Abstract
We studied variation in plumage colour and life history in a population of tawny owls (Strix aluco) in southern Finland, using 26 years of data on individually marked male and female owls. Colour was scored on a semi-continuous scale from pale grey to reddish brown. Colour scoring was repeatable and showed a bimodal distribution (grey and brown morph) in both sexes. During the study period, colour composition was stable in the study population in both sexes. The sexes did not mate assortatively with respect to their colour. Colour was a highly heritable trait and was under selection. Grey-coloured male and female owls had a higher lifetime production of fledglings, and grey-coloured male (but not female) owls produced more recruits during their lifetime than brown individuals. Selection on colour was mediated through viability selection and not through fecundity selection. Our results reveal remarkably strong selection on a genetically determined phenotypic trait.
Keywords: natural selection, phenotypic polymorphism, mating system, quantitative genetics, fitness
1. Introduction
Phenotypic polymorphism (i.e. within-population variation in a trait across individuals independent of age and sex) is common in many taxa. Evolutionary biologists have long been interested in the persistence of genetically determined phenotypic polymorphism in natural populations (e.g. Ford 1945; Cooch 1961; Mayr 1963; Rockwell et al. 1985; Sinervo & Lively 1996; Krüger et al. 2001). Part of this interest centres upon the question whether the (often clearly visible) different morphs also vary in aspects other than their appearance. In particular, do morphs differ in their fitness, or is polymorphism neutral to selection?
In birds, plumage colour is a genetically determined phenotypic polymorphism, but it remains largely unclear whether variation in colour across individuals has any fitness consequences (Roulin 2004a). Strong fitness difference between colour morphs in terms of lifetime production of fledglings has been found in buzzards, where the intermediate morph produces about twice as many fledglings during its lifetime as the extreme morphs (Krüger & Lindström 2001). However, most studies have found that colour variation is associated with more subtle differences in fitness components (reviewed in Roulin 2004a). For example, in the Arctic skua, morphs differ in their age at first breeding (O'Donald 1983) and, in the barn owl, female spottiness signals her offspring's immunocompetence (Roulin et al. 2000). In the tawny owl, descriptive data show that lighter-coloured tawny owl females produce offspring of a higher somatic condition (Roulin et al. 2003), although a cross-fostering experiment indicates the opposite (Roulin et al. 2004). In addition, plumage polymorphism may have behavioural and fitness consequences. For example, colour morphs mate assortatively in the lesser snow goose (Cooke et al. 1995), Arctic skua (Phillips & Furness 1998), barn owl (Roulin 1999) and buzzard (Krüger et al. 2001). In the barn owl, light colour morphs differ in the composition of their prey species from dark morphs, suggesting that they differ in their hunting behaviour (Roulin 2004b).
Importantly, however, any association between colour and a component of fitness (such as age at first breeding or aspects of offspring condition) need not reflect overall selection pressures on colour. This is because variation in colour may be associated with different reproductive strategies that all amount to the same final fitness and are thus selectively equivalent in the long term. For example, notwithstanding differences in age at first breeding in the Arctic skua, no long-term fitness differences across morphs are detectable (e.g. Philips & Furness 1998). Hence, to show that plumage polymorphism is a non-neutral phenomenon, empirical examination of the consequences that morph has for an individual's lifetime fitness is needed.
Colour polymorphism in birds is phylogenetically most common in owls (Strigiformes), where one-third of all described species are polymorphic in colour (Galeotti et al. 2003). Tawny owls differ in their plumage colour from pale grey to red (König et al. 1999), but they are commonly viewed as two morphs: a grey and a brown morph. This variation in colour is found in all populations of tawny owls in Europe (Galeotti & Cesaris 1996; König et al. 1999). Tawny owl coloration probably has a genetic component (Wallin 1988), and is thought to be maintained by fluctuating selection pressures owing to small-scale spatial or temporal variation, alternately benefiting the light and darker morphs (Galeotti & Cesaris 1996; Roulin et al. 2003, 2004). However, to date, no study has used individual-based data to describe selection on tawny owl colour using proper lifetime estimates of fitness, and little is known about the mating pattern or genetics behind colour polymorphism in this species. In this paper, we use 26 years of life-history data on a population in southern Finland where both sexes have been individually marked and colour scored to study (i) variation in colour and temporal stability of colour polymorphism, (ii) the genetic base of plumage colour, (iii) mating pattern with respect to coloration and (iv) selection on colour using lifetime fitness estimates.
2. Material and methods
(a) Study population and methods
Tawny owls were studied from 1978 onwards in a study area of about 250 km2 in west Uusimaa, southern Finland (60°15′ N, 24°15′ E). About 120 nest-boxes or other cavities were available for breeding. The area consisted of a mixture of agricultural land and spruce-dominated or mixed forests. A territory was considered occupied in early spring if an individual or both members of a pair responded to a playback recording. Regular checks of all nest-boxes and other cavities suitable for breeding were performed. All nests within the study area were located each year. The laying of at least one egg was considered a breeding attempt. Annually, between six and 34 pairs were present, although not all of them attempted to breed each year.
Female owls were caught on their nest soon after the nestlings hatched. Male owls were caught at the same time by using a swingdoor trap attached to the nest-box (see Saurola 1987). All offspring at the age of 2–3 weeks, and all unringed adults that were handled, were ringed with a numbered aluminium ring that allowed individual identification.
(b) Colour scoring
An individual's colour was scored using a modification of the approach of Savolainen (1978). This scoring method focuses on one aspect of tawny owl colour; namely, that caused by phaeomelanins (brown or red pigments) in different parts of the plumage. A higher score indicated more brown or red pigmented feathers. Four parts were colour scored: facial disc (1 point, less than 20% of feathers pigmented; 2 points, 20–40% pigmented; 3 points, 40% or more pigmented), back (1–4 points), breast (1–2 points) and the general appearance including wings, tail and head (1–5 points). Hence, the total colour score could vary from 4 (a pale grey-coloured individual virtually without brown pigments) to 14 (red-coloured individual). All colour scoring was done by either K.A. or T.K. or both. In total, the dataset on colour scores consisted of 449 observations on a male and 478 observations on a female, out of 605 breeding attempts. For female and male owls whose colour was scored in more than one season, repeatability of the colour score was calculated as described by Lessells & Boag (1987). For each individual, the average colour score over all times it was scored was used as an indicator of its colour (henceforth termed colour). This resulted in 190 male and 212 female owls of known colour. This method of scoring colour is not typically used; instead, tawny owls are categorized as either grey or brown. Therefore, we a posteriori categorized individuals into these two categories to make our results more comparable with other studies.
(c) The heritability of colour
Because colour was scored only on adults, we considered heritability by comparing the phenotypes of parents and their offspring that were recruited back into the breeding population. Only pairs where both parents were colour scored were included in the analysis. For parents that had more than one offspring recruited, the mean colour of their offspring was used (sexes combined). Colour scores of offspring and parents were each standardized to zero mean and unit standard deviation (Roff 1997). Heritability was calculated as the slope of a regression of standardized mean offspring colour score on standardized mid-parent colour score (Roff 1997).
(d) Mating pattern with respect to colour
We first tested whether there were systematic differences across female owls in their mate choice with respect to colour by calculating—for females that had paired with more than one male—the repeatability of her partner's colour. We further assessed assortative mating by testing whether the colour of a female was dependent on that of her mate. For female owls that had paired with more than one male during their breeding life, we randomly selected one of her male partners for analysis. To make sure that long-lived individuals did not have a higher probability to be recognized as a mate, we used a randomization approach that was independent of how many times a female had paired with her respective partners; that is, for m different male partners, the probability per male to be included in the analysis was 1/m. We categorized each male–female pair as grey–grey, grey–brown, brown–grey or brown–brown, and tested whether the observed proportions differed from random by a 2×2 table χ2 test.
(e) Quantifying lifetime individual fitness
An individual was assumed to have died if it had not been found breeding for 2 consecutive years. This criterion is justified because breeding tawny owls in Finland seldom move territory (Saurola 1987). In the selection analyses, we considered all individuals that were last observed before 2001. The complete life history of these individuals was therefore known, and almost all offspring had presumably recruited into the breeding population. As estimates of fitness, we calculated an individual's lifetime fledgling production (LFP; the sum of all fledglings produced during an individual's lifetime), and an individual's lifetime recruit production (LRP; the sum of all offspring of both sexes produced during an individual's lifetime that were recruited back into the breeding population). Although there are other estimates of fitness that take into account reproductive timing (e.g. Brommer et al. 2002), empirical studies have shown that LFP and LRP perform better than other such measures (Brommer et al. 2004).
To test whether selection operated mainly through the fecundity component or the viability component, we estimated viability as the number of years an individual was present in the breeding population (although not necessarily breeding each year), and its average fecundity as the average number of fledglings it produced per breeding attempt.
(f) Capture–recapture analysis
We analysed the complete dataset (1978–2003) on colour-scored male and female owls in the breeding population using the Cormack–Jolly–Seber (CJS) model (e.g. Jolly 1965), implemented by the program Mark (http://www.cnr.colostate.edu/~gwhite/mark/mark.htm). The CJS model separates survival probability from capture probability using a maximum-likelihood approach. Male and female owls were analysed separately because the high mate fidelity of the tawny owl increases the probability of observing a male–female pair whose partners are non-independent of each other, which violates one of the assumptions behind the CJS model. We categorized individuals as belonging to one of two colour morphs (see below). We followed the approach outlined by Cooch & White (2001). In brief, both survival probabilities (ϕ) and recapture probabilities (p) can be either constant (.), colour dependent (c), time dependent (t), or both colour and time dependent (c×t). All 16 possible permutations were fitted to the data. Models were ranked on the basis of their quasi-likelihood Akaike information criterion (QAIC) calculated as , where L is the likelihood of the model, K the number of parameters and n the effective sample size. The parameter ĉ estimates overdispersion, and was calculated as the ratio of observed model deviance over the average model deviance of 100 bootstrap simulations of the basic time-dependent survival and capture model [ϕ(t)p(t)]. Normalized weights were used to express the proportional support for each model compared with all alternative models. For more details on the rationale behind this modelling approach, see Burnham & Anderson (1998).
(g) Further statistics
Two-sample t-tests were calculated on the basis of separate variances in case the variance in the two groups was unequal. All values were presented as means±1 s.e.m., and all probabilities were two-tailed.
3. Results
(a) Colour polymorphism in the tawny owl: repeatability and heritability
There were 94 female and 94 male owls that were colour scored in more than one season (female, scored 2–11 times; male, 2–13 times). Colour scoring was highly repeatable (females r=0.90; males r=0.92). Colour varied across the entire observable range (from 4 to 14) in both male and female owls (figure 1). In both sexes, there was evidence of a bimodal distribution, where colour scores around 6 and around 12–13 were common, but there were few individuals of intermediate or of extreme colour (figure 1). Based on this distribution, we categorized individuals into two morphs, either grey (colour less than 10) or brown (colour 10 or more), thus recognizing two discrete morphs with approximately normal variation within each morph. In total, about 70% of the population was grey (70.5% (134/190) of male and 69.2% (146/211) of female owls).
Figure 1.
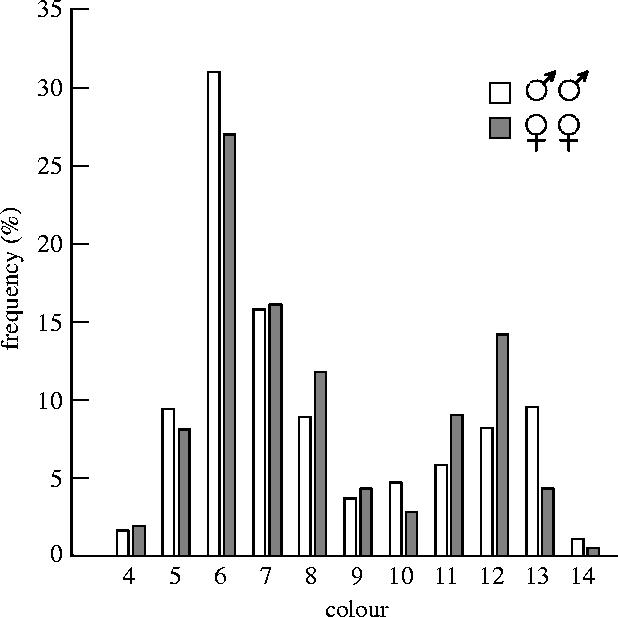
Frequency distribution of the colour of 190 male owls (open bars) and 212 female owls (shaded bars) in the breeding population in the period between 1978 and 2003. Colour is scored as the sum of points given for the degree of brown and red pigmentation in different body parts of the individual, and varies from 4 (light grey) to 14 (red).
There were 50 colour-scored pairs that recruited at least one offspring. Offspring colour strongly resembled the colour of their parents; colour was a heritable trait (figure 2; h2=0.72±0.15, t=4.7, n=50, p<0.001). Heritability was probably not inflated by the effect of a shared rearing environment because most parents only produced one recruit that was colour scored, and only 18% (9/50) of the mid-offspring values consisted of two offspring from the same nest.
Figure 2.
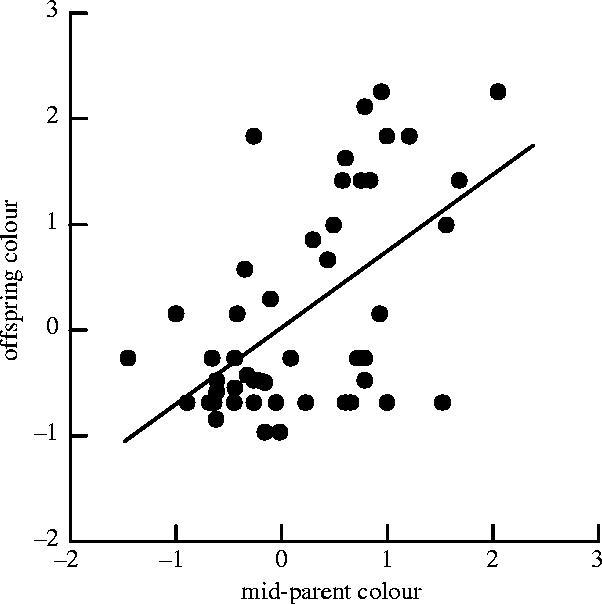
The relationship between offspring colour and parental colour. Plotted are the standardized values of the average colour of offspring recruited against the standardized average colour of their parents (n=50). The regression line indicates the heritability of colour (see text for coefficient and test).
There were no differences across years in the colour composition of the breeding population or any evidence of sexes being different in colour (generalized linear model on colour: year, F25,875=1.14, p=0.29; sex, F1,875=1.01, p=0.32). The colour of neither male nor female owls differed across years (year×sex: F25,875=0.77, p=0.79). Hence, colour polymorphism in this population was stable during the 26 years studied with respect to both sexes.
(b) Non-assortative mating
There were 422 breeding attempts where the colour of both the female and her male partner was scored. In most (67%, 283/422) of these attempts, a female paired with the same male. Breeding attempts between a female and a second male were not uncommon (24.9%, 105/422). Few female owls had breeding attempts with more than two different male owls during their lifetime (6.4% (27/422) of attempts with a third male and 1.7% (7/422) with a fourth male). There was little evidence of consistent differences across female owls in the choice of their mate's colour score (repeatability r=0.007, n=52 females). For each female, we randomly selected one of her male partners, following the criteria outlined above. We considered 201 breeding attempts between a female and male of known colour. There was no evidence that female and male owls formed a pair with respect to their colour, as 77.5% (110/142) of grey females and 72.9% (43/59) of brown females paired with a grey male (χ2=0.5, d.f.=1, p=0.5).
(c) Colour morph, individual fitness and breeding lifespan
On average, grey males produced 2.1 more fledglings (figure 3a; t84.7=2.0, p=0.05) and 0.4 more recruits (figure 3b; t101.1=2.2, p=0.03) than brown males. Grey females produced 2.0 more fledglings than brown ones during their lifetimes (figure 3a; t114.3=2.2, p=0.03), but did not differ in the number of recruits produced per lifetime (figure 3b; t104.6=0.9, p=0.36).
Figure 3.
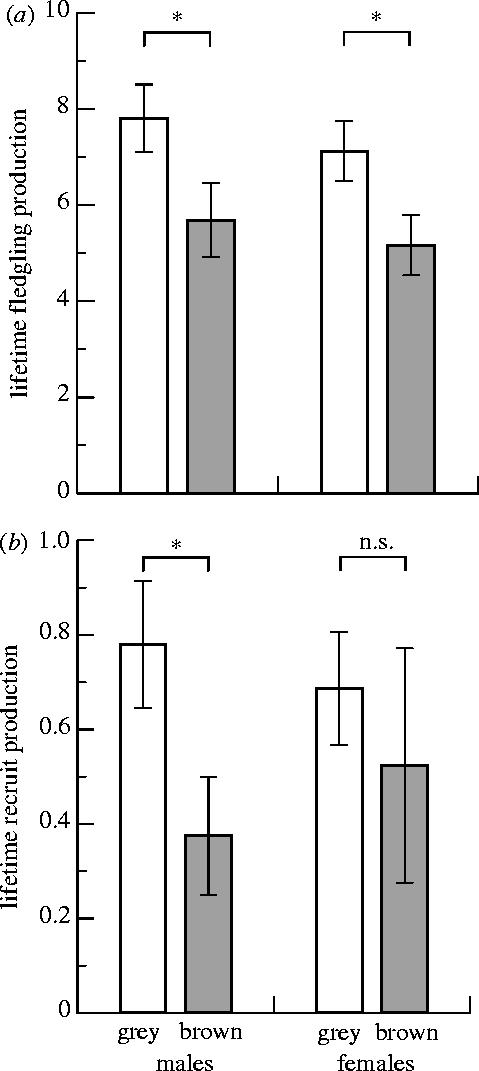
Estimates of individual fitness: (a) lifetime fledgling production and (b) lifetime recruit production of male and female grey morph (colour less than 10, light bar; male, n=100; female n=96) and brown morph (colour 10 or more, shaded bar; male, n=32; female, n=42). Graph denotes means±1 s.e.m. Significance of two-sample t-tests between the colour morphs are indicated (*p≤0.05; n.s.=not significant).
The effect of colour on fitness appeared to be mediated through viability selection as darker birds tended to have a shorter breeding lifespan (sexes combined: rs=−0.11, n=270, p=0.07), but did not produce fewer offspring per breeding attempt (rs=−0.03, n=270, p=0.58). Grey individuals had a longer breeding lifespan than brown individuals (grey males (n=100), 3.1±0.27; brown males (n=32), 2.0±0.32, t78.3=2.4, p=0.02; grey females (n=96), 2.7±0.3; brown females (n=42), 1.9±0.2, t127.9=2.7, p=0.009). Both colour morphs produced on average similar brood sizes (grey males, 3.1±0.1; brown males, 3.2±0.16; t130=−0.9, p=0.4; grey females, 3.1±0.1; brown females, 3.1±0.17; t136=0.19, p=0.9).
Roulin et al. (2003) found that capture probability was dependent on colour morph. Hence, any difference in fitness or breeding lifespan could therefore be an artefact of one colour morph being less likely to be recorded. We applied a capture–recapture analysis on the data for breeding male and female owls, categorized by colour morph (grey or brown). For both sexes, we found good support for colour-morph dependent survival probability (ϕ(c); table 1). The model that included colour-morph dependent survival and time-dependent capture probability [ϕ(c)p(t)] had in both sexes over 50% support, and received 41% (0.512/0.361) in male owls and 89% (0.654/0.346) in female owls higher support than a model without colour-dependent survival probability [ϕ(.)p(t)]. Moreover, for both sexes capture probability was only dependent on time (p(t); table 1) and not on colour in all models which, combined, had a support of 95% (male) and 100% (female). Hence, the observed differences in lifespan between the morphs were not an artefact of differential capture probability.
Table 1.
Results of a capture–recapture analysis for data on breeding male and female tawny owls between 1978 and 2003. (Owls are categorized by their colour (grey or brown). The analysis separates between survival probabilities (ϕ) and recapture probabilities (p) that can be either constant (.), colour dependent (c), time dependent (t), or both colour and time dependent (c×t). The best four (out of 16 possible) models are displayed in order of their fit to the data. Statistics given for each model include quasi-likelihood adjusted Akaike information criterion (QAIC), proportional support of the model (i.e. the QAIC weight), number of parameters and likelihood. Models are corrected for overdispersion ( for male and for female owls).)
| male owls | female owls | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| model | QAIC | support | n par | likelihood | model | QAIC | support | n par | likelihood |
| ϕ(c) p(t) | 776.5 | 0.512 | 28 | 407.3 | ϕ(c) p(t) | 836.3 | 0.654 | 28 | 414.2 |
| ϕ(.) p(t) | 777.2 | 0.361 | 27 | 410.3 | ϕ(.) p(t) | 837.6 | 0.346 | 27 | 417.7 |
| ϕ(c) p(.) | 781.3 | 0.047 | 3 | 466.1 | ϕ(t) p(t) | 858.0 | 0.000 | 51 | 380.5 |
| ϕ(.) p(.) | 782.2 | 0.031 | 2 | 469.0 | ϕ(t) p(.) | 861.9 | 0.000 | 27 | 442.0 |
4. Discussion
We have studied variation, genetics and selection of colour polymorphism in a wild population of tawny owls in southern Finland during a 26 year period. We have shown that plumage colour of tawny owls is an individual trait that can be reliably scored on a semi-continuous scale. Using this method of colour scoring, we have shown that tawny owls consist of two morphs (grey and brown) with some variation in the degree of pigmentation within each morph. Our method of scoring colour allowed us to use parent–offspring regression to show, for the first time (to our knowlege), that variation in tawny owl colour has a strong additive genetic component (h2=0.72). Furthermore, colour is not selectively neutral, but grey tawny owls of both sexes produce about 33% more offspring during their lifetime than brown ones, and grey males produce about twice as many recruits as brown males. To our knowledge, this is the first evidence that colour in the tawny owl has lifetime fitness consequences. Here, we discuss further implications of our results.
We used a semi-continuous colour score and found an apparent bimodal distribution of colour. This bimodal distribution fits well with a previously used categorization of colour morphs into two categories, grey and brown (e.g. Galeotti & Cesaris 1996). This bimodal distribution of colour is most probably a reflection of a relatively simple genetic mechanism where some individuals are highly pigmented across all body parts, whereas other individuals are much less pigmented. In general, it has been suggested that avian melanistic plumage coloration is determined by Mendelian (typically one locus biallelic) inheritance (Roulin 2004a). Indeed, molecular genetic work has shown, for three divergent avian lineages, that colour polymorphism in melanistic morphs is associated with variation in a single gene (Theron et al. 2001; Mundy et al. 2004). In owls, plumage colour can be considered a continuous trait with relatively little non-additive genetic variation (h2=0.65−0.85; Roulin & Dijkstra 2003; this paper). Nevertheless, additional work is needed to illucidate whether tawny owl plumage colour is caused by a single or relatively few major genes or whether it is of a more polygenic nature.
The fitness advantage of the grey morph is a result of viability selection. In fact, lifespan is the main determinant of lifetime reproductive success in a variety of taxa (e.g. Clutton-Brock 1988). Grey tawny owls do not produce more offspring per breeding attempt, but have a longer breeding lifespan than brown individuals, and therefore manage to produce more fledglings and recruits during their lifetime. Importantly, by capture–recapture analysis, we were able to show that it is indeed the survival probability that is colour-morph dependent in our population, and not, as Roulin et al. (2003) found, the capture probability. Survival differences across tawny owl morphs may stem from either external or internal factors.
First, colour may be associated with predation risk, where a dark individual suffers a higher predation risk than a lighter one. Tawny owls can be predated by several species of large birds of prey and owls (Mikkola 1983), and brown individuals may be more visible to predators, especially when the landscape is covered by snow during the winter months. However, darker morphs are typically thought to be less visible than light-coloured morphs under low-light conditions, which would prevail in boreal forests (Galeotti et al. 2003). Thus, it remains unclear how colour translates into predation risk under Finnish conditions.
Second, intrinsic (physiological) capacities may differ between colour morphs. In an Italian tawny owl population, the grey morph has a higher count of white blood cells (an indicator of immunocompetence) and a lower load of parasitaemia than the brown morph (Galeotti & Sacchi 2003). In the screech owl, the light morph has lower metabolic requirements and suffers a lower mortality than the dark morph during severe winters (Mosher & Henny 1976). In addition, light plumage feathers may themselves provide a better insulation capacity than dark feathers (Salomonsen 1972). Since this population of tawny owls lives at the northernmost margin of its European distribution, any potential immunonological, energetic and thermoregulatory benefits of the light morph may greatly facilitate its winter survival. Indeed, organisms are generally lighter in the northern parts of their range (Gloger's rule; Gloger 1833, c.f. Rensch 1960). Therefore, our results provide a good example of individual fitness advantages for the light morph in the northern margin of a genetically based, colour polymorphic species.
5. Conclusion
Directional selection on a strongly heritable trait (e.g. colour) should lead to monomorphy. Nevertheless, the colour composition of either male or female owls breeding in the population did not change markedly during the study period. Three factors appear to have contributed to this phenomenon. First, we found no evidence that tawny owls mate assortatively. Given the high heritability of colour, mating with a grey tawny owl partner would actually be advantageous for all morphs because the offspring of such a mating would, on average, have a higher fitness value than offspring of a mating with a brown morph. Fixation of colour will be deterred as long as a light individual has a reasonable probability to mate with a darker-coloured individual, and thereby produce offspring that are darker than itself. Our finding that tawny owls mate non-assortatively contrasts with other studies on avian colour-polymorphic species that often mate (dis)assortatively (Roulin 2004a). Second, selection (in terms of recruits) is sex dependent, which may reduce the total impact on the population. Third, the main hypothesis on the maintenance of tawny owl colour polymorphism concerns small-scale variation in either space (Galeotti & Cesaris 1996) or time (Roulin et al. 2003) that would benefit either the grey or the brown morph. Since we have shown that there is a net selection pressure for lighter-coloured individuals over a period of 26 years, temporal fluctuations in selection would have to be on a fairly long time-scale to be a major factor for the maintenance of colour polymorphism in this population. Immigration of individuals from other populations, where the selection regime would differ from our study population, is a factor that probably maintains colour variation. In fact, 89% (108/121) of all brown individuals come from outside our population, whereas less (78%, 221/281) grey individuals are immigrants (, p=0.01). However, a larger-scale perspective is needed to establish whether the brown morph is indeed performing better outside our population.
Acknowledgments
This is report number 5 of Kimpari Bird Projects. We thank the other members of KBP—Juhani Ahola, Pentti Ahola, Bo Ekstam, Arto Laesvuori and Martti Virolainen—for the many hours spent conducting fieldwork. We thank Jari Valkama and Seppo Niiranen from the Finnish Ringing Bureau for kindly providing data on tawny owl recruits. We are also grateful to Martin Fowlie, Jan Lindström, Kai Lindström, Patrik Karell and Hannu Pietiäinen for discussion and comments.
References
- Brommer J.E, Merilä J, Kokko H. Reproductive timing and individual fitness. Ecol. Lett. 2002;5:802–810. [Google Scholar]
- Brommer J.E, Gustafsson L, Pietiäinen H, Merilä J. Single-generation estimates of fitness as proxies for long-term genetic contribution. Am. Nat. 2004;163:505–517. doi: 10.1086/382547. [DOI] [PubMed] [Google Scholar]
- Burnham K.P, Anderson D.R. Springer; New York: 1998. Model selection and inference—a practical information theoretical approach. [Google Scholar]
- Clutton-Brock T.H, editor. Reproductive success. University of Chicago Press; Chicago: 1988. [Google Scholar]
- Cooch F.G. Ecological aspects of the blue-snow goose complex. Auk. 1961;78:72–89. [Google Scholar]
- Cooch E, White G. 2nd edn. 2001. Program MARK. A gentle introduction. See http://www.phidot.org/software/mark/docs/book/. [Google Scholar]
- Cooke F, Rockwell R.F, Lank D.B. Oxford University Press; Oxford: 1995. The snow geese of La Pérouse Bay. [Google Scholar]
- Ford E.B. Polymorphism. Biol. Rev. Camb. Phil. Soc. 1945;20:73–88. [Google Scholar]
- Galeotti P, Cesaris C. Rufous and grey colour morphs in the Italian tawny owl: geographical and environmental influences. J. Avian Biol. 1996;27:15–20. [Google Scholar]
- Galeotti P, Sacchi R. Differential parasitaemia in the tawny owl (Strix aluco): effects of colour morph and habitat. J. Zool. 2003;261:91–99. [Google Scholar]
- Galeotti P, Rubolini D, Dunn P.O, Fasola M. Colour polymorphism in birds: causes and functions. J. Evol. Biol. 2003;16:635–646. doi: 10.1046/j.1420-9101.2003.00569.x. [DOI] [PubMed] [Google Scholar]
- Gloger C.L. 1833. Das Abändern der Vögel durch Enfluss des Klima. Breslau: August Schulz. [Google Scholar]
- Jolly G.M. Explicit estimates from capture–recapture data with both death and immigration stochastic model. Biometrika. 1965;52:225–247. [PubMed] [Google Scholar]
- König C, Weick F, Becking J.-H. Pica Press; Sussex: 1999. Owls. A guide to the owls of the world. [Google Scholar]
- Krüger O, Lindström J. Lifetime reproductive success in common buzzard, Buteo buteo: from individual variation to population demography. Oikos. 2001;93:260–273. [Google Scholar]
- Krüger O, Lindström J, Amos W. Maladaptive mate choice maintained by heterozygote advantage. Evolution. 2001;55:1207–1214. doi: 10.1111/j.0014-3820.2001.tb00640.x. [DOI] [PubMed] [Google Scholar]
- Lessells C.M, Boag P.T. Unrepeatable repeatabilities: a common mistake. Auk. 1987;104:416–423. [Google Scholar]
- Mayr E. Harvard University Press; Cambridge, MA: 1963. Animal species and evolution. [Google Scholar]
- Mikkola H. T & AD Poyser; Calton, London: 1983. Owls of Europe. [Google Scholar]
- Mosher J.A, Henny C.J. Thermal adaptiveness of plumage color in screech owls. Auk. 1976;93:614–619. [Google Scholar]
- Mundy N.I, Badcock N.S, Hart T, Scribner K, Janssen K, Nadeau N.J. Conserved genetic basis for a quantitative plumage trait involved in mate choice. Science. 2004;303:1870–1873. doi: 10.1126/science.1093834. [DOI] [PubMed] [Google Scholar]
- O'Donald P. Cambridge University Press; Cambridge: 1983. The Arctic skua. A study of the ecology and evolution of a seabird. [Google Scholar]
- Philips R.A, Furness R.W. Polymorphism, mating preferences and sexual selection in Arctic skua. J. Zool. 1998;245:245–252. [Google Scholar]
- Rensch B. Columbia University Press; New York: 1960. Evolution above the species level. [Google Scholar]
- Rockwell R.F, Findlay C.S, Cooke F, Smith J.A. Life history studies of the lesser snow goose (Anser caerulescens caerulescens). IV. The selective value of plumage polymorphism: net viability, the timing of maturation, and breeding propensity. Evolution. 1985;39:178–189. doi: 10.1111/j.1558-5646.1985.tb04090.x. [DOI] [PubMed] [Google Scholar]
- Roff D.A. Chapman & Hall; New York: 1997. Evolutionary quantitative genetics. [Google Scholar]
- Roulin A. Nonrandom pairing by male barn owls (Tyto alba) with respect to a female plumage trait. Behav. Ecol. 1999;10:688–695. [Google Scholar]
- Roulin A. The evolution, maintenance and adaptive function of genetic colour polymorphism in birds. Biol. Rev. 2004;79:815–848. doi: 10.1017/s1464793104006487. [DOI] [PubMed] [Google Scholar]
- Roulin A. Covariation between plumage colour polymorphism and diet in the barn owl Tyto alba. Ibis. 2004;146:509–517. [Google Scholar]
- Roulin A, Dijkstra C. Genetic and environmental components of variation in eumelanin and phaeomelanin sex-traits in the barn owl. Heredity. 2003;90:359–364. doi: 10.1038/sj.hdy.6800260. [DOI] [PubMed] [Google Scholar]
- Roulin A, Jungi T.W, Pfister H, Dijkstra C. Female barn owls (Tyto alba) advertise good genes. Proc. R. Soc. B. 2000;267:937–941. doi: 10.1098/rspb.2000.1093. doi:10.1098/rspb.2000.1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulin A, Ducret B, Ravussin P.-A, Altwegg R. Female colour polymorphism covaries with reproductive strategies in the tawny owl Strix aluco. J. Avian Biol. 2003;34:393–401. [Google Scholar]
- Roulin, A., Bize, P., Ravussin, P.-A. & Broch, L. 2004 Genetic and environmental effects on the covariation between colour polymorphism and a life history trait. Evol. Ecol. Res.6, 1253–1260.
- Salomonsen F. Proceedings of the XV International Ornithological Congress, The Hague, The Netherlands. 1972. Zoogeographical and ecological problems in arctic birds. pp. 160–174. [Google Scholar]
- Saurola P. Mate and nest-site fidelity in ural and tawny owls. In: Nero R.W, Clark R.J, Knapton R.J, Hamre R.H, editors. Biology and conservation of northern forest owls, symposium proceedings. USDA forest service general technical report RM-142, Winnipeg, Manitoba. 1987. pp. 81–86. [Google Scholar]
- Savolaine, J. 1978 [On colour morphs in the tawny owl Lintuviesti]. 1, 5–8. [In Finnish.]
- Sinervo B, Lively C.M. The rock–paper–scissors game and the evolution of alternative male strategies. Nature. 1996;380:240–243. [Google Scholar]
- Theron E, Hawkins K, Bermingham E, Ricklefs R.E, Mundy N.I. The molecular basis of an avian plumage polymorphism in the wild: a melanocortin-1-receptor point mutation is perfectly associated with the melanic plumage morph of the bananaquit, Coereba flaveola. Curr. Biol. 2001;11:550–557. doi: 10.1016/s0960-9822(01)00158-0. [DOI] [PubMed] [Google Scholar]
- Wallin, K. 1988 Life history evolution and ecology in the tawny owl Strix aluco Ph.D. thesis, University of Göteborg, Sweden.


