of correlation coefficient sign change observed here are available in the Electronic Appendix.
The number of breeding pairs for snow petrel and emperor penguin was positively correlated with SIE at a lag of 7 and 6 years, respectively (table 1). For snow petrel and southern fulmar, the correlation coefficients between SIE and the number of breeding pairs were not significant for the overall period (table 1), but showed a change of sign from negative to positive starting at the beginning of the 1980s ( pc=0.073 and pc=0.030, respectively; Electronic Appendix; figure 4a).
For SOI during the whole data period (1963–2002), the number of breeding pairs of southern fulmars was positively correlated at a lag of 9 years (table 1). The number of breeding emperor penguin pairs was positively related to SOI without any lag (table 1), particularly between 1975 and 1995 (figure 4b), with significant changes from negative to positive correlation coefficient in the mid-1970s, and from a positive to a negative value during the mid-1990s ( pc<0.001, Electronic Appendix). The next higher correlation occurred at a lag of 5 years (table 1), that is consistent with the previous lagged positive relation highlighted with SIE. In contrast to this, no significant correlations were found for snow petrels during any time-period.
(ii) Breeding success
Snow petrel breeding success was not significantly related to environmental variable during the overall period (table 1), but showed a strong positive correlation with SIE after 1990 (figure 4c), with a significant change to a negative relationship before the mid-1980s to a positive one afterwards ( pc=0.015, Electronic Appendix). For the whole period, the breeding success of southern fulmar was negatively related with SOI, whereas it was positively correlated for emperor penguin (table 1). However, the correlation between southern fulmar breeding success and SOI was strongly negative only before 1980 and after 1990 (figure 4d ), and this change in correlation pattern during the 1980s was highly significant ( pc<0.001, Electronic Appendix). For the emperor penguin, there was also a significant change of correlation during the 1980s, with a strong positive one before 1980 (figure 4d, pc<0.001, Electronic Appendix).
4. Discussion and conclusions
The breeding populations of three seabird species breeding in sympatry in coastal Antarctica and their breeding success strongly fluctuated from year to year during the past 40 years. All shared a dominant periodicity around 3 years for both population and breeding success time-series. Snow petrel and emperor penguin populations also shared a marginal periodicity around 5 years, and in addition emperor penguin breeding success showed a cyclicity around 6 years. Population models showed that the breeding success variations have a limited impact on breeding population sizes on short time-scales for such long-lived species (Lebreton & Clobert 1991; Jenouvrier et al. 2003), and consequently the cyclic variations around 3 years of breeding success cannot be a primary determinant of the same periodicity observed in breeding population size.
These common periodicity patterns across species suggested the existence of a common environmental forcing on the demography of this seabird community. However, wavelet analysis clearly indicated that the interannual variability of the population and breeding success of the three species have significantly changed since 1980, although the intensity and periodicity of these changes were not uniform but varied considerably depending on the species. The cyclicity for the snow petrel disappeared after 1980, while at the same time, it appeared for the southern fulmar. It was almost the opposite pattern for breeding success. For the emperor penguin, population periodicity showed a strong periodicity change from 3 to 4 years. Together, these significant changes in periodicity of demographic parameters around the 1980s suggested a change in environmental conditions, perhaps indicating a regime shift.
Indeed, environmental parameters also showed a significant change in cyclicity around 1980. Winter SIE presents a well-known periodicity between 3 and 5 years (Zwally et al. 2002), which was especially marked around 3 years from 1980 to 1995 in Terre Adélie. The periodicity of SOI also changed from 3 years before 1975 to 5 years after 1980, which was accompanied by an unprecedented, significant decadal variability of low SOI values appearing since 1975.
Our results are consistent with those of Weimerskirch et al. (2003) who interpreted the concomitant changes in climate and Southern Ocean ecosystems during the late 1970s in the light of a regime shift. Based on an analysis of ice core data in Antarctica, Masson-Delmotte et al. (2003) suggested a change in meridional atmospheric circulation during the 1970s, bringing more moisture from warm subtropical moisture sources to the Antarctic coast. Our local data are consistent with this, as the winter SIE decreased steadily from 1973 to 1981, reaching the smallest SIE in 1981. Moreover, SST over the Southern Ocean as a whole rose abruptly during the same period (Weimerskirch et al. 2003). Finally, the SOI decadal trend since 1866 reached maximum values in the early 1970s, but experienced an important decrease during the late 1970s. It then shifted to a negative trend since 1980 with unprecedented lower values, especially during the 1979–1984 and 1990–1996 periods.
The hypothesis that changes in cyclic patterns of demographic parameters of the three species are linked to changes in environmental cyclic patterns is supported by the observed variations in the correlation patterns between demographic and environmental variables. In Antarctic ecosystems, environmental conditions may have an important influence on food availability either directly or indirectly, and thus are likely to influence population dynamics of seabirds. SST and sea-ice anomalies could affect food availability of seabirds, since reduced SIE and warm SST negatively affect the abundance of krill (Loeb et al. 1997), a major prey species for Antarctic top predators. Fraser & Hofmann (2003) showed that there is a direct causal relationship between variability in sea-ice cover, krill recruitment, prey availability and seabird foraging ecology. Krill populations are sustained by strong age class that emerges episodically after years of heavy sea-ice condition.
Southern fulmar and snow petrel populations showed very similar correlation patterns with environmental variables without lag, especially with SIE. These similarities may be explained by the fact that the life cycles of these two fulmarine petrels are nearly identical (similar age at first breeding, breeding frequency, adult survival, etc.). They both forage in the pack ice during the breeding season in summer, although snow petrel is a more pagophilic species. Moreover, the key demographic parameter that explained the number of breeding pair fluctuations was the proportion of breeders for both species ( Jenouvrier et al. 2005). These species behave as prudent parents (Drent & Dann 1980) in that they never allow their body condition to deteriorate to a level that may jeopardize their lifetime reproductive success. Breeding abstention among long-lived species may be a response to poor feeding conditions early in the breeding season. Indeed, other studies showed that southern fulmars and snow petrels are able to skip reproduction when environmental conditions are poor (Chastel et al. 1993; Jenouvrier et al. 2003).
On the other hand, only the two pagophilic species (i.e. snow petrel and emperor penguin) were strongly related to SIE with a lag. The lagged relations between environmental variables and the number of breeding pairs may be explained by an effect of environmental fluctuations on recruitment (e.g. Wilson et al. 2001; Thompson & Ollason 2001). Our results highlighted a positive relationship between snow petrel breeding success and SIE. Moreover, Barbraud & Weimerskirch (2001a,b) showed that the overall breeding success and fledging body condition of snow petrel were improved during the year with extensive sea-ice cover in July. Therefore, when SIE during winter increased, recruitment was enhanced and population size increased several years later.
Although SIE is a reasonable proxy for investigating the impact of Antarctic climate on population dynamics through its effect on food availability, it is only one manifestation of a host of associated processes that might explain variability in marine populations (Nicol et al. 2000). However, using a global climate index (Stenseth et al. 2003), the SOI, we showed an environmental influence on populations at different lags, and on breeding success for both southern fulmar and emperor penguin.
The number of breeding pairs was positively related with SOI at a lag of 9 years for southern fulmars, and at a lag of 5 years for emperor penguins. These lags correspond for both species to the age at first breeding attempt (Mougin & Vanbeveren 1979; Jenouvrier et al. 2003). During negative SOI, reduced SIE and warm SST negatively affect the abundance of krill (Loeb et al. 1997), a major prey species for emperor penguins and southern fulmars but not for snow petrels (Offredo & Ridoux 1986; Ridoux & Offredo 1989; Kirkwood & Robertson 1997). Therefore, in years of lower SOI and reduced SIE, juvenile inexperienced birds may have difficulties in finding food, which could decrease the number of recruits several years later. Similarly, emperor penguin breeding success was negatively affected by warm events.
Emperor penguin population was also strongly positively related to SOI without lag, especially between 1975 and 1995. The dramatic decrease in the emperor penguin population during 1975 to 1980 was thus probably related to the high decrease of SOI, and the reduced winter SIE, suggesting that this species may be very susceptible to environmental variability, and particularly to sudden changes in their environment. The mechanism proposed by Barbraud & Weimerskirch (2001a) was a high decrease in adult survival linked to warm events (higher SST and lower SIE). Southern fulmars and snow petrels showed their lowest number in 1976, but they are able to skip reproduction, and seem to be less sensitive to major changes in their environment.
Most of the demographic parameters and environmental variables are related, but showed significant shift in their correlation. The majority of changes started at the end of the 1970s and at the beginning of the 1980s. These results are consistent with the variability in the demographic and environmental times-series described above, and highlight major changes around the 1980s. Based on the abrupt change of the SOI during 1975 and 1979, with an amplitude never experienced since 1866, and the dramatic 50% decrease in the emperor penguin population starting in 1975, we suggest that a major regime shift occurred from 1975.
In conclusion, after a significant change during the late 1970s, the Antarctic environmental factors have shifted into a relatively stable, new state since the end of the 1980s. In response to this shift in climate, the demographic parameters of three Antarctic seabird species have also changed significantly in their cyclic periodicities and relations with the environmental variables, although the causal mechanisms underlying such changes remain unclear. This regime shift around 1980 may have affected the dynamics of the Southern Ocean, but more work is needed to understand the demographic and ecological mechanisms that could explain the changes in correlation patterns observed during this shift. For this, in situ observations of the foraging tactics of the Antarctic seabirds together with their prey are necessary.
Acknowledgments
We acknowledge all the wintering fieldworkers involved in the long-term monitoring programmes in Terre Adélie since 1963, and Dominique Besson for her constant support and help in the management of the database. This long-term study was supported by Expéditions Polaires Françaises, by Institut Paul Emile Victor (Programme IPEV no. 109), and by Terres Australes et Antarctiques Françaises.
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
Supplementary Material
References
- Barbraud C, Chastel O. Early body condition and hatching success in the snow petrel Pagodroma nivea. Polar Biol. 1999;21:1–4. [Google Scholar]
- Barbraud C, Weimerskirch H. Emperor penguins and climate change. Nature. 2001;411:183–186. doi: 10.1038/35075554. [DOI] [PubMed] [Google Scholar]
- Barbraud C, Weimerskirch H. Contrasting effects of the extent of sea-ice on the breeding performance of an Antarctic top predator, the snow petrel Pagodroma nivea. J. Avian Biol. 2001;32:297–302. [Google Scholar]
- Barbraud C, Weimerskirch H, Guinet C, Jouventin P. Effect of sea-ice extent on adult survival of an Antarctic top predator, the snow petrel Pagodroma nivea. Oecologia. 2000;125:483–488. doi: 10.1007/s004420000481. [DOI] [PubMed] [Google Scholar]
- Berryman A. Oxford University Press; Oxford: 2002. Population cycles. The case for trophic interactions. [Google Scholar]
- Chastel O, Weimerskirch H, Jouventin P. High annual variability in reproductive success and survival of an Antarctic seabird, the snow petrel Pagodroma nivea. Oecologia. 1993;94:278–285. doi: 10.1007/BF00341328. [DOI] [PubMed] [Google Scholar]
- Croxall J.P. Southern ocean environmental changes, effects on seabird, seal and whale populations. Phil. Trans. R. Soc. B. 1992;338:319–328. [Google Scholar]
- Croxall J.P, Trathan P.N, Murphy E.J. Environmental change and Antarctic seabirds populations. Science. 2002;297:1510–1514. doi: 10.1126/science.1071987. [DOI] [PubMed] [Google Scholar]
- Drent R.H, Dann S. The prudent parent: energetic adjustments in avian breeding. Ardea. 1980;68:225–252. [Google Scholar]
- Fraser W.R, Hofmann E.E. A predator's perspective on causal links between climate change, physical forcing and ecosystem response. Mar. Ecol. Prog. Ser. 2003;265:1–15. [Google Scholar]
- Guinet C, Chastel O, Koudil M, Durbec J.P, Jouventin P. Effects of warm sea-surface temperature anomalies on the blue petrel at the Kerguelen Islands. Proc. R. Soc. B. 1998;265:1001–1006. http://dx.doi.org/10.1098/rspb.1998.0390 [Google Scholar]
- Hare S.R, Mantua N.J. Empirical evidence for a North Pacific regime shifts in 1977 and 1989. Prog. Oceanogr. 2000;47:103–145. [Google Scholar]
- Hughes L. Biological consequences of global warming, is the signal already apparent? Trends Ecol. Evol. 2000;15:56–61. doi: 10.1016/s0169-5347(99)01764-4. [DOI] [PubMed] [Google Scholar]
- Jenouvrier S, Barbraud C, Weimerskirch H. Effects of climate variability on the temporal population dynamics of southern fulmars. J. Anim. Ecol. 2003;72:576–587. doi: 10.1046/j.1365-2656.2003.00727.x. [DOI] [PubMed] [Google Scholar]
- Jenouvrier S, Barbraud C, Cazelles B, Weimerskirch H. Modelling population dynamics of seabirds: importance of the effects of climate fluctuations on breeding proportions. Oikos. 2005;108:511–522. [Google Scholar]
- Kirkwood R, Robertson G. The foraging ecology of female emperor penguins in winter. Ecol. Monogr. 1997;67:155–176. [Google Scholar]
- Klvana I, Berteaux D, Cazelles B. Porcupine feeding scars and climatic data show ecosystem effects of the solar cycle. Am. Nat. 2004;164:283–297. doi: 10.1086/423431. [DOI] [PubMed] [Google Scholar]
- Kwok R, Comiso J.C. Southern ocean climate and sea ice anomalies associated with the southern oscillation. J. Clim. 2002;15:487–501. [Google Scholar]
- Lebreton J.-D, Clobert J. Birds population dynamics, management, and conservation: the role of mathematical modelling. In: Perrins C.M, Lebreton J.D, Hirons G.J.M, editors. Bird population studies: relevance to conservation and management. Oxford University Press; Oxford: 1991. pp. 105–125. [Google Scholar]
- Liu J, Yuan X, Rind D, Martison D.G. Mechanism study of the ENSO and southern high latitude climate teleconnections. Geophys. Res. Lett. 2002;29:24.1–24.3. [Google Scholar]
- Loeb V.J, Siegel V, Holm-Hansen O, Hewitt R, Fraser W, Trivelpiece S.G. Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature. 1997;387:897–900. [Google Scholar]
- Masson-Delmotte V, Delmotte V, Morgan V, Etheridge D, Van Ommen T, Tartarin S, Hoffmann G. Recent southern Indian Ocean climate variability inferred from a Law Dome ice core, new insights for the interpretation of coastal Antarctic isotopic records. Clim. Dyn. 2003;21:153–166. http://dx.doi.org/10.1007/s00382-003-0321-9 [Google Scholar]
- Mougin J.L, Vanbeveren M. Structure et dynamique de la population de Manchots empereurs Aptenodytes forsteri de la colonie de l'archipel de Pointe Géologie, Terre Adélie. C. R. Acad. Sci. 1979;289:157–160. [Google Scholar]
- Nicol S, Pauly T, Bindoff N.L, Wright S, Thiele D, Hosie G.W, Strutton P.G, Woehler E. Ocean circulation off east Antarctica affects ecosystem structure and sea-ice extent. Nature. 2000;406:504–507. doi: 10.1038/35020053. [DOI] [PubMed] [Google Scholar]
- Offredo C, Ridoux V. The diet of Emperor penguins Aptenodytes forsteri in Adélie Land, Antarctica. Ibis. 1986;128:409–413. [Google Scholar]
- Park Y.-H, Gambéroni L. Large-scale circulation and its variability in the south Indian Ocean from TOPEX/POSEIDON altimetry. J. Geophys. Res. 1995;100:24 911–24 929. [Google Scholar]
- Park Y.-H, Roquet F, Vivier F. Quasi-stationary ENSO wave signals versus the Antarctic Circumpolar Wave scenario. Geophys. Res. Lett. 2004;31:L09315. http://dx.doi.org/10.1029/2004GL019806 [Google Scholar]
- Percival D.P. On the estimation of the wavelet variance. Biometrika. 1995;82:619–631. [Google Scholar]
- Reid K, Croxall J.P. Environmental response of upper trophic-level predators reveals a system change in an Antarctic marine ecosystem. Proc. R. Soc. B. 2001;268:377–384. doi: 10.1098/rspb.2000.1371. http://dx.doi.org/10.1098/rspb.2000.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridoux V, Offredo C. The diets of five summer breeding seabirds in Adelie Land, Antarctica. Polar Biol. 1989;9:137–145. [Google Scholar]
- Scheffer M, Carpenter S.R. Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol. Evol. 2003;18:648–656. http://dx.doi.org/10.1016/j.tree.2003.09.002 [Google Scholar]
- Spiegel M.R. McGraw-Hill; New York: 1972. Theory and problems of statistics. [Google Scholar]
- Stenseth N.C, Ottersen G, Mysterud A, Hurell J.W, Chan K.S, Lima M. Ecological effects of climate fluctuations. Science. 2002;297:1292–1295. doi: 10.1126/science.1071281. [DOI] [PubMed] [Google Scholar]
- Stenseth N.C, Ottersen G, Hurell J.W, Mysterud A, Lima M, Chan K.S, Yoccoz N.G, Adlandsvik B. Studying climate effects on ecology through the use of climate indices, the North Atlantic Oscillation, El Nino Southern Oscillation and beyond. Proc. R. Soc. B. 2003;270:2087–2096. doi: 10.1098/rspb.2003.2415. http://dx.doi.org/10.1098/rspb.2003.2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P.M, Ollason J.C. Lagged effects of ocean climate change on fulmar population dynamics. Nature. 2001;413:417–420. doi: 10.1038/35096558. [DOI] [PubMed] [Google Scholar]
- Torrence C, Compo G.P. A practical guide to wavelet analysis. Bull. Am. Meteorol. Soc. 1998;79:61–78. [Google Scholar]
- Walther G.R, Post E, Convey P, Menzel A, Parmesan C, Beebee T.J.C, Fromentin J.M, Hoegh-Guldberg O, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- Weimerskirch H, Inchausti P, Guinet C, Barbraud C. Trends in bird and seal populations as indicators of a system shift in the southern ocean. Ant. Sci. 2003;15:249–256. [Google Scholar]
- Wilson P.R, Ainley D.G, Nur N, Jacobs S.S, Barton K.J, Ballard J.C, Comiso J.C. Adélie penguin population change in the pacific sector of Antarctica: relation to sea-ice extent and the Antarctic Circumpolar Current. Mar. Ecol. Prog. Ser. 2001;213:301–309. [Google Scholar]
- Zwally H.J, Comiso J.C, Parkinson C.L, Cavalieri D.J, Gloersen P. Variability of Antarctic sea ice 1979–1998. J. Geophys. Res. 2002;107:9.1–9.18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.