Abstract
The first avian fossil recovered from high-temperature hot spring deposits is a three-dimensional external body mould of an American coot (Fulica americana) from Holocene sinters of Yellowstone National Park, Wyoming, USA. Silica encrustation of the carcass, feathers and colonizing microbial communities occurred within days of death and before substantial soft tissue degradation, allowing preservation of gross body morphology, which is usually lost under other fossilization regimes. We hypothesize that the increased rate and extent of opal-A deposition, facilitated by either passive or active microbial mediation following carcass colonization, is required for exceptional preservation of relatively large, fleshy carcasses or soft-bodied organisms by mineral precipitate mould formation. We suggest physico-chemical parameters conducive to similar preservation in other vertebrate specimens, plus distinctive sinter macrofabric markers of hot spring subenvironments where these parameters are met.
Keywords: hot spring deposits, silicification, fossilization, geomicrobiology, avian fossil
1. Introduction
We report the first avian body fossil described from ancient siliceous sinter deposits1. The American coot (Fulica americana) that we describe is preserved in a unique manner, in that 3D preservation of feathers and soft body morphology allows diagnosis of features normally lost during fossilization. Preservation of vertebrate remains in this manner has not been previously described, and suggests a suite of abiotic and biotic environmental conditions that have combined to bring about rapid specimen encrustation by opaline silica (opal-A) at a rate far outpacing organic decay. This encrustation served to stabilize the body fossil against collapse even as the microscopically porous nature of the mould allowed infiltration by chemical and biological agents of decay.
(a) Geologic setting
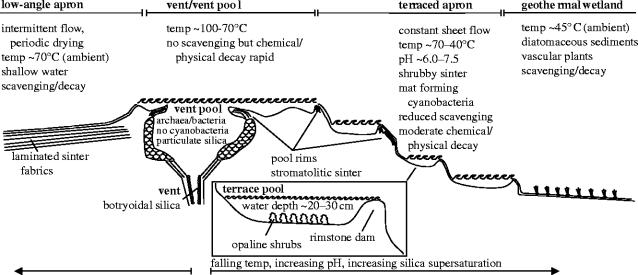
The coot was discovered in extinct and degrading hot spring deposits that lie above the upper member of the 600–640 Kyr Lava Creek Tuff (White et al. 1988; Christiansen 2001), southwest of the Norris Geyser Basin, Yellowstone National Park, Wyoming, USA (Christiansen 1975). The stepped topography of the silica sinter mound from which the specimen was collected indicates that the bird died and was preserved in either a hot spring vent-pool or (more likely) an associated high-temperature sinter terrace pool, as illustrated in figure 1. In such settings, scalding, chemical burns, CO/CO2/H2S asphyxiation or biological poisoning are potential causes of death.
Figure 1.
Diagram illustrating typical hot spring subenvironments, their physical/chemical characteristics, biota, diagnostic sediments and sediment fabrics and potential taphonomic fate of vertebrate carcasses (adapted from Guidry & Chafetz 2003b, fig. 4). Only the terrace pool environment has the requisite hydrodynamic, physico-chemical, biotic and sedimentological characteristics to have allowed mouldic preservation of the coot carcass.
Precise dating of the fossil or surrounding sediments is not available. We have constrained the maximum age of the specimen to Late Pleistocene or Early Holocene by identifying the encrusting silica phase as amorphous opal-A by X-ray diffraction studies (figure 2). The opal-A to opal-C/CT silica phase transformation of sinter occurs approximately 10 Kyr after deposition (Herdianita et al. 2000). Although no minimum age is implied by XRD data, hot springs are no longer active in the immediate area, and the degrading sinter deposits are covered with old-growth lodgepole pine (Pinus contorta) forest. The seral development stage (Despain 1990) and approximately 35 cm diameter trunks (Kaufmann 1996) of trees growing on the sinter mound from which the specimen was collected imply a conservative minimum age of approximately 300 years.
Figure 2.
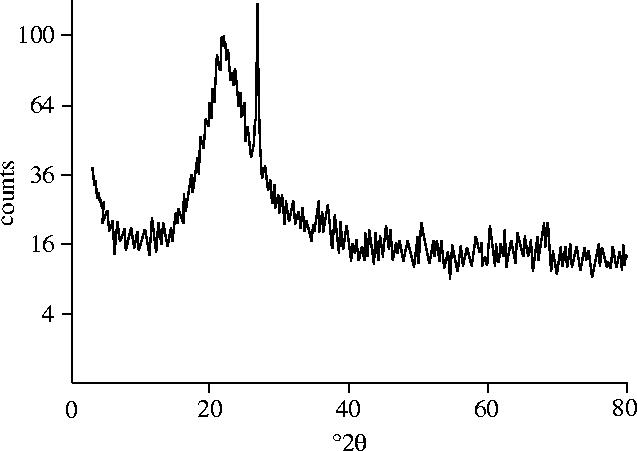
XRD diffractogram of sinter surrounding the bird indicates that the mineral is in the form of opal-A, thus constraining a maximum age of the specimen to approximately 10 000 years.
(b) Specimen description
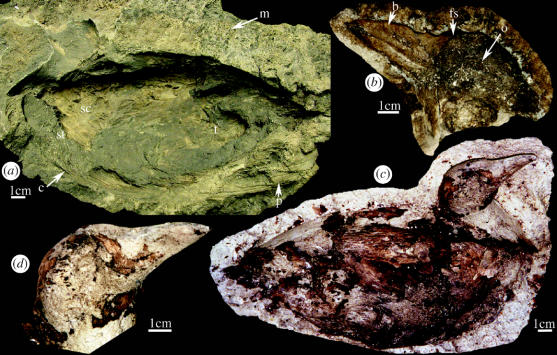
The soft body morphology of the coot is preserved as an external mould that appears to have completely encased the body of the bird prior to erosion (figure 3a). Figure 3b depicts detail of the head and neck of the bird, clearly representing aspects of facial anatomy. When silicon polymers were injected into the body mould, the original soft tissue anatomical features of the bird were perfectly represented in the resulting cast (figure 3c,d ). These included the torso, the right upper leg, right wing, exposed sternum and the head, including the fleshy facial shield, orbit, upper and lower mandibles of the bill and nostrils (figure 3d ). Based on the specimens size (estimated extended length from tip of tail to tip of bill, 32 cm), stout duck-like body with convex back, short tail and relatively short neck, short conical beak and fleshy facial shield extending onto forehead, we identify the specimen as F. americana. Recent erosion of the mould has removed the left wing and leg. Posterior curvature of the cervical vertebrae indicates early post- or peri-mortem rigor mortis.
Figure 3.
External mould and external cast of Fulica americana. (a) External mould (National Park Service accession number YELL 147421), c, contour feathers; m, microbially mediated silica fabric; p, primary tail feathers; sc, scapular; st, sternum; t, thigh. (b) External mould of head, o, orbit; b, bill (upper and lower mandible plus nostril visible); fs, facial shield. (c–d) cast of carcass and head.
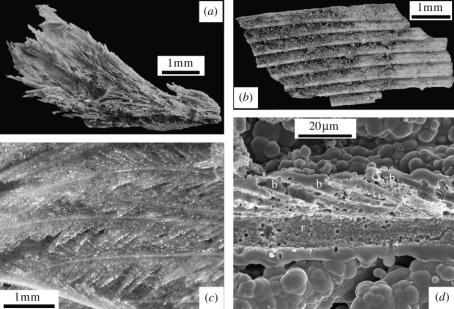
Preservation extends from the gross to the microscopic level (figure 4). Both body contour feathers (figure 4a,c) and flight feathers (figure 4b) are also preserved as external moulds. Scanning electron microscopy reveals that preservation is due to complete silica encrustation of the feathers with silica films or accumulations of opal-A spheres with aggregated botryoidal morphologies (figure 4d ). Later degradation of the encased organics left hollow moulds that faithfully depict macroscopic and microscopic features of the feathers (rachis, barbs, barbules), as the body mould does on a larger scale.
Figure 4.
Feather preservation fabrics. (a) Contour/down feathers preserved by silica encrustation. (b) Flight feather moulds fractured to reveal a fine layer of carbon grains. (c) Light microscope image of plumage encrusted by glassy silica. (d) Scanning electron photomicrograph of external feather mould, fractured to reveal internal surfaces, r, rachis; b, barbs. Botryoidal silica fabric of external surface comprises partly coalesced silica particles.
(c) Taphonomic interpretation
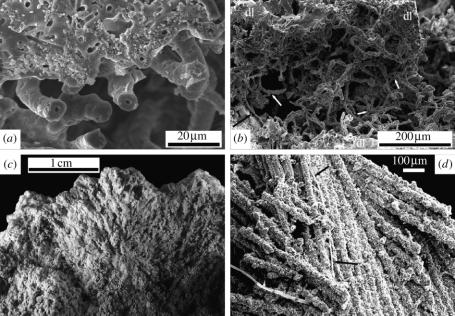
Close examination of the opal-A fabrics surrounding the bird gives evidence of microbial mediation in the unusual preservation of this vertebrate specimen. Silica encrustation of filamentous microbes is clearly visible (figure 5a), revealing the concentric pattern characteristic of these organisms (e.g. Jones & Renaut 2003). Both non-branching (figure 5a) and branching (figure 5b) microbial mould morphotypes are common. Small (ca 1–5 μm) rod and sphericalshaped moulds (not illustrated) indicate further diversity and suggest that multiple taxa of coexisting micro-organisms may have contributed to the unusual preservation of this specimen. Fungi are less prone to silicification in hot spring environments than other micro-organisms (e.g. Jones et al. 1999; Jones & Renaut 2003), but fungal influence is prevalent in this specimen in regions adjacent to the carcass. Silicified fungi can be seen intertwined with both the filamentous microbes (figure 5b) and feather barbs and barbules (figure 5d ). The presence of shrubby opal-A macrofabrics that characterize more distal areas of the mould (figure 5c) are commonly associated with cyanobacterial populations (Guidry & Chafetz 2003a,b).
Figure 5.
Microbial fabrics. (a) Scanning electron photomicrograph of short non-branching microbes adjacent to dense silica laminae illustrating mouldic preservation and post-encrustation removal of organic components. (b) Scanning electron photomicrograph of branching (e.g. white arrows) and intertwined silicified microbes forming open filament network between dense silica laminae (dl), silicified fungal hypha (black arrow). (c) Microbially mediated shrubby silica fabric created by silicification of carcass-encrusting microbial population dominated by cyanobacteria. (d) Scanning electron photomicrograph of silica encrusted fungal hypha (arrows) intertwined with silicified feather barbs.
(d) Timing of silica encrustation
Taphonomic studies of extant avian carcasses record little loss of soft tissue body mass until approximately 2–3 days post-mortem, when decomposition becomes obvious. The loss of loosely attached down and contour feathers likewise begins at about day two or three (Davis & Briggs 1998). Therefore, retention of feathers and 3D preservation of this specimen (figures 3 and 4) constrains the maximum time between death and encrustation, and indicates that silica precipitation was sufficient to stabilize the body mould within days of death. Apparently, the restricted aerial extent of the pool constrained transport-induced damage, while precipitation and accretion of silica on the exposed surfaces of this specimen offset influences of high temperatures, pH factors and rapid biological degradation that normally prevent preservation of vertebrate remains in hot spring environments.
(e) Microbial influences on silica precipitation
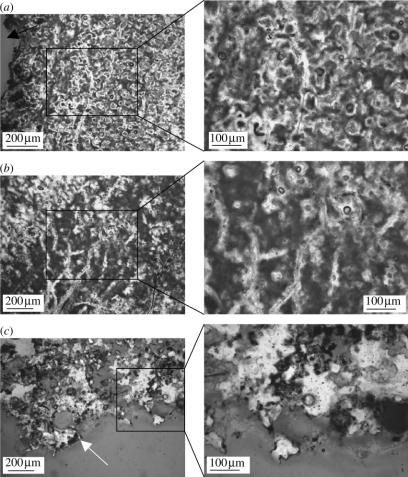
The extremely rapid mineral precipitation necessary to preserve this body fossil is not commonly observed. We propose that this unusual preservation may be mitigated by rapid colonization of the carcass by saprophytic fungi and mat-forming thermophilic and photosynthetic microbes immediately after death. Figure 6 depicts differential distribution of microbial overgrowth, varying from proximal (figure 6a,b) to distal (figure 6c) with respect to the bird carcass. The cavity containing the head of the bird is represented by the black arrow in figure 6a. As is evident from the photomicrographs, density and distribution of microbial populations, correlated to the presence of brown organic fabric, is directly correlated to proximity of the decaying carcass. In addition, sinter precipitation is denser and more uniform closer to the bird body, with fewer visible pores.
Figure 6.
Light microscopy photomicrographs of ground thin sections of sinter surrounding bird. Insets represent high magnification images of the boxed regions. (a) Ground section of region of sinter immediately adjacent to bird carcass shows evidence of organic substrate, including fungal filaments. Black arrow represents cavity where body of bird was situated. (b) Branching pattern of filaments, consistent with fungi. (c) Ground thin section of region distal to bird body shows obvious decrease in density of sinter fabric and corresponding decrease of organic incorporation. No fungal influence can be seen in this region. White arrow indicates external surface of sinter block.
Microbes can influence mineral precipitation in a number of ways. Some are capable of altering the pH of their immediate environment, thus favouring precipitation of minerals directly from saturated waters (e.g. Fortin et al. 1997; Phoenix et al. 1999). Alternatively, because of their very large surface area to volume ratios, prokaryotic micro-organisms can passively increase precipitation by acting as sites for the precipitation of minerals (Fortin et al. 1997). Microbes also produce biofilms, exopolymeric substances that help attach microbes to surfaces. These biofilms collect and concentrate nutrients, and contribute anions that can change surface chemistry to favour precipitation (Fortin et al. 1997). Finally, microbes can directly influence precipitation through catalysis of chemical reactions, or through reactive sites on cell surfaces, such as the abundant hydroxyl groups on the surface of biomolecules (e.g. polysaccharides) contained in bacterial sheaths. These functional moieties promote hydrogen bond formation with silicic acids in solution, thereby facilitating silica nucleation and precipitation (Cady & Farmer 1996; Fortin et al. 1997).
2. Discussion
Although fossils of microbes (Cady & Farmer 1996), plants (Powell et al. 2000; Wellman 2004) and arthropods (Anderson & Trewin 2003) are common in ancient hot spring deposits, thus indicating that these environments are conducive to exceptional morphological preservation, there are few records of vertebrate remains from similar deposits. The dearth of vertebrate material preserved in hot spring deposits is related to a combination of factors. First, most vertebrates avoid or can escape hostile regions of geothermal systems, minimizing influx of source material. Extant vertebrate material observed in active hot spring areas suggests that inexperienced/young animals and migratory water birds are most likely to die in hot spring pools, which was probably the case for the specimen we describe. Second, vertebrate remains would be scavenged from all but the hottest, wettest and deepest regions of hot spring environments, resulting in taphonomic loss. Third, vertebrate remains that are occasionally observed in hot spring vent-pools in regions where water temperatures and/or pH preclude tissue removal by macro-scavengers usually possess no associated soft tissue. This suggests that, normally, soft tissue degradation proceeds by microbial and/or chemical means, and in the vast majority of cases of vertebrate death in hot spring environments, soft tissue degradation outpaces mineral deposition and body fossils are not preserved. Additionally, initial mineral precipitates may not fully seal a carcass from circulating hot spring fluids or microbial decomposers, thus prolonging the potential window of soft tissue degradation. Some or all of these processes were arrested in the specimen that we describe, preserving 3D anatomy and plumage of the bird, encased within dense sinter mineral precipitate.
Preservation of the bird and feathers as 3D external moulds requires unusual conditions. In extant hot spring terrace pool environments similar to the site of preservation of this specimen, dissolved silica in solution is often supersaturated with respect to crystalline quartz and opaline silica phases (e.g. Guidry & Chafetz 2002, 2003a,b). This supersaturation is mainly the result of the cooling that ground waters, chemically equilibrated with silica minerals at geothermally constrained temperatures and pressures, undergo as they rise into and erupt from hot spring vent pools. High degrees of supersaturation and opal-A nucleation on suspended solids (e.g. mineral grains and Mn/Fe hydroxides) encourage homogenous and heterogeneous nucleation of opal-A in the water column, respectively (e.g. organic matter, mineral grains and Mn/Fe hydroxides), homogeneous and heterogeneous nucleation of silica in the water column is encouraged (e.g. Jones & Renaut 2003 and references therein). The resultant opal-A nanospheres grow via Ostwald ripening to microspheres (e.g. Mountain et al. 2003). When the microspheres become too large to remain suspended, they precipitate from the water column to form opal-A sediment either as isolate microspheres or groups of aggregated microspheres (e.g. Jones & Renaut 2003). This path of opal-A precipitation/sediment formation occurs without any discernible biotic influence, but even high rates of accretion appear insufficient to outpace carcass degradation without biological mediation.
We suggest a series of events that proceeded in parallel with and augmented abiotic opal-A precipitation, contributing to rapid silica accretion and subsequent preservation. Deposition began as a passive process, when the pH and temperature of hot spring waters caused partial denaturing of external keratin proteins and exposed protein functional groups, allowing them to serve as binding sites for heterogeneous opal-A nucleation and precipitation. Gross body morphology was replicated as silica precipitated upon the plumage, a process enhanced by the large surface area of the feathers. This passive process is illustrated by opal-A films, which immediately surround the feather barbs (figure 4d). Microbial populations formerly living on the feathers and body added further organic nucleation surfaces at this stage.
Following initial deposition of an opal-A monolayer, further opal-A nucleated/precipitated abiotically, as evidenced by botryoidal surface textures of the feather-encrusting films. Botryoidal aggregates comprising submicron to micron sized silica spheres are visible evidence that nucleation, growth, precipitation and sedimentation of opal-A microspheres from the water column also continued after the initial encrustation of the coot's plumage. As incipient opal-A films were developing passively, rapid microbial colonization of the carcass continued. The increasing density and metabolic activity of the colonizing microbial populations facilitated precipitation of opal-A by directly or indirectly altering the chemical microenvironments surrounding the body and feathers. The role of microbes in opal-A precipitation in waters that are supersaturated with respect to silica is at present a topic of much debate (e.g . Guidry & Chafetz 2002, 2003a,b; cf. Mountain et al. 2003). Regardless of whether the microbial role is an active or passive one, we hypothesize that the rate and extent of opal-A precipitation around the carcass that resulted in mould formation and stabilization was, in this instance, greatly enhanced by microbial colonization. Perhaps most significantly, opal-A precipitation was facilitated by a concomitant increase in organic and (following initial opal-A film formation) mineral surface area for deposition, as microbial bodies and biofilms provided fresh nucleation sites. Microbial fabrics associated with this fossil indicate that the first wave of post-mortem colonization and microbially influenced opal-A precipitation apparently involved saprophytic fungi (figure 6a,b) in addition to populations of bacteria and cyanobacteria. As organic substrates used in fungal metabolism became less accessible owing to increasingly thick, dense and widespread opal-A film deposition, a new secondary microbial population, this time dominated by cyanobacteria, used the carcass-encrusting mineral deposits as an attachment site, continuing the cycle of precipitation. The microbial fossils and fabric depicted in figure 4a,b provide evidence of this secondary microbial influence. Following secondary colonization of the silica film, precipitation probably became more rapid, resulting in shrubby macrofabrics (figure 5c) that, in active springs, have recorded growth rates of 5–10 mm month−1 (White et al. 1988). These fabrics completely encased the specimen, forming a physically stable mould that replicated body morphology at macro- and microscopic levels. Following this process, the porous mineral matrix allowed waters and/or a microbiota to penetrate, dissolve and remove soft tissues and bones. Feather moulds of this specimen are often covered with a fine layer of carbon grains that may be diagenetic products of the degradation process (figure 4b).
From the presence of shrub-like microbial fabrics surrounding this specimen, certain aspects of the palaeoenvironment can be inferred. Shrubby fabrics indicate encrustation of the bird in a terraced pool at least 20–30 cm deep that formed behind a mineral deposit dam immediately adjacent to a silica-depositing hot spring vent (Guidry & Chafetz 2003b). However, because encrustation of the carcass required total immersion, the pool may have been deeper. Terrace-forming hot springs are characterized by high volume, sheet-flow fluid discharge and high silica deposition rates (e.g. ca 5 cm yr−1 for silica‐depositing features; White et al. 1988). Cyanobacterial populations, dominated by Synechococcus spp., Phormidium spp. and Calothrix spp. (e.g. Walter & DesMarais 1993; Cady & Farmer 1996; Guidry & Chafetz 2003a,b), mediate silica shrub growth and morphology in extant settings. Therefore, it is reasonable to assume that these or closely related organisms were responsible for the fabrics seen in association with this fossil specimen. The presence of these microbial taxa (Cady & Farmer 1996) and the apparent absence of diatoms suggest water temperatures of the death pool ranging from about 40 to 70 °C.
This fossil suggests that hot springs may act as preservational traps; self-contained environments that lure, kill and preserve vertebrates. Vertebrates are drawn to hot springs for numerous reasons. They may be the sole water source in otherwise arid, frozen or saline environments, or they may support plentiful vegetation or provide a source of otherwise unavailable mineral nutrients. However, the physical and chemical properties of hot spring waters make them potentially lethal as well. If death occurs in subaerial hot spring settings, such as on sinter sheet surfaces, carcass decay progresses at ‘normal’ or even elevated rates. However, in terrace pools with sufficient depth to cover a carcass with silica-supersaturated spring water, and with temperatures both high enough to deter macro-/micro-scavengers and low enough to allow colonization of the carcass surfaces by fungi and photosynthetic cyanobacteria (below 60–70 °C), hot springs can also promote extremely rapid preservation of vertebrate fossils. Further, silica hot spring deposits provide a geologically durable medium for the preservation of fossil organisms. Precambrian cherts hold body fossil evidence of life on a very young Earth (e.g. Westall et al. 2001) and Lower Palaeozoic cherts (Trewin 1996; Walter et al. 1998; Powell et al. 2000; Anderson & Trewin 2003) provide evidence of the earliest terrestrial ecosystems. Thus, hot springs have provided an environment for potential preservation throughout vertebrate history. Shrubby macrofabrics similar to those we report have a geological record extending at least to the Lower Palaeozoic (Trewin 1996; Walter et al. 1998). These morphologically distinctive and persistent fabrics, when identified in later Palaeozoic to recent deposits, may indicate ancient hot spring environments where water temperatures (ca 40–70 °C), microbial communities and chemistry (silica/carbonate-depositing) were conducive to this unusual mode of vertebrate preservation. This fossil bird represents the first reported vertebrate remains described from ancient hot spring settings, but using associated fabrics as an indicator of where to prospect, it will most probably not be the last.
Acknowledgments
We thank Carolyn Davies, Smokey Sturtevant, Bill Wise, NPS staff of Yellowstone National Park, Kevin Padian, John Fountain, Jim Schmitt, Jennifer Wittmeyer, Recep Avci, the staff of ICAL and MSU, and two anonymous reviewers. This research was supported through an NERC studentship grant (A.C.) and an NSF grant (M.H.S.).
Endnote
The specimen is housed at Yellowstone Centre for Resources, Mammoth Hot Springs, Yellowstone National Park. Accession number YELL 147421.
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
References
- Anderson L.I, Trewin N.H. An Early Devonian arthropod fauna from the Windyfield cherts, Aberdeenshire, Scotland. Paleontology. 2003;46:1–43. [Google Scholar]
- Cady S.L, Farmer J.D. Fossilisation processes in siliceous thermal springs: trends in preservation along the thermal gradient. In: Bock G.R, Goode J, editors. Evolution of hydrothermal ecosystems on Earth (and Mars?) Wiley; Chichester: 1996. pp. 150–173. [DOI] [PubMed] [Google Scholar]
- Christiansen R.L. Map GQ-1193. Department of the Interior, United States Geological Survey; Washington, DC: 1975. Geologic map of the Norris junction Quadrangle, Yellowstone National Park, Wyoming. [Google Scholar]
- Christiansen R.L. 2001 Geology of Yellowstone National Park: the Quaternary and Pliocene Yellowstone plateau volcanic field of Wyoming Idaho and Montana USGS professional paper 729-G.
- Davis P.G, Briggs D.E.G. The impact of decay and disarticulation on the preservation of fossil birds. Palaios. 1998;13:3–13. [Google Scholar]
- Despain D.G. Roberts Rinehart; Boulder, CO: 1990. Yellowstone vegetation: consequences of environment and history in a natural setting. [Google Scholar]
- Fortin D, Ferris F.G, Beveridge T.J. Surface-mediated mineral development by bacteria. In: Banfield J.F, Nealson K.H, editors. Geomicrobiology: interactions between microbes and minerals. Mineralogical Society of America; Washington, DC: 1997. pp. 161–180. [Google Scholar]
- Guidry S.A, Chafetz H.S. Factors governing subaqueous siliceous sinter precipitation in hot springs: examples from Yellowstone National Park, Wyoming, USA. Sedimentology. 2002;49:1253–1267. [Google Scholar]
- Guidry S.A, Chafetz H.S. Anatomy of siliceous hot springs: examples from Yellowstone National Park, Wyoming, USA. Sediment. Geol. 2003a;157:71–106. [Google Scholar]
- Guidry S.A, Chafetz H.S. Siliceous shrubs in hot springs from Yellowstone National Park, Wyoming, USA. Can. J. Earth Sci. 2003b;40:1571–1583. [Google Scholar]
- Herdianita N.R, Browne P.R.L, Rodgers K.A, Campbell K.A. Mineralogical and textural changes accompanying ageing of silica sinter. Miner. Deposita. 2000;35:48–62. [Google Scholar]
- Jones B, Renaut R.W. Hot spring and geyser sinters: the integrated product of precipitation, replacement and deposition. Can. J. Earth Sci. 2003;40:1549–1569. [Google Scholar]
- Jones B, Renaut R.W, Rosen M.R. Role of fungi in the formation of siliceous coated grains, Waiotapu geothermal area, North Island, New Zealand. Palaios. 1999;15:450–475. [Google Scholar]
- Kaufmann M.R. To live fast or not: growth, vigor and longevity of old-growth ponderosa pine and lodgepole pine trees. Tree Physiol. 1996;16:139–144. doi: 10.1093/treephys/16.1-2.139. [DOI] [PubMed] [Google Scholar]
- Mountain B.W, Benning L.G, Boerema J. Experimental studies on New Zealand hot spring sinters: rates of growth and textural development. Can. J. Earth Sci. 2003;40:1643–1667. [Google Scholar]
- Phoenix V.R, Konhauser K.O, Adams D.G. Photosynthetic controls on the silicification of cyanobacteria. In: Ármannsson H, editor. Geochemistry of the Earth's surface. A. A. Balkema; Rotterdam: 1999. pp. 275–278. [Google Scholar]
- Powell C.L, Trewin N.H, Edwards D. Palaeoecology and plant succession in a borehole through the Rhynie cherts, Lower Old Red Sandstone, Scotland. In: Friend P.F, Williams B.P.J, editors. New perspectives on the Old Red Sandstone. Special publication 180. Geological Society; London: 2000. pp. 439–457. [Google Scholar]
- Trewin N.H. The Rhynie cherts: an early Devonian ecosystem preserved by hydrothermal activity. In: Bock G.R, Goode J, editors. Evolution of hydrothermal ecosystems on Earth (and Mars?) Wiley; Chichester: 1996. pp. 131–149. [DOI] [PubMed] [Google Scholar]
- Walter M.R, DesMarais D.J. Preservation of biological information in thermal spring deposits: developing a strategy for the search for fossil life on Mars. Icarus. 1993;101:129–143. doi: 10.1006/icar.1993.1011. [DOI] [PubMed] [Google Scholar]
- Walter M.R, McLoughlin S, Drinnan A.N, Farmer J.D. Palaeontology of Devonian thermal spring deposits, Drummond Basin, Australia. Alcheringa. 1998;22:285–314. [Google Scholar]
- Wellman C.H. Palaeoecology and palaeophytogeography of the Rhynie chert plants: evidence from integrated analysis of in situ and dispersed spores. Proc. R. Soc. B. 2004;271:985–992. doi: 10.1098/rspb.2004.2686. http://dx.doi.org/10.1098/rspb/2004.2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westall F, DeWit M.J, Dann J, Van der Gaast S, De Ronde C.E.J, Gerneke D. Early Archean fossil bacteria and biofilms in hydrothermally-influenced sediments from the Barberton greenstone belt, South Africa. Precamb. Res. 2001;106:93–116. [Google Scholar]
- White D.E, Hutchinson R.A, Keith T.E.C. USGS professional paper, 1456. Yellowstone National Park; Wyoming: 1988. The geology and remarkable thermal activity of Norris Geyser Basin. [Google Scholar]