Abstract
Maternal and physical factors play a significant role in animal life-history variability, which means that large scale climate change has the potential to affect the size and dynamics of animal populations indirectly through maternal investment and directly through conditions that animals are exposed to. However, little is known about the effects of large-scale oceanographic events such as the El-Niño southern oscillation (ENSO) that influence productivity in the Southern Ocean and the abundance, quality and distribution of prey. The possible mechanisms by which physical factors and primary productivity could influence life-history traits, such as survival of apex predators, includes direct influences such as food availability and foraging success and indirect influences such as stored maternal investment and resource transfer during lactation. Here, we quantify the relative contribution of maternal investment and climate conditions at remote foraging sites to survival in the first year of life for southern elephant seals. We present evidence linking climate (ENSO) and variations in a key demographic parameter—first-year survival—and demonstrate that survival was highest during ENSO events and that the ability of mothers to store and acquire resources, which is typically related to ocean productivity, is the most important determinant of survival in the first year. This functional link provides valuable insights that can be used to model the responses of the seal populations to climate change scenarios.
Keywords: environmental variability, survival, maternal investment
1. Introduction
An evaluation of the links between regular climatic variations, such as El Niño southern oscillation (ENSO), and predator demography allows us to assess, from relatively short-term datasets, the effects of long-term climate change on the life-history of animals. In the first instance, this requires the identification of the links between oceanic and atmospheric events in distant parts of the globe (Gloersen 1995; Kwok & Comiso 2002) because predators such as southern elephant seals (SESs) range widely across the worlds ocean basins (Hindell & McMahon 2000). ENSO events affect the physical environment, including the sea-ice (Carleton & Carpenter 1990; Ledley & Huang 1997; Turner 2004) and the biological environment (Wilson et al. 2001; Atkinson et al. 2004) in Antarctica where elephant seals forage. In the case of elephant seals from Macquarie Island, whose foraging zones are located near the Ross Sea (Hindell et al. 1991; Slip et al. 1994), as much as 76% of the variation in sea-ice extent can be explained by ENSO (Arrigo & van Dijken 2004). The sea-ice environment is an important part of the southern ocean (SO) ecosystem because all biological activity is associated with the physical components of the sea-ice in the SO (Arrigo et al. 1997; Tynan 1998; Fraser & Hofmann 2003; Atkinson et al. 2004). In this general region, SES foraging zones are commonly concentrated around oceanic frontal zones and determined by the prey distribution within these zones (Hindell et al. 1999; Field et al. 2001, 2004), much of which is dictated by the thermal structure of the water column (Sokolov & Rintoul 2002) which in turn is influenced by ENSO through pressure and sea temperature changes (Carleton & Carpenter 1990; Ledley & Huang 1997; Turner 2004). The thermal structure and the long-term predictability of the relatively high productivity in these areas means that elephant seals have adopted a non-random foraging strategy so that they centre their foraging effort in areas to which they habitually return (Bradshaw et al. 2004), even though the position and the productivity around these frontal zones changes annually (Sokolov & Rintoul 2002; Constable et al. 2003). Such a strategy may have evolved because of the evolutionary advantage elephant seals gain from the (generally) higher productivity associated with these variable sites (Bradshaw et al. 2004), which enables female seals to, on average (i.e. over their lifetimes), produce and rear offspring that have the highest probability of surviving by producing offspring with higher than average weaning masses (McMahon et al. 2000, 2003; Bradshaw et al. 2004). We use the variability in the productivity of SESs foraging zones quantified as: (i) weaning mass (Fedak et al. 1996; Burton et al. 1997; Crocker et al. 2001) and (ii) ENSO as enumerated by the southern oscillation index (SOI) to assess the effect of varying climates on a key demographic parameter—survival—and to study the functional relationship between environmental conditions and animal demography. ENSO was selected because it is an accessible and, therefore, easily quantifiable environmental perturbation that is known to affect productivity in the SO through variations in, for example, ocean temperature and winter sea-ice extent. Variations (in particular, variations in sea-ice extent) mediated by ENSO are biologically significant because: (i) productivity in the SO is allied to sea-ice extent (Loeb et al. 1997; Atkinson et al. 2004) and (ii) the ecological influence of sea-ice extent has been demonstrated at all trophic levels (Ross et al. 1996; Fraser & Hofmann 2003). Thus, environmental perturbations such as ENSO events are likely to influence higher order predator life history traits such as survival.
The survival of naive seals (i.e. less than 1 year) was quantified because: (i) foraging success in the first year of life is not influenced by previous foraging experience, as has been demonstrated for adult elephant seals (Bradshaw et al. 2004) and (ii) recently weaned seals have finite stored resources (McMahon et al. 2000; McConnell et al. 2002) with which to successfully locate patchily distributed foraging areas and food (Constable et al. 2003) after which they will starve. Moreover, naive seals require more resources, at least in mass specific terms, for maintenance and growth (McLaren 1993) than adults and therefore environmental variability is more likely to affect juvenile survival than adult survival (Gaillard et al. 1998). Indeed, the consequences of such changes in survival, especially of young animals, are often far-reaching and have been associated with changes in population structure and size (Caughley 1977; Hindell 1991). Understanding the relationship between environmental conditions and the demography of an apex predator, such as the SES, is vital to our understanding and management of the SO ecosystem (Fraser & Hofmann 2003; Hindell et al. 2003), especially given the present predictions of dramatic global climate change in the next few decades (Levitus et al. 2000).
2. Materials and methods
At the Macquarie Island isthmus (54°30′S, 158°50′E) in the SO, 6504 recently weaned SES pups were branded and weighed from 1993 to 1999. Both rear flanks were branded with a four-character alphanumeric brand that uniquely identified individual seals (McMahon et al. 1999).
Daily searches of the isthmus beaches and tussock areas (main study area; McMahon et al. 1999, 2000) and once monthly searches of the entire island beaches and tussock areas were made to resight (recapture) these marked seals over 8 years (1993–2001). The recapture rates varied between years (, p < 0.001) but this variation was taken into account (compensated for) when estimating seal survival by using the standard practise of including time-dependent recapture rates in the design matrices when estimating survival in the capture–mark–recapture programme—Mark (White & Burnham 1999). Capture-history matrices were constructed from the resight histories and used as input files for Mark (White & Burnham 1999). Mark provides survival (ϕ) and recapture (ρ) estimates under the Cormack–Jolly–Seber (CJS) model and under several models that appear as special cases of the CJS-model (White & Burnham 1999). Likelihood ratio tests within Mark were used to test between nested models (White & Burnham 1999). In this case, the model ϕ (first-year survival constant for all cohorts) ρ (time-dependent) was nested/constrained within the general model; ϕ (cohort-specific 1st-year survival) ρ (time-dependent).
To quantify the direct affect of environmental variation (ENSO) on the response variable (first-year survival), we constrained the survival model (White & Burnham 1999) using the annual SOI values as a proxy measure for ENSO (Deser & Wallace 1987). In so doing, the fit of the constrained (i.e. including the direct measure of environmental variation) model can be compared with the fits of unconstrained models that included the indirect estimates of environmental variability (weaning mass) using the general rules of model selection (Burnham & Anderson 2002). The amount of variation accounted for by the covariates, i.e. the direct estimation and quantification of the relative effects of environmental variability on first-year survival, was determined using an analysis of deviance (Burnham & Anderson 2002).
The SOI values we used (January–October, i.e. when the seals were at sea during their first foraging trips) are available from the Australian Bureau of Meteorology at http://www.bom.gov.au/climate/current/soihtm1.shtml. These SOI values are, therefore, a direct representation of the environmental conditions when the naive seals are at sea for the first time, e.g. seals branded in 1993 were foraging in 1994 and the survival anomaly for the 1993 cohort is related to the SOI anomaly in 1994 (January–October). The annual SOI anomaly was calculated by subtracting the mean annual January–October values from the 50 year (1950–2000) mean value for the January–October period. Similarly, the survival anomaly was the annual survival value subtracted from the mean of all the annual values. Sustained negative values of the SOI indicate ENSO (El-Niño) episodes (Deser & Wallace 1987) which are manifested in the SO as lower sea-level air pressure, cooler air temperatures above the ocean surface, cooler sea-temperatures and greater sea-ice extent in the Ross Sea (Kwok & Comiso 2002).
3. Results
The mean first-year survival estimate for each of the seven seal cohorts was 0.7461 (±s.e. 0.0035, 95% CI 0.7393 and 0.7528), from this and the first-year survival estimates for each of the cohorts (1993–1999; table 1) we calculated the annual survival anomaly (figure 1a). First-year survival varied significantly (, p < 0.0001) between cohorts and was highest for the 1993 and 1996 cohorts. There were two candidate models (i.e. ΔAIC < 2; Burnham & Anderson 2002) that described the variability in first year survival. The best model excluded the direct measure of environmental variable to which naive seals were exposed—SOI—but included wean-mass, i.e. the indirect measure of environmental variability, while the next best model included environmental variability (SOI) and wean mass. From these two models, the generic model and following Burnham & Anderson's (2002) analysis of deviance, we were able to determine that wean mass explained 88% (r2=0.877) of the variation in first-year survival and that SOI explained 12% (r2=0.123) of the variation in first-year survival. Seal survival in the first year of life was primarily dependent on wean mass (87.7%) and therefore maternal foraging success in the previous year is the fundamental determinant of survival (McMahon et al. 2000, 2003). However, while maternal investment was important for survival in the first year, environmental conditions to which naive seals were exposed were also important in determining survival (model ΔAIC<2), such that survival was highest during ENSO events (1993–1994 and 1996–1997) and lowest in between ENSO events (figure 1a,b), i.e. first-year survival of the 1993 and 1996 cohorts.
Table 1.
The mean (±standard error, s.e.) first-year survival estimates and the lower and upper 95% confidence intervals (CI) for seven cohorts of southern elephant seals at Macquarie Island.
| cohort | mean estimate | s.e. | lower 95% CI | upper 95% CI | mean wean mass (kg)±s.d. | sample (n) |
|---|---|---|---|---|---|---|
| 1993 | 0.8070 | 0.0101 | 0.7864 | 0.8260 | 118.17±26.10 | 1061 |
| 1994 | 0.7517 | 0.0099 | 0.7317 | 0.7707 | 117.41±26.43 | 585 |
| 1995 | 0.7622 | 0.0101 | 0.7419 | 0.7813 | 115.56±26.37 | 816 |
| 1996 | 0.7993 | 0.0092 | 0.7808 | 0.8167 | 117.36±26.75 | 962 |
| 1997 | 0.7627 | 0.0091 | 0.7444 | 0.7802 | 122.85±28.09 | 953 |
| 1998 | 0.7456 | 0.0086 | 0.7284 | 0.7621 | 119.11±28.78 | 1126 |
| 1999 | 0.7172 | 0.0089 | 0.6994 | 0.7343 | 120.03±27.01 | 1001 |
| all cohorts | 0.7461 | 0.0034 | 0.7393 | 0.7528 | 118.79±27.24 | 6504 |
Figure 1.
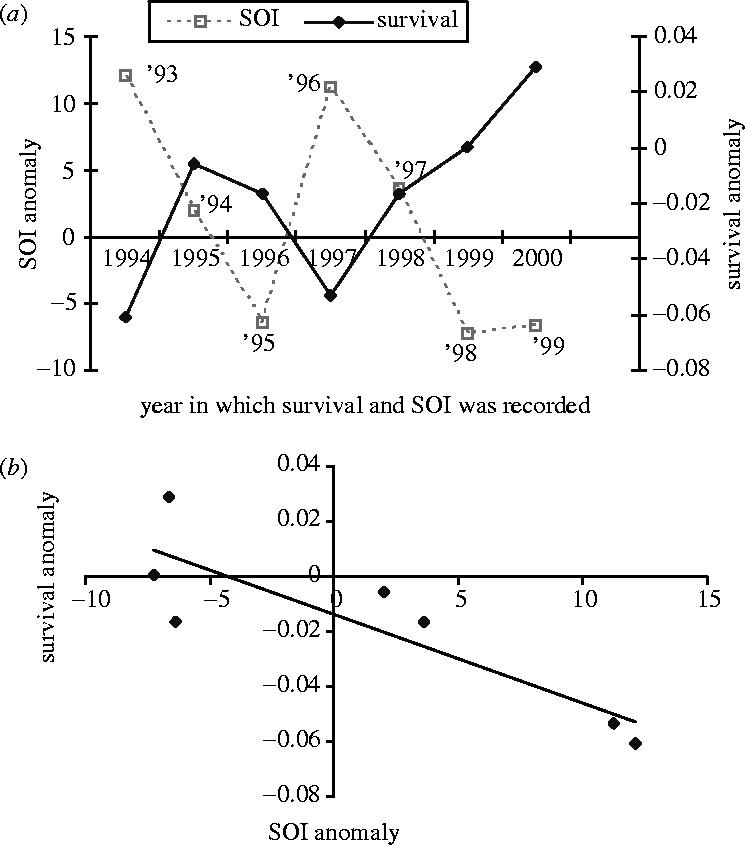
The general nature of the relationship between first-year survival (open squares) and the SOI (solid diamonds) recorded during January–October for each year of the first foraging trip for the seven cohorts (1993–1999) of southern elephant seals at Macquarie Island. The figure shows that survival is highest during ENSO events, i.e when the SOI is lowest. However, we recognize that the time-series is relatively short, making rigorous time-series analysis difficult. Nevertheless, even when we take account of the temporal autocorrelation within the two time-series and calculate the effective degrees of freedom (Pyper & Peterman 1998), the strong correlation coefficient (r=−0.868) remains robust (p=0.05). The data labels associated with the survival curve represent cohort membership for the seven seal cohorts we studied.
4. Discussion
Here, for the first time, we have shown a correlation between a quasi-cyclic event (ENSO as measured by the SOI) and an important demographic parameter (first-year survival) of a large Antarctic predator. For the first time, we have also quantified the relative importance of pre-breeding foraging conditions (during which time females accumulate and store resources) and the post-breeding foraging conditions that offspring are exposed to, to survival in the first year of life of an apex, albeit naive, predator. We showed that the conditions to which pregnant female seals were exposed and typically enumerated as weaning mass (Fedak et al. 1996; Arnbom et al. 1997; Crocker et al. 2001) were more important to survival in the first year than the conditions to which pups were directly exposed during their first foraging trips (88% versus 12%). This is because weaning masses (maternal foraging conditions) are a true reflection of environmental conditions rather than a reflection of the age/experience or foraging zone in which females feed (Fedak et al. 1996; Arnbom et al. 1997; Crocker et al. 2001). Ours is a key finding because it describes a functional response of an apex predator in Antarctica to environmental conditions that can be used to predicatively model population responses to climate changes, which is rarely achievable (Barbraud et al. 1999; Barbraud & Weimerskirch 2001; Wilson et al. 2001). The importance of our study lies in the quantification of the links between high order predators and environmental conditions, providing conservationists, ecologists and managers with the vital information, which in many instances is lacking (e.g. see Turner 2004 for a review), that they need to understand, conserve and manage ecosystems. Given that both the intensity and frequency of inter-annual sea-surface temperatures are expected to intensify with global warming (IPCC 2001), it follows that SES populations, in general, are likely to be negatively affected by the predicted warmer than average temperatures (Karl & Trenberth 2003).
ENSO can affect apex predators in several ways. Firstly, ENSO is an extreme Pacific Ocean climate state which is triggered from blocking weather systems in the west Pacific Region of the SO (Smith & Stearns 1993). These Antarctic blocking systems relate directly to sea-ice extent, which, in turn, results in the inter-annual variability of the position of ocean frontal zones and productivity (Sokolov & Rintoul 2002). Secondly, ENSO and its associated blocking systems may influence ocean stratification and the subsequent nutrient availability and therefore primary and secondary productivity which is available to apex predators (Richardson & Schoeman 2004; Sarmiento et al. 2004). Disruptions are therefore likely to alter nutrient balances and productivity in the SO between 45 and 60°S where elephant seals forage (e.g. Hindell et al. 2003; Field et al. 2004). Disruptions to the biological pump and the subsequent nutrient supply to the thermocline, where much biological productivity occurs (Sarmiento et al. 2004), will have further direct effects on elephant seals because elephant seals, concentrate their foraging effort at the thermocline (Boyd & Arnbom 1991; Slip 1997a,b). Variability in ocean productivity has been linked to variations in foraging performances in elephant seals by measuring mass gain in female elephant seals, which is highest when productivity is greatest and vice versa (Bradshaw et al. 2004). The positive relationship between ENSO events and mean first-year cohort survival has two possible explanations. First, weaning mass is an indirect cause because the resources that determine survival (McMahon et al. 2000, 2003) have been acquired and stored in the preceding year by the seal mothers and transferred to the pups during lactation (Fedak et al. 1996; Crocker et al. 2001). These resources are gathered on foraging trips to areas between the Antarctic Polar Front and the sub-Antarctic Front (Hindell et al. 1999; McConnell et al. 2002; Hindell et al. 2003), an area of high but variable productivity which is influenced by oceanographic phenomena such as the Antarctic Circumpolar Current and ENSO (Constable et al. 2003; Hosie et al. 2003). It is such variability that has been linked to variations in weaning masses (Vergani et al. 2001; McMahon et al. 2003) and first-year survival in elephant seals (McMahon et al. 2000). The difference in survival that results from weaning mass is probably owing to ontogeny differences, such that heavier pups are more precocious, make deeper and longer dives than lighter pups (Hindell et al. 1999) and are capable of spending longer periods (on average 10–11 days more) in search of food (McMahon et al. 2000; McConnell et al. 2002). The second reason is more direct: survival of the pups will be higher if prey are more plentiful or of higher quality. We suggest that prey may be easier to acquire (greater abundance), or more nutritious, during ENSO events because sea-ice is more abundant, and because the subsequent decay of the sea-ice in summer releases particulate and dissolved organic matter to the water column, therefore enhancing biogeochemical cycling as well as the seeding of the water column with nutrients and phytoplankton (Brierley & Thomas 2002). The effects of such greater abundances or quality of prey will be easily observed in elephant seals because the principal prey of SESs is squid and squid have short annual life-cycles (Waluda et al. 1999), i.e. lag effects are unlikely to dilute the immediate effects of increased productivity. Recently, it has been shown that krill stocks in the South Atlantic sector of the SO have decreased in accordance with an increase in water temperature (Atkinson et al. 2004) and if these decreases and climate changes were to propagate westward it is conceivable that elephant seal populations would be negatively affected as the decreases in krill numbers cascade up through the food chain.
Acknowledgments
We are indebted to our colleagues of all the Australian National Antarctic Research Expeditions (ANARE) to Macquarie Island from 1993–2002 that so ably assisted us in the field by marking seals and collecting resights in often miserable conditions. The Australian Antarctic Animal Ethics Committee (AAS 2265) and the Tasmanian Parks and Wildlife Service approved and permitted all aspects of this research at Macquarie Island. We would like to express our sincere gratitude to two anonymous reviewers for their positive and constructive comments that have improved the manuscript. We also thank Dr Graeme Hays for his insightful comments and assistance with the autocorrelation analysis.
Footnotes
School of Biological Sciences, Institute of Environmental Sustainability, University of Wales Swansea, Singleton Park, Swansea SA2 8PP, UK.
References
- Arnbom T, Fedak M.A, Boyd I.L. Factors affecting maternal expenditure in southern elephant seals during lactation. Ecology. 1997;78:471–483. [Google Scholar]
- Arrigo K.R, van Dijken G.L. Annual changes in sea-ice, chlorophyll a, and primary production in the Ross Sea, Antarctica. Deep Sea Res. II Topical Stud. Oceanogr. 2004;51:117–138. [Google Scholar]
- Arrigo K.R, Worthen D.L, Lizotte M.P, Dixon P, Dieckman G. Primary production in Antarctic sea-ice. Science. 1997;276:394–397. doi: 10.1126/science.276.5311.394. [DOI] [PubMed] [Google Scholar]
- Atkinson A, Siegel V, Pakhomov E, Rothery P. Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature. 2004;432:100–103. doi: 10.1038/nature02996. [DOI] [PubMed] [Google Scholar]
- Barbraud C, Weimerskirch H. Emperor penguins and climate change. Nature. 2001;411:183–186. doi: 10.1038/35075554. [DOI] [PubMed] [Google Scholar]
- Barbraud C, Weimerskirch H, Robertson G.G, Jouventin P. Size-related life history traits: insights from a study of snow petrels (Pagodroma nivea) J. Anim. Ecol. 1999;68:1179–1192. [Google Scholar]
- Boyd I.L, Arnbom T. Diving behaviour in relation to water temperature in the southern elephant seal: foraging implications. Polar Biol. 1991;11:259–266. [Google Scholar]
- Bradshaw C.J.A, Hindell M.A, Sumner M.D, Michael K. Loyalty pays: life-history consequences of fidelity to marine foraging areas by elephant seals. Anim. Behav. 2004 [Google Scholar]
- Brierley A.S, Thomas D.N. Ecology of southern ocean pack ice. Adv. Mar. Biol. 2002;43:171–276. doi: 10.1016/s0065-2881(02)43005-2. [DOI] [PubMed] [Google Scholar]
- Burnham K.P, Anderson D.R. Springer; New York: 2002. Model selection and inference: a practical information-theoretic approach. [Google Scholar]
- Burton H.R, Arnbom T, Boyd I.L, Bester M.N, Vergani D, Wilkinson I. Significant differences in the weaning mass of southern elephant seals from five sub-Antarctic islands in relation to population declines. In: Battaglia B, Valencia J, Watton D.W.H, editors. Antarctic communities: species structure and survival. Cambridge University Press; Cambridge: 1997. pp. 335–338. [Google Scholar]
- Carleton A.M, Carpenter D.A. Satellite climatology of ‘polar lows’ and broadscale climatic associations for the southern hemisphere. Int. J. Climatol. 1990;10:219–246. [Google Scholar]
- Caughley G. Wiley; London: 1977. Analysis of vertebrate populations. [Google Scholar]
- Constable A.J, Nicol S, Strutton P.G. Southern ocean productivity in relation to spatial and temporal variation in the physical environment. J. Geophys. Res. 2003;108(C4):8079. http://dx.doi.org/doi:10.1029/2001JC001270 [Google Scholar]
- Crocker D.E, Williams J.D, Costa D.P, Le Boeuf B.J. Maternal traits and reproductive effort in northern elephant seal. Ecology. 2001;82:3541–3555. [Google Scholar]
- Deser C, Wallace J.M. El-Niño events and their relation to the southern oscillation: 1925–1986. J. Geophys. Res. 1987;92:14 189–14 196. [Google Scholar]
- Fedak M.A, Arnbom T.A, Boyd I.L. The relation between the size of southern elephant seal mothers, the growth of their pups and the use of maternal energy, fat and protein during lactation. Physiol. Zool. 1996;69:887–911. [Google Scholar]
- Field I.C, Hindell M.A, Slip D.J, Michael K. Foraging strategies of southern elephant seals (Mirounga leonina) in relation to frontal zones and water masses. Antarct. Sci. 2001;13:371–379. [Google Scholar]
- Field I.C, Bradshaw C.J.A, Burton H.R, Hindell M.A. Seasonal use of oceanographic and fisheries management zones by juvenile southern elephant seals (Mirounga leonina) from Macquarie Island. Polar Biol. 2004;27:432–440. [Google Scholar]
- Fraser W.R, Hofmann E.E. A predator's perspective on causal links between climate change, physical forcing and ecosystem response. Mar. Ecol. Prog. Ser. 2003;265:1–15. [Google Scholar]
- Gaillard J.-M, Festa-Blanchet M, Yoccoz N.G. Population dynamics of large herbivores: variable recruitment with constant adult survival. Trends Ecol. Evol. 1998;13:58–64. doi: 10.1016/s0169-5347(97)01237-8. [DOI] [PubMed] [Google Scholar]
- Gloersen P. Modulation of hemispheric sea-ice cover by ENSO events. Nature. 1995;373:503–506. [Google Scholar]
- Hindell M.A. Some life-history parameters of a declining population of southern elephant seals, Mirounga leonina. J. Anim. Ecol. 1991;60:119–134. [Google Scholar]
- Hindell M.A, McMahon C.R. Long distance movement of a southern elephant seal (Mirounga leonina) from Macquarie Island to Peter 1 ØY. Mar. Mamm. Sci. 2000;16:504–507. [Google Scholar]
- Hindell M.A, Burton H.R, Slip D.J. Foraging areas of southern elephant seals, Mirounga leonina, as inferred from water temperature data. Aust. J. Mar. Freshwater Res. 1991;42:115–128. [Google Scholar]
- Hindell M.A, McConnell B.J, Fedak M.A, Slip D.J, Burton H.R, Reijnders P.J.H, McMahon C.R. Environmental and physiological determinants of successful foraging by naive southern elephant seal pups during their first trip to sea. Can. J. Zool. 1999;77:1807–1821. [Google Scholar]
- Hindell M.A, Bradshaw C.J.A, Sumner M.D, Michael K, Burton H.R. Dispersal of female southern elephant seals and their prey consumption during the austral summer: relevance to management and oceanographic zones. J. Appl. Ecol. 2003;40:703–715. [Google Scholar]
- Hosie G.W, Fukuchi M, Kawaguchi S. Development of the southern ocean continuous plankton recorder survey. Prog. Oceanogr. 2003;58:263–283. [Google Scholar]
- IPCC. Cambridge University Press; Cambridge: 2001. Climate change 2001: synthesis report. [Google Scholar]
- Karl T.R, Trenberth K.E. Modern global climate change. Science. 2003;302:1719–1723. doi: 10.1126/science.1090228. [DOI] [PubMed] [Google Scholar]
- Kwok R, Comiso J.C. Southern ocean climate and sea-ice anomalies associated with the southern oscillation. J. Clim. 2002;15:487–501. [Google Scholar]
- Ledley T.S, Huang Z. A possible ENSO signal in the Ross Sea. Geophys. Res. Lett. 1997;24:3253–3256. [Google Scholar]
- Levitus S, Antonov J.I, Boyer T.P, Stephens C. Warming of the world ocean. Science. 2000;287:2225–2229. [Google Scholar]
- Loeb V, Siegel V, Holm-Hanson O, Hewitt R, Fraser W, Trivelpiece W, Trivelpiece S. Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature. 1997;387:897–900. [Google Scholar]
- McConnell B.J, Fedak M.A, Burton H.R, Engelhard G.H, Reijnders P.J.H. Movements and foraging areas of naive, recently weaned elephant seals pups. J. Anim. Ecol. 2002;71:65–78. [Google Scholar]
- McLaren I.A. Growth in pinnipeds. Biol. Rev. 1993;68:1–79. doi: 10.1111/j.1469-185x.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- McMahon C.R, Burton H.R, Bester M.N. First-year survival of southern elephant seals, Mirounga leonina, at sub-Antarctic Macquarie Island. Polar Biol. 1999;21:279–284. [Google Scholar]
- McMahon C.R, Burton H.R, Bester M.N. Weaning mass and the future survival of juvenile southern elephant seals, Mirounga leonina, at Macquarie Island. Antarct. Sci. 2000;12:149–153. [Google Scholar]
- McMahon C.R, Burton H.R, Bester M.N. A demographic comparison of two southern elephant seal populations. J. Anim. Ecol. 2003;72:61–74. [Google Scholar]
- Pyper B.J, Peterman R.M. Comparison of methods to account for autocorrelation in correlation analyses of fish data. Can. J. Fish. Aquat. Sci. 1998;55:2127–2140. [Google Scholar]
- Richardson A.J, Schoeman D.S. Climate impact on plankton ecosystems in the Northeast Atlantic. Science. 2004;305:1609–1612. doi: 10.1126/science.1100958. [DOI] [PubMed] [Google Scholar]
- Ross R.M, Hofmann E.E, Quetin L.B, editors. Foundations for ecological research west of the Antarctic Peninsula. Antarct. Res. Ser. American Geophysical Union; Washington, DC: 1996. [Google Scholar]
- Sarmiento J.L, Gruber N, Brzezinski M.A, Dunne J.P. High-latitude controls of thermocline nutrients and low latitude biological productivity. Nature. 2004;427:56–60. doi: 10.1038/nature02127. [DOI] [PubMed] [Google Scholar]
- Slip D.J. Diving and foraging behaviour of juvenile southern elephant seals from Heard Island. In: Hindell M.A, Kemper C, editors. Marine mammal research in the southern hemisphere volume 1: status ecology and medicine. Surrey Beatty and Sons; Chipping Norton: 1997. pp. 114–124. [Google Scholar]
- Slip D.J. University of Tasmania; Hobart: 1997. Foraging ecology of southern elephant seals from Heard Island, pp. 182. [Google Scholar]
- Slip D.J, Hindell M.A, Burton H.R. Diving behaviour of southern elephant seals from Macquarie Island: an overview. In: LeBoeuf B.J, Laws R.M, editors. Elephant seals: population ecology, behaviour, and physiology. University of California Press; Berkeley: 1994. pp. 253–270. [Google Scholar]
- Smith S, Stearns C. Antarctic pressure and temperature anomalies surrounding the minimum in the Souther Oscillation Index. J. Geophys. Res. 1993:98. [Google Scholar]
- Sokolov S, Rintoul S.R. Structure of the southern ocean fronts at 140°E. J. Mar. Syst. 2002;37:151–184. [Google Scholar]
- Turner J. The El Niño-southern oscillation and Antarctica. Int. J. Climatol. 2004;24:1–31. [Google Scholar]
- Tynan C.T. Ecological importance of the southern boundary of the Anarctic circumpolar current. Nature. 1998;392:708–710. [Google Scholar]
- Vergani D.F, Stanganelli Z.B, Bilenca D. Weaning mass variation of southern elephant seals at King George Island and its possible relationship with El Niño and La Niña events. Antarct. Sci. 2001;13:37–40. [Google Scholar]
- Waluda C.M, Trathan P.N, Rodhouse P.G.K. Influence of oceanographic variability on recruitment in the Illex argentinus (Cephalopoda: Ommastrephidae) fishery in the south Atlantic. Mar. Ecol. Prog. Ser. 1999;183:159–167. [Google Scholar]
- White G.C, Burnham K.P. Program Mark: survival estimation from populations of marked animals. Bird Stud. 1999;46:120–138. [Google Scholar]
- Wilson P.R, Ainley D.G, Nur N, Jacobs S.S, Barton K.J, Ballard G, Comiso J.C. Adélie penguin population change in the Pacific sector of Antarctica: relation to sea-ice extent and the Antarctic circumpolar current. Mar. Ecol. Prog. Ser. 2001;213:301–309. [Google Scholar]


