Abstract
The synchronization of the dynamics of spatially subdivided populations is of both fundamental and applied interest in population biology. Based on theoretical studies, dispersal movements have been inferred to be one of the most general causes of population synchrony, yet no empirical study has mapped distance-dependent estimates of movement rates on the actual pattern of synchrony in species that are known to exhibit population synchrony. Northern vole and lemming species are particularly well-known for their spatially synchronized population dynamics. Here, we use results from an experimental study to demonstrate that tundra vole dispersal movements did not act to synchronize population dynamics in fragmented habitats. In contrast to the constant dispersal rate assumed in earlier theoretical studies, the tundra vole, and many other species, exhibit negative density-dependent dispersal. Simulations of a simple mathematical model, parametrized on the basis of our experimental data, verify the empirical results, namely that the observed negative density-dependent dispersal did not have a significant synchronizing effect.
Keywords: habitat fragmentation, metapopulation, population synchrony, population cycles, tundra voles
1. Introduction
To what extent do biological populations exhibit spatially synchronous dynamics, and what is the synchronizing mechanism that is currently subject to intensive research (e.g. Grenfell et al. 1998; Blasius et al. 1999; Post & Forchammer 2002; Schwartz et al. 2002)? Studies of population synchrony provide a valuable approach to the most fundamental question in population biology: by which mechanisms are populations regulated? From a more applied point of view, the degree of population synchrony determines the extinction risk of fragmented populations (Earn et al. 2000) and the opportunities for control of pests and diseases (Earn et al. 1998). A distinguished body of theory suggests that dispersal movement is a powerful synchronizing mechanism (Ranta et al. 1995, 1997; Blasius et al. 1999; Lande et al. 1999; Bjørnstad & Bolker 2000; Kendall et al. 2000; Sherratt et al. 2000). Indeed, among three main causes of population synchrony that have been identified largely based on theoretical studies (Bjørnstad et al. 1999a); i.e. dispersal movements, regionalized disturbances (the so-called Moran effect) and trophic interactions (e.g. predator–prey interactions), dispersal movements have been claimed to be the most parsimonious (Schwartz et al. 2002). Theoretical studies have demonstrated that even spatially restricted dispersal movements can account for large-scale synchrony under certain circumstances (Ranta et al. 1997; Bjørnstad & Bolker 2000; Kendall et al. 2000). Moreover, dispersal-induced population synchrony is compatible with the pattern of decaying synchrony with distance that is commonly observed in population survey data (Ranta et al. 1995; Sutcliffe et al. 1996; Lambin et al. 1998; Paradis et al. 1999; Cattadori et al. 2000), yet dispersal is generally the most poorly known demographic parameter. It is notoriously difficult to measure in the field and, in most cases, estimates of dispersal rate have to be derived from indirect methods that are associated with large uncertainties and potential biases (Ims & Yoccoz 1997). To the best of our knowledge, no previous study has been able to map independent estimates of distance-dependent dispersal rates on equivalent estimates of population synchrony.
In this study, we use an experimental setting to examine the relationship between dispersal and population synchrony. Experimental studies conducted on small spatial and temporal scales (so-called experimental model systems) have proved to be instrumental in population biology, especially for facilitating a better dialogue between theory and data (Ims & Stenseth 1989; Wiens et al. 1993; Lawton 1995). For instance, such experiments have been used to verify the theoretical conjecture that dispersal can promote metapopulation persistence and species coexistence (Burkey 1997; Gonzales et al. 1998). Although the issue of population synchrony typically concerns large-scale and long-term phenomena beyond the realm of controlled experiments, we justify our relatively small-scale study on the following grounds: in order to be a powerful agent of region-wide synchrony, dispersal must at least be able to exert its synchronizing effect on a more local scale (Bjørnstad, Ims & Lambin 1999). As has been carried out successfully in conjunction with model system experiments previously (Ellner et al. 2001), we use mathematical modelling as a tool for evaluating the consistency of our experimental results.
2. Material and methods
Data on dispersal movements and population synchrony was obtained from a study of 14 experimentally fragmented populations of tundra voles, Microtus oeconomus, at Evenstad Landscape Ecological Field Station in southern Norway (Johannesen et al. 2003). Voles and lemmings are among the best-known species with large-scale population synchrony (Krebs & Myers 1974; Ranta & Kaitala 1997; Lambin et al. 1998; Ims & Andreassen 2000). Each of the experimental populations was studied in 50 × 100 m2 enclosed plots with six meadow patches arranged according to a gradient of inter-patch distances (range: 1.5–80 m) in a non-habitat matrix (figure 1). The habitat patches were large enough (225 m2) to harbour small subpopulations of voles similar to size of natural tundra vole patches in boreal and arctic landscapes (Lambin et al. 1992).
Figure 1.
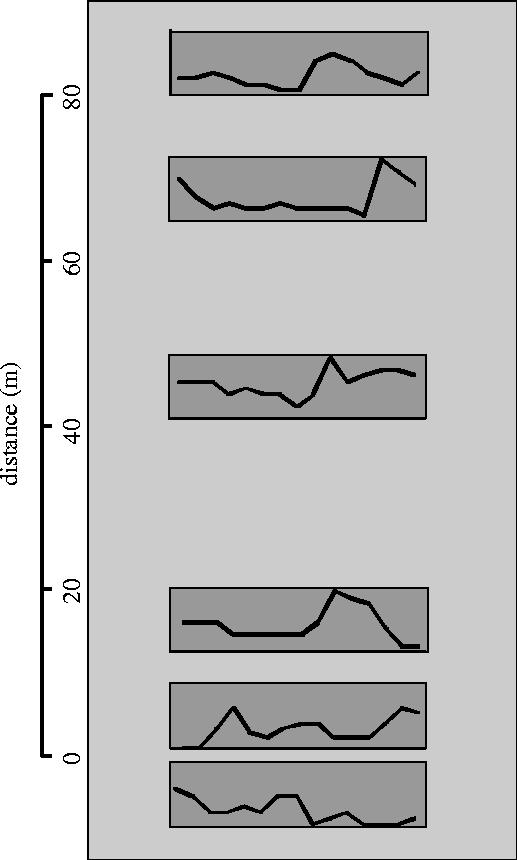
The spatial arrangement of the six habitat fragments (=subpopulations) within 1 of the 14 replicates of fragmented populations that were studied. Dark grey rectangles are the habitat patches, embedded in a matrix area (light grey), which were uninhabitable for voles. Subpopulation density trajectories over the 3.5 month breeding season for one arbitrarily chosen replicate are superimposed on the habitat fragments.
For each of the years 1992 and 1993, seven experimental, fragmented populations were initiated by releasing five laboratory-raised mothers with weaned litters into separate enclosures (figure 1). Population dynamics and dispersal movements between patches/subpopulations were monitored on a weekly basis with 2 days of trapping and six trap checks per week from early July (week 26) until mid October (week 42). The 3.5 month monitoring period per year encompassed three vole generations (Johannesen et al. 2003). In total, 1407 individuals were captured 16 519 times. We recorded dispersal movements according to whether an individual had changed patch from one week to the next. If an individual was captured in more than one patch in a week, the home patch was assigned as the patch in which it had been captured most in that week. In the few cases where a living animal was not captured in one week it was assigned its previous week's home patch. Animals captured equally in two patches were assigned the previous week's home patch, as this was always one of the two. In total, we recorded 553 weekly dispersal movements by 351 individuals. Potential aberrant effect of frustrated movements beyond the enclosures was avoided by removing animals frequently trapped in particular fence traps (Johannesen et al. 2003). Removed animals did not make up more than 5.6% of the total number of individuals in any population, and the removals did not affect demography (Johannesen et al. 2003).
The vegetation was burned and fertilized every spring prior to the introduction of voles to standardize habitat productivity among patches and populations. The spatial habitat configuration with a gradient of inter-patch distances (figure 1) was already set at the start of the experimental period for half of the 14 experimental populations, while it was created by fragmenting one initial large habitat block after six weeks for the other half. The latter manipulation was carried out as a part of a study of the effect of habitat destruction on local demography (Johannesen et al. 2003). Only data from the period after habitat fragmentation was used for the latter seven populations. The matrix between the patches was maintained uninhabitable for voles by frequently mowing the vegetation.
3. Results
(a) Experiment
The intensive live-trapping programme allowed us to accurately monitor dispersal movements and population dynamics on a weekly basis through the 3.5 month breeding season (early July to late October) in 2 years. The size of the 84 subpopulations varied from 0 to 49 individuals/patch over the season. A Gompertz-type model fitted the population time-series adequately (see Lebreton 1991 for model fitting procedure) and there was clear evidence for density-dependent regulation (test for log-linear negative density-dependence: F=66.9, p<0.001, estimated model parameters are given below).
Weekly dispersal rate between pairs of patches (e.g. exchange rate) was quantified as the proportion of the animals in a patch/subpopulation that had dispersed to another patch/subpopulation during a week. The dispersal rate was highest between the closest subpopulations and it dropped steeply with increasing distance (figure 2a). Synchrony in the weekly growth rates among subpopulations was quantified by the cross-correlation coefficient equivalent to Kendall's tau proposed by Buonaccorsi et al. (2001). This measure of population synchrony (which focuses on short-term, week-to-week dynamics) was generally statistically indistinguishable from zero, and there was no trend that could be ascribed to distance (figure 2b). In order to test whether there was any cumulative effect of dispersal that acted to spatially homogenize subpopulation sizes on the time-scale of a full breeding season, we also computed the spatial autocorrelation (Cliff & Ord 1973) in subpopulation sizes at the end of the experimental period (i.e. week 42). However, not even this measure of population synchrony was significant at any inter-patch distance (figure 2c). These results imply that dispersal had no synchronizing effects either on a short (a week) or on a longer time-scale (a 3.5 month breeding season=three vole generations).
Figure 2.
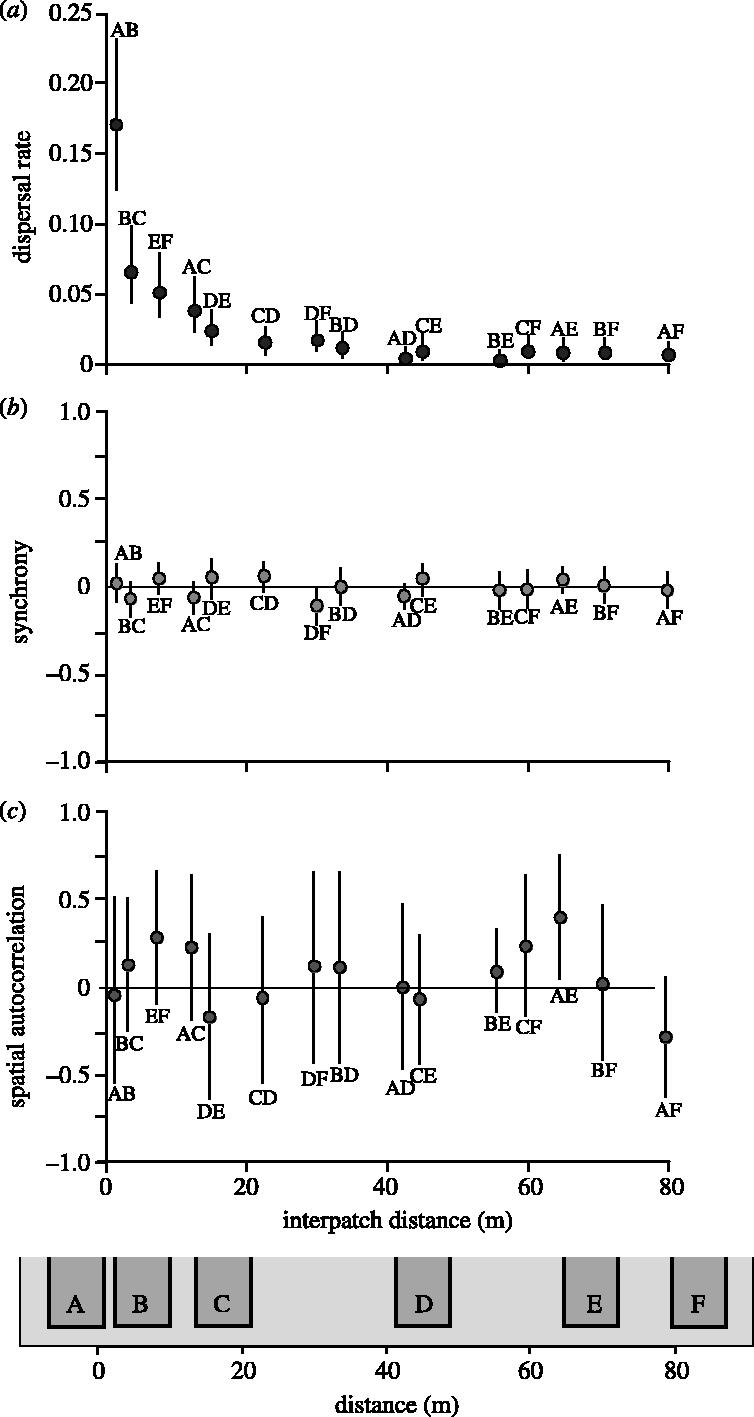
Dispersal rate and degree of synchrony between subpopulations as a function of inter-patch distance. (a) weekly dispersal rate among subpopulations. (b) The degree of synchrony quantified as cross-correlations between weekly growth rates among subpopulations. (c) spatial autocorrelation in subpopulation sizes at the end of the breeding season. All estimates are given with 95% confidence intervals based on the 14 replicates of the fragmented populations.
Most theoretical studies demonstrating that dispersal movements can act as a powerful synchronizing mechanism, assume an arbitrary, constant (i.e. density-independent) dispersal rate. However, density-dependent dispersal is common in nature (Ims & Hjermann 2001; Clobert et al. 2004). We tested for density-dependent dispersal applying a mixed logistic-binomial model (SAS GLIMMIX macro; Littell et al. 1996) with proportion dispersers as a response variable. In addition to subpopulation density, we included week in season as a fixed predictor variable to control for possible season effects, and subpopulation identity as a random effect to control for non-independence between the repeated measurements of dispersal within subpopulations. We rejected the assumption of a constant dispersal rate in favour of a negative density-dependent dispersal rate (F=21.79, p<0.001; figure 3a, the estimated parameter for the density-dependence is given below). A negative density-dependent movement rate may imply that the demographic importance of dispersal relative to survival and mortality will decrease with increasing population density. Indeed, this was evident in our data (figure 3b,c), although most clearly for growing subpopulations (figure 3b).
Figure 3.
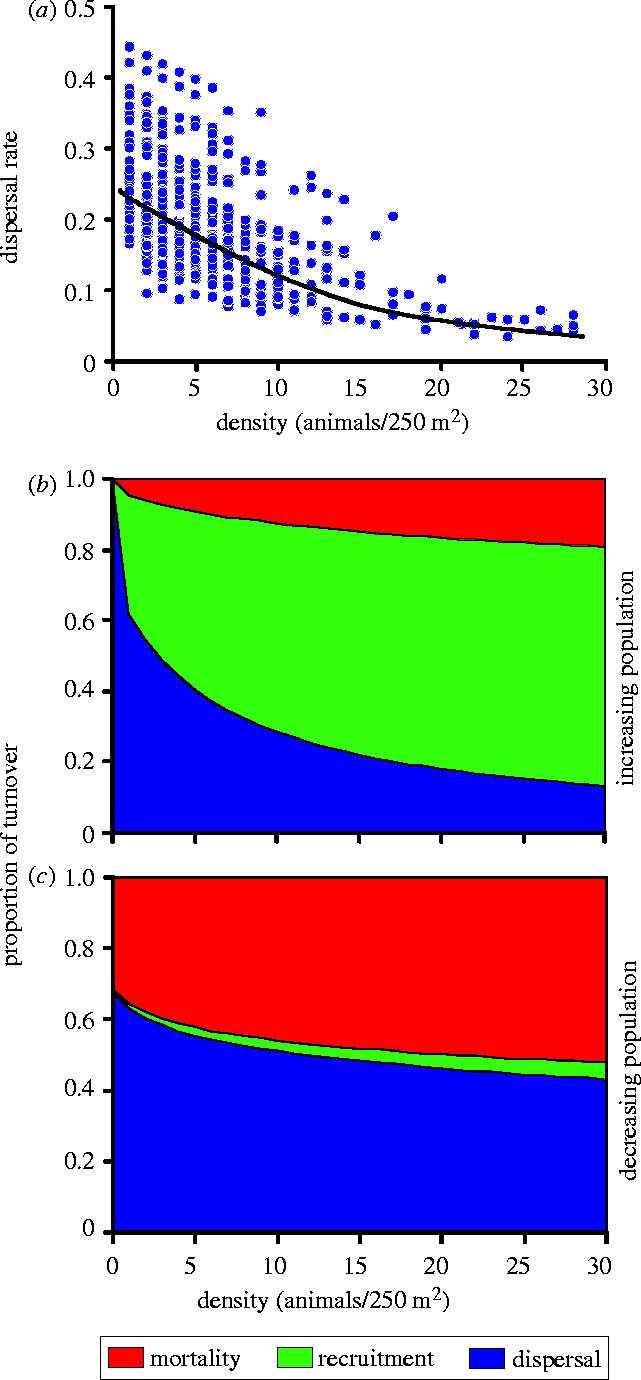
Negative density-dependent dispersal and its proportionate effect on the dynamics of the subpopulation. (a) Density-dependent dispersal rate function fitted by logistic-binomial modelling. (b) and (c) Density-dependent proportionate contribution of dispersal (emigration and immigration), mortality and recruitment to total turnover of increasing (b) and decreasing (c) subpopulations. Total turnover is defined as the sum of individuals recruited, immigrating, dying and emigrating to/from a subpopulation during a week. The density-dependent functions are obtained from a repeated measures logistic regression with subpopulation identity as the subject-level random effect and the demographic parameter (dispersal, mortality and recruitment) as the within-subject, repeated effect (cf. Andreassen & Ims 2001).
(b) Mathematical modelling
We used a simple stochastic difference equation model to evaluate the consequences of the observed density-dependent dispersal rate on population synchrony. The model was formulated and parametrized so as to mimic the main features of the observed dynamics of our experimental subpopulations; i.e. density-dependent subpopulation growth and dispersal rates. However, to simplify the analysis we modelled a system consisting of two patches only. This simplification is justified by the fact that most of the movements in our experimental systems took place between the two closest subpopulations/patches (figure 2a).
The dynamics in the system consisting of the two patches/subpopulations i and j was modelled as
| 3.1 |
where λ(Nt,i) is the density-dependent per capita growth rate owing to patch-specific survival and reproduction, φ(Nt,i) is the density-dependent dispersal rate (the proportion of animals dispersing from one patch to the other during a week) and εt,j is a noise term owing to stochastic patch-specific sources of variability. A Gompertz-type model described the density-dependence in the weekly subpopulation growth:
| 3.2 |
The parameters in this growth model were estimated from population time-series for which all individuals immigrating onto, or emigration from, subpopulations were excluded to highlight subpopulation dynamics without dispersal.
The density-dependent dispersal was estimated by the logistic function
| 3.3 |
The standard deviation of the residuals from (3.2) was used as an estimate of additive local white noise operating on each subpopulation (ε=s.d.=0.75). Thus, in the simulations, independent values for ε were drawn from a normal distribution with mean=0 and s.d.=0.75 and added to the weekly growth rate of each population. Each simulation was run for 100 time-steps and the degree of synchrony in population growth between the patches was quantified for the last 50 time-steps, employing the same cross-correlation coefficient used to estimate growth rate synchrony in our experimental data (Buonaccorsi et al. 2001). Mean±95% confidence intervals for this coefficient were obtained from 1000 independent simulations. To contrast the synchronizing effect of negative density-dependent dispersal with that of constant dispersal, we also ran simulations assuming a constant dispersal rate. The constant rate was set to the average dispersal rate obtained in the density-dependent simulations (φ=0.18).
Simulating the model with the observed negative density-dependent dispersal rate was not able to produce statistically significant synchrony (mean cross-correlation coefficient: 0.14±0.15), even in a tightly coupled two-patch system. On the other hand, equivalent simulations with the same amount of constant (density-independent) dispersal yielded a significant synchronizing effect of dispersal (0.27±0.13).
4. Discussion
According to current theory (e.g. Haydon & Steen 1997; Lande et al. 1999) the degree of synchrony in fragmented populations is determined by a tension between local synchrony-disrupting factors, such as demographic stochasticity, and large-scale synchrony-inducing factors, such as dispersal and regional disturbances. Despite the fact that the dispersal rate was high in our experimental study, especially among the closest subpopulations, dispersal was not able to counteract the influence of the variance in local growth rates. Our model analysis showed that this was not because the average dispersal rate was not sufficiently high. Indeed, a constant dispersal rate of the same magnitude as the average of the density-dependent rate yielded significant population synchrony against the background of the observed local variance in subpopulation growth. Hence, it was the negative density-dependent nature of dispersal rather than its average magnitude that precluded its synchronizing effect. Also a recent purely theoretical analysis, using Richer-type local dynamics, many subpopulations and other dispersal functions, has hinted that the synchronizing power of dispersal is likely to be conditional on the specific dispersal rule implemented in models (Ylikarjula et al. 2000).
Emerging empirical evidence indicates that negative density-dependent dispersal is prevalent in voles (Andreassen & Ims 2001; Lin & Batzli 2001) and in several other taxa as well (Hanski 1999; Ims & Hjermann 2001). Negative density-dependent dispersal may be expected on different biologically justifiable grounds (Hanski 1999). For example, both scarcity of mates and inbreeding avoidance may result in enhanced dispersal at low population density. Moreover, reduced dispersal at high densities may result from suppressed sexual maturation in species such as voles where natal dispersal is induced at puberty (Clobert et al. 2004).
As noted above, cyclic vole and lemming populations are among the best-known cases of large-scale population synchrony. Recent analyses of large-scale survey data and some experimental results have indicated that mobile predators (Norrdahl & Korpimäki 1996; Ims & Andreassen 2000) or climate (Krebs et al. 2002; Sundell et al. 2003) are the main synchronizing factors. The relative unimportance of dispersal in this context has been inferred from two sources of information: (i) The extent of the synchrony domain is usually much larger than would be expected based on the assumed dispersal range of voles (Bjørnstad et al. 1999b; Sundell et al. 2003); (ii) Population synchrony has in some cases been found to be unaffected by dispersal barriers (Heikkilä et al. 1994; Aars et al. 1999). In this study, we have provided more direct evidence for the lacking effect of dispersal on population synchrony than any previous study. Moreover, based on our combined experimental and theoretical analyses, we can now explain why dispersal, at least under these circumstances, does not act to synchronize population dynamics by elucidating the role of negative density-dependence.
Our findings are so far restricted to dispersal during the breeding season and do not incorporate the multi-annual dynamics of cyclic vole populations. Indeed, dispersal rate and its density-dependence may depend on season and phases of the cycle, but it is not known whether such dependencies exist and what their effects could be on spatial population dynamics. Clearly, a new perspective in the study of role dispersal in population dynamics is warranted by including its dynamic interaction with population density and other time-dependent processes (Andreassen & Ims 2001; Haydon et al. 2003).
5. Conclusion
Our results challenge the common generalization that dispersal is a parsimonious cause of population synchrony (e.g. Schwartz et al. 2002), and that the synchronizing power of dispersal only depends on its average rate and distance in combination with other synchronizing and de-synchronizing factors (Bjørnstad et al. 1999a; Koenig 1999). As shown in this study, whether dispersal is density-dependent also matters. Specifically, the synchronizing effect of dispersal in a metapopulation context becomes significantly diminished when dispersal is negatively density-dependent. Recent reviews have testified for the richness of conditional responses to both internal and external drivers of dispersal in animal and plant populations (e.g. Ims & Hjermann 2001; Clobert et al. 2004). Population biologists, therefore, need to take into account the fact that dispersal is a more dynamic and complex factor than they conventionally have assumed when posing applied and fundamental problems in spatial population dynamics.
Acknowledgments
The manuscript was improved by the comments of three anonymous referees. We thank all the people that helped us during the fieldwork at Evenstad Landscape Ecological Field Station. The Research Council of Norway funded the study.
References
- Aars J, Johannesen E, Ims R.A. Demographic consequences of movements in subdivided root vole populations. Oikos. 1999;85:204–216. [Google Scholar]
- Andreassen H.P, Ims R.A. Dispersal in patchy vole populations: role of patch configuration, density dependence, and demography. Ecology. 2001;82:2911–2926. [Google Scholar]
- Bjørnstad O.N, Bolker B. Canonical functions for dispersal induced synchrony. Proc. R. Soc. Lond. B. 2000;267:1787–1794. doi: 10.1098/rspb.2000.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørnstad O.N, Ims R.A, Lambin X. Spatial population dynamics: analysing patterns and processes of population synchrony. Trends Ecol. Evol. 1999;14:427–432. doi: 10.1016/s0169-5347(99)01677-8. [DOI] [PubMed] [Google Scholar]
- Bjørnstad O.N, Stenseth N.C, Saitoh T. Synchrony and scaling in dynamics of voles and mice in northern Japan. Ecology. 1999;80:622–637. [Google Scholar]
- Blasius B, Huppert A, Stone L. Complex dynamics and phase synchronization in spatially extended ecological systems. Nature. 1999;399:354–359. doi: 10.1038/20676. [DOI] [PubMed] [Google Scholar]
- Buonaccorsi J.P, Elkingston J.P, Evans S.R, Liebhold A.M. Measuring and testing for spatial synchrony. Ecology. 2001;82:1668–1679. [Google Scholar]
- Burkey T.V. Metapopulation extinction in fragmented landscapes: using bacteria and protozoa communities as model ecosystems. Am. Nat. 1997;150:568–591. doi: 10.1086/286082. [DOI] [PubMed] [Google Scholar]
- Cattadori I.M, Merler S, Hudson P.J. Searching for mechanisms of synchrony in spatially structured game bird populations. J. Anim. Ecol. 2000;69:620–638. [Google Scholar]
- Cliff A.D, Ord J.K. Pion Limited; London: 1973. Spatial autocorrelation. [Google Scholar]
- Clobert J, Ims R.A, Rousset F. Causes, mechanisms and consequences of dispersal. In: Hanski I, Gaggiotti O, editors. Ecology, genetics and evolution of metapopulations. Academic Press; London: 2004. pp. 307–336. [Google Scholar]
- Earn D.J.D, Rohani P, Grenfell B.T. Persistence, chaos and synchrony. Proc. R. Soc. Lond. B. 1998;265:7–10. doi: 10.1098/rspb.1998.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earn D.J.D, Levin S.A, Rohani P. Coherence and conservation. Science. 2000;290:1360–1364. doi: 10.1126/science.290.5495.1360. [DOI] [PubMed] [Google Scholar]
- Ellner S.P, et al. Habitat structure and population persistence in an experimental community. Nature. 2001;412:538–543. doi: 10.1038/35087580. [DOI] [PubMed] [Google Scholar]
- Gonzales A, Lawton J.H, Gilbert F.S, Blackburn T.M, Evans-Freke I. Metapopulation dynamics abundance and distribution in microecosystem. Science. 1998;281:2045–2047. doi: 10.1126/science.281.5385.2045. [DOI] [PubMed] [Google Scholar]
- Grenfell B.T, et al. Noise and determinism in synchronized sheep dynamics. Nature. 1998;394:674–677. [Google Scholar]
- Hanski I. University Press; Oxford: 1999. Metapopulation ecology. [Google Scholar]
- Haydon D, Steen H. The effects of large- and small-scale random events on the synchrony of metapopulation dynamics: a theoretical analysis. Proc. R. Soc. Lond. B. 1997;264:1375–1381. [Google Scholar]
- Haydon D, Greenwood P.E, Stenseth N.C, Saitoh T. Spatio-temporal dynamics of the grey-sided vole in Hokkaido: identifying coupling using state-based Markov-chain modelling. Proc. R. Soc. Lond. B. 2003;270:435–445. doi: 10.1098/rspb.2002.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkilä J, Below A, Hanski I. Synchronous dynamics of microtine rodent populations on islands in Lake Inari in northern Fennoscandia: evidence for regulation by mustelid predators. Oikos. 1994;70:245–252. [Google Scholar]
- Ims R.A, Andreassen H.P. Spatial synchronization of vole population dynamics by predatory birds. Nature. 2000;408:194–196. doi: 10.1038/35041562. [DOI] [PubMed] [Google Scholar]
- Ims R.A, Hjermann D.Ø. Condition dependent dispersal. In: Clobert J, Nichols J.D, Dancin E, Dhondt A, editors. Dispersal. Oxford University Press; Oxford: 2001. pp. 203–216. [Google Scholar]
- Ims R.A, Stenseth N.C. Divided the fruitflies fall. Nature. 1989;342:21–22. doi: 10.1038/342021a0. [DOI] [PubMed] [Google Scholar]
- Ims R.A, Yoccoz N.G. The study of tranfer processes in metapopulations: emigration, dispersal and colonization. In: Hanski I.A, Gilpin M.E, editors. Metapopulation dynamics: ecology, genetics and colonization. Academic Press; San Diego, CA, USA: 1997. pp. 247–265. [Google Scholar]
- Johannesen E, Aars J, Andreassen H.P, Ims R.A. A demographic analysis of vole population responses to fragmentation and destruction of habitat. Popul. Ecol. 2003;45:47–58. [Google Scholar]
- Kendall B.E, Bjørnstad O.B, Bascompte J, Keitt T.H, Fagan W.E. Dispersal, environmental correlation, and spatial synchrony in population dynamics. Am. Nat. 2000;155:628–636. doi: 10.1086/303350. [DOI] [PubMed] [Google Scholar]
- Koenig W.D. Spatial autocorrelation of ecological phenomena. Trends Ecol. Evol. 1999;14:22–26. doi: 10.1016/s0169-5347(98)01533-x. [DOI] [PubMed] [Google Scholar]
- Krebs C.J, Myers J. Population cycles in small mammals. Adv. Ecol. Res. 1974;8:267–399. [Google Scholar]
- Krebs C.J, Kenney A.J, Gilbert S, Danell K, Angerbjörn A, Erlinge S, Bromley R.G, Shank C, Carriere S. Synchrony in lemming and vole populations in the Canadian Arctic. Can. J. Zool. 2002;80:1323–1333. [Google Scholar]
- Lambin X, Krebs C.J, Scott B. Spacing system of the tundra vole Microtus oeconomus during the breeding season in Canada's western Arctic. Can. J. Zoology. 1992;70:2068–2072. [Google Scholar]
- Lambin X, et al. Spatial asynchrony and periodic travelling wave in cyclic field vole populations. Proc. R. Soc. Lond. B. 1998;265:1491–1496. doi: 10.1098/rspb.1998.0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R, Engen S, Sæther B.E. Spatial scale of population synchrony: environmental correlation versus dispersal and density regulation. Am. Nat. 1999;154:271–281. doi: 10.1086/303240. [DOI] [PubMed] [Google Scholar]
- Lawton J.H. Ecological experiments with model systems. Science. 1995;269:328–331. doi: 10.1126/science.269.5222.328. [DOI] [PubMed] [Google Scholar]
- Lebreton J.-D. Modelling density dependence, environmental variablity and deomgraphic stachasticity from population counts: an example using Wyhtam Woods great tits. In: Blondel J, Gosler A, Lebreton J.-D, McCleery R, editors. Population biology of passerine birds. Springer; Berlin: 1991. pp. 89–102. [Google Scholar]
- Lin Y.T.K, Batzli G.O. The influence of habitat quality on dispersal demography, and population dynamics of voles. Ecol. Monogr. 2001;71:245–275. [Google Scholar]
- Littell R.C, Milliken G.A, Stroup W.W, Wolfinger R.D. SAS Institute, SAS Campus Drive; Cary, NC: 1996. SAS systems for mixed models. [Google Scholar]
- Norrdahl K, Korpimäki E. Do nomadic avian predators synchronize population fluctuations of small mammals? A field experiment. Oecologia. 1996;107:478–483. doi: 10.1007/BF00333938. [DOI] [PubMed] [Google Scholar]
- Paradis E, Baillie S.R, Sutherland W.J, Gregory R.D. Dispersal and spatial scale affect synchrony in spatial population dynamics. Ecol. Lett. 1999;2:112–114. [Google Scholar]
- Post E, Forchhammer M.C. Synchronization of animal populations by large-scale climate. Nature. 2002;420:168–171. doi: 10.1038/nature01064. [DOI] [PubMed] [Google Scholar]
- Ranta E, Kaitala V. Travelling waves in vole population dynamics. Nature. 1997;390:456. [Google Scholar]
- Ranta E, Kaitala V, Lindström J, Linden H. Synchrony in populations. Proc. R. Soc. Lond. B. 1995;262:113–118. [Google Scholar]
- Ranta E, Kaitala V, Lundberg P. The spatial dimension in population dynamics. Science. 1997;278:1621–1623. doi: 10.1126/science.278.5343.1621. [DOI] [PubMed] [Google Scholar]
- Schwartz M.K, Mills L.S, McKelvey K.S, Ruggiero L.F, Allendorf F.W. DNA reveals high dispersal synchronizing the population dynamics of Canada lynx. Nature. 2002;415:520–522. doi: 10.1038/415520a. [DOI] [PubMed] [Google Scholar]
- Sherratt N.T, Lambin X, Petty S.J, MacKinnon J.L, Coles C.F, Thomas C.J. Use of coupled oscillator models to understand synchrony and travelling waves in populations of the field vole in northern England. J. Appl. Ecol. 2000;37:148–158. [Google Scholar]
- Sundell J, Huitu O, Henttonen H, Kaikusalo A, Korpimäki E, Pietiäinen H, Saurola P, Hanski I. Large-scale spatial dynamics of vole populations in Finland revealed by the breeding success of vole-eating avian predators. J. Anim. Ecol. 2003;73:167–178. [Google Scholar]
- Sutcliffe O.L, Thomas C.D, Moss D. Spatial synchrony and asynchrony in butterfly population dynamics. J. Anim. Ecol. 1996;65:85–95. [Google Scholar]
- Wiens J.A, Stenseth N.C, Van Horne B, Ims R.A. Ecological mechanisms and landscape ecology. Oikos. 1993;66:369–380. [Google Scholar]
- Ylikarjula J, Alaja S, Laakso J, Tesar D. Effects of patch number and dispersal patterns on population dynamics and synchrony. J. Theor. Biol. 2000;207:377–387. doi: 10.1006/jtbi.2000.2181. [DOI] [PubMed] [Google Scholar]


