Abstract
A growing body of research on humans suggests that exposure to a stressful family environment or father absence from home during childhood is associated with early female puberty and greater interest in infants among adolescent girls. This effect may be mediated by early exposure to harsh and inconsistent maternal care, but the mechanisms by which maternal care affects female reproductive maturation are not known. The present study reports sex differences in interest in infants among juvenile rhesus macaques similar to those observed in human adolescents. Furthermore, juvenile females that were exposed to harsh and inconsistent maternal care in infancy showed higher interest in infants than controls. Evidence from cross-fostered females indicated that these effects resulted from early experience and not genetic inheritance from the mother. There were no significant differences in female age at first conception in relation to the quality of maternal care received during infancy. Macaque females exposed to harsh and inconsistent maternal care in infancy tended to have higher cortisol responses to stress and to corticotropin-releasing hormone than controls in the first three years of life. Furthermore, females with higher cortisol responses to stress exhibited higher interest in infants. These findings suggest that some of the effects of early parental care on female reproductive maturation may be mediated by developmental changes in the activity of the hypothalamic–pituitary–adrenal axis.
Keywords: rhesus macaques, early experience, maternal care, female reproductive maturation, interest in infants
1. Introduction
In human and non-human primates, experience with infants obtained during early development is important for the acquisition of parenting skills and the survival of first-born offspring (e.g. Pryce 1996). Thus, interest in infants should appear early in life and remain elevated at least until the first reproductive event. In the vast majority of primate species, females are the primary infant caregivers. Therefore, females should be more interested in infants and more likely to engage in interactions with them prior to reproduction than males.
Previous studies of human and non-human primates have reported not only sex differences in early interest in infants but also considerable within-sex variation (e.g. Maestripieri & Pelka 2002; Roney & Maestripieri 2003). One possibility is that within-sex variation in early interest in infants is associated with variation in reproductive development, and that both are linked to early experience. From the perspective of life-history theory, the quality of the early environment may affect the trajectories of developing individuals and their reproductive strategies, leading in some cases to early, and in others, to late sexual maturation, reproduction and parental investment (e.g. Stearns 1992).
Several different hypotheses (or ‘mid-level theories’) have been derived from life-history theory so as to predict the timing of female pubertal maturation in relation to the quality of the early environment (see Ellis (2004) for a review). According to the energetics theory, a chronically poor early nutritional environment should result in delayed puberty and reproduction (e.g. Ellison 2001). Harsh physical or psychosocial stress during early development should also be associated with delayed puberty (stress-suppression theory; e.g. MacDonald 1999). Most human longitudinal studies of early psychosocial stress and female reproductive maturation, however, have shown that lack of economic resources, early family conflict or father absence from home are associated with early menarche as well as with early onset of sexual activity and first pregnancy (e.g. Jones et al. 1972; Moffitt et al. 1992; Wierson et al. 1993; Graber et al. 1995; Kim et al. 1997; Quinlan 2003). These studies, therefore, support the psychosocial acceleration theory of pubertal development (Belsky et al. 1991), according to which, early menarche is part of a reproductive strategy that emphasizes precocious reproduction in conjunction with cues of low probability of adult survival or in situations in which male commitment to relationships or male parental investment are not expected (see also Chisholm 1993). Adolescent girls who grow up without a father also exhibit greater interest in pictures of infants (Maestripieri et al. 2004), suggesting that by being more attracted to infant stimuli, or by expressing interest in infants earlier during development, rapidly maturing girls may acquire crucial parenting skills earlier in life and be better equipped for early reproduction and child-rearing.
It has been argued that the effects of early stressful environment on reproductive maturation are mediated by the quality of parental care (e.g. Belsky et al. 1991). In other words, lack of resources or high levels of conflict in the home environment would lead to harsh and inconsistent parenting behaviour, and this in turn would affect the child's behavioural and reproductive development. Differences in the amount and quality of parental care between single-parent and two-parent households may also mediate the effects of father absence on early menarche and precocious sexual maturation (Belsky et al. 1991; Quinlan 2003). However, early menarche and early interest in infants in girls who grow up in stressful environments or without a father may also be traits that are genetically inherited from the girls' mothers or fathers. Although there is some evidence for genetic heritability of timing of menarche in humans (e.g. Rowe 2002), possible genetic influences on interest in infants have not been investigated.
In this study, I investigated the role of biological and experiential variables in the development of interest in infants and reproductive maturation in rhesus macaques (Macaca mulatta). First, I hypothesized that interest in infants should appear early on and that young females should exhibit greater interest in infants than do males. Consistent with the results of most human studies in this area, I also hypothesized that juvenile females exposed to harsh and inconsistent maternal behaviour early in life should exhibit greater interest in infants and earlier age at first conception than control females. To assess the relative contribution of genetic and experiential factors to interindividual variability in interest in infants and age at first conception, I compared these variables among female juveniles that were cross-fostered at birth between mothers that differed in the quality of maternal care. Finally, I investigated the possibility that the effect of early experience on female behavioural and reproductive development may be mediated by long-term alterations of the hypothalamic–pituitary–adrenal (HPA) axis.
2. Material and methods
(a) Subjects
This study was conducted with rhesus macaques living in several different social groups at the Field Station of the Yerkes National Primate Research Center in Lawrenceville, GA, USA. The groups were housed in 38×38 m outdoor compounds and consisted of 30–35 adult females with their immature offspring and 2–5 unrelated adult males. All groups had a stable matrilineal structure and a linear dominance hierarchy. Female dominance ranks were assessed using data on unidirectional aggression and submission collected during previous studies.
Study subjects were one cohort of 20 juveniles (12 females, 8 males) reared by their biological mothers and one cohort of 15 female juveniles cross-fostered at birth and reared by unrelated foster mothers in social groups different from the group of origin. Half of the subjects in the first cohort (6 females and 4 males) were born to multiparous mothers with a previous history of abusive parenting and were abused by them in the first 2–3 months of life. The other half of the subjects were born to non-abusive mothers and were matched to the abused subjects for age, sex, and maternal parity and dominance rank. Seven subjects in the second cohort were born to abusive mothers and reared by non-abusive mothers. The other eight subjects were born to non-abusive mothers and reared by abusive mothers, who abused them in the first 2–3 months of life (for more information on subject recruitment, the cross-fostering procedure and the early infant abuse, see Maestripieri et al. 2000). The adult females with a previous history of abusive parenting were identified during the course of previous research. During the first few months of their infants' lives, these mothers typically alternated nurturing maternal care with high rates of infant rejection and potentially harmful behaviours, such as infant dragging, throwing or stepping on (Maestripieri 1998; similar behaviours have also been observed among free-ranging rhesus macaques on the island of Cayo Santiago, Puerto Rico; D. Maestripieri, personal observation).
(b) Procedure
All subjects, with the exception of one cross-fostered female, were observed on a continuous basis in their social groups during their first three years of life. During the first three months of life, all infants and their mothers were observed once a week for 1 h. After the first three months, the subjects were observed once a month for 1 h until the end of their third year of life. Behavioural data were collected by three observers using a portable computer. The three observers were tested for intra‐ and inter‐observer reliability prior to the beginning of data collection.
Two types of behavioural data were used in the present study. Infant handling refers to interactions between the juveniles and infants within the group, which were initiated by the juvenile and involved touching, holding, grooming or carrying the infant. Infants were defined as individuals of less than six months of age that were born a year or more after the juveniles. Infant‐handling data were analysed as hourly rates, and average scores for each of the first three years of life, or across the three years, were used. Data for the first year of life refer to interactions that occurred towards the end of the first year, when yearlings were exposed to the next cohort of infants born in their group.
Data on maternal behaviour refer to interactions between the juveniles and their mothers that were initiated by the mothers. They included hourly rates of contact-making, contact-breaking, cradling, restraining, rejection, grooming and abuse (see Maestripieri (1998), for behavioural definitions). A composite measure of maternal protectiveness was obtained by adding together the scores of maternal contact-making, restraining and grooming. A composite measure of maternal rejection was obtained by adding together the scores of maternal contact-breaking and rejection. These measures typically cluster together in principal components analysis (e.g. Schino et al. 1995). In all data analyses, only data on maternal behaviour during the first three months of life were used, unless otherwise indicated.
Reproductive development of female juveniles was assessed from the age at first conception, which was estimated counting back 165 days from the day in which the subjects gave birth to their first offspring (for reference on the length of pregnancy in rhesus macaques, see Ardito 1976). Exact dates of birth were known for all of the juveniles and their first offspring.
Hormonal data were available only for the cohort of juveniles reared by their biological mothers and not for the cross-fostered juveniles. For these 20 individuals, blood samples were collected every six months for three years. Samples were collected in consecutive weeks, under the following conditions, and in this order: under basal conditions, in response to mild stress, in response to a corticotropin-releasing hormone (CRH) challenge, and in response to an adrenocorticotropin hormone (ACTH) challenge (only in the second and third years). All samples were taken between 09.00 and 10.00 h, unless otherwise noted. Animals were trained for capture and handling, and the blood samples under basal conditions and in response to stress were obtained quickly and without anaesthesia. The mild stress test involved removing the animal from the group and placing it alone into a novel cage and room for 20 min. Blood samples were collected at the beginning and at the end of the test. For the CRH challenge, subjects were captured and immediately anaesthetized with an intramuscular injection of ketamine (10 mg kg−1). Then, they received either CRH (50 μg kg−1) or saline solution IV (as a control) in a balanced design. One basal blood sample was taken at the beginning of the procedure, and others 30 and 60 min after the injections. The following week, the procedure was repeated, but subjects received ACTH (1 μg kg−1 IV) instead of CRH. Plasma cortisol concentrations were assayed at the Yerkes Endocrine Core Laboratory using commercially available kits (Diagnostic Products, Los Angeles, CA). The sensitivity of the assay was 0.50 μg ml−1. Between- and within-assay coefficients of variation were 10% and less than 3%, respectively.
Statistical analyses included analysis of variance (ANOVA), Pearson's correlations, Student's t-tests and regression analyses. Whenever the data were non-normally distributed or the variances were non-homogeneous, the data were log transformed. All tests were two-tailed and probabilities of 0.05 or less were considered statistically significant, unless otherwise indicated.
3. Results
(a) Sex differences in interest in infants
A repeated-measures ANOVA revealed main effects of sex and age on infant handling as well as a significant interaction between these variables (ANOVA, sex, F1,32=11.26, p=0.002; age, F2,64=15.72, p<0.0001; sex×age: F2,64=4.22, p=0.01). As figure 1 illustrates, the average rates of infant handling for females across the three years were higher than the rates for males. Infant‐handling rates increased with infant age, but the increase was steadier and more obvious for females than for males. Sex differences in infant handling may have potentially resulted from differences in the availability of infant siblings or differences in maternal behaviour that influenced the activity levels and social interactions of male and female juveniles. However, male and female juveniles did not differ in their numbers of younger siblings (male=2.0±0.33; female=2.08±0.21), or in any interactions with their mothers over their first three years of life, with the exception that females tended to receive more grooming from their mothers than males did (females=1.31±0.18; males=0.67±0.10; t=−1.88, p=0.06).
Figure 1.
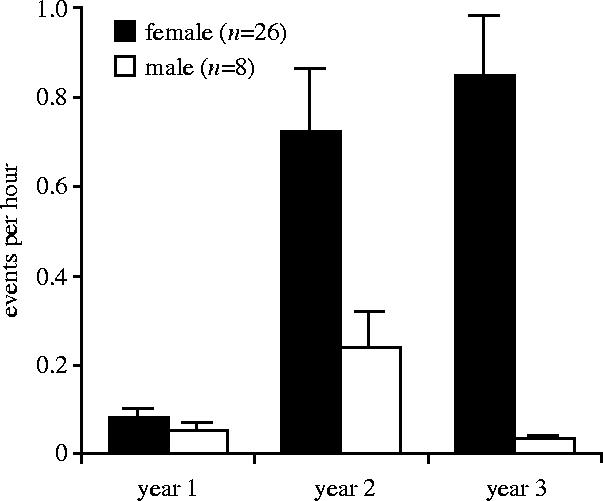
Mean (+ s.e.m.) number of infant-handling episodes by female and male juveniles in the first three years of life.
(b) Effects of early experience on interest in infants
A two-way factorial ANOVA revealed a significant main effect of early abuse on interest in infants (F1,30=6.98, p=0.01) as well as a significant interaction between abuse and sex (F1,30=3.98, p=0.05). As figure 2a illustrates, abused juveniles had higher average rates of infant handling over the first three years of life than controls, and the difference was greater for females than for males. A repeated-measures ANOVA run with data only for the females (n=26) revealed a significant interaction between early abuse and age (F2,48=5.16, p<0.01), such that the difference in infant‐handling rates between abused females (n=13) and controls (n=13) became greater as a function of age (figure 2b).
Figure 2.
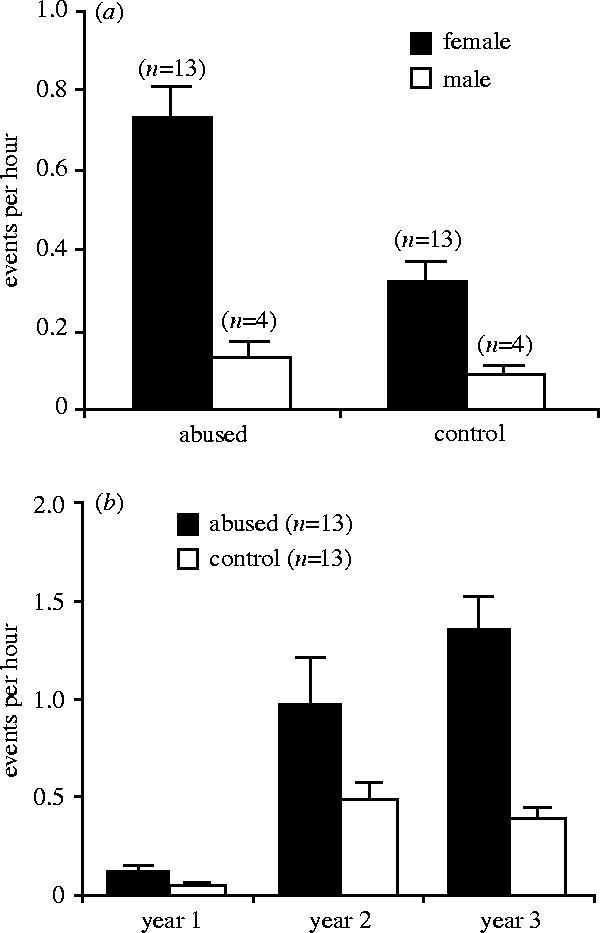
(a) Mean (+ s.e.m.) number of infant‐handling episodes by abused and control juveniles. Average scores across the first three years of life for male and female juveniles are presented. (b) Mean (+ s.e.m.) number of infant‐handling episodes by abused and control females in the first three years of life.
To assess the relative contributions of genetic inheritance and early experience to female interest in infants, the average rates of infant handling over the first three years of life were compared among females born to abusive mothers and reared by them (abuse/abuse, n=6), females born to control mothers and reared by abusive mothers (control/abuse, n=7), females born to abusive mothers and reared by control mothers (abuse/control, n=7), and females born and reared by control mothers (control/control, n=6). A one-way ANOVA revealed statistically significant differences among the four groups (F3,22=8.77, p=0.000 5) and Bonferroni–Dunn post hoc analyses indicated that the infant‐handling rates of the abuse/abuse and the control/abuse groups were significantly higher than those of the abuse/control and the control/control groups (all p<0.05), with no significant difference between the abuse/abuse and the control/abuse group, or between the abuse/control and the control/control group (figure 3).
Figure 3.
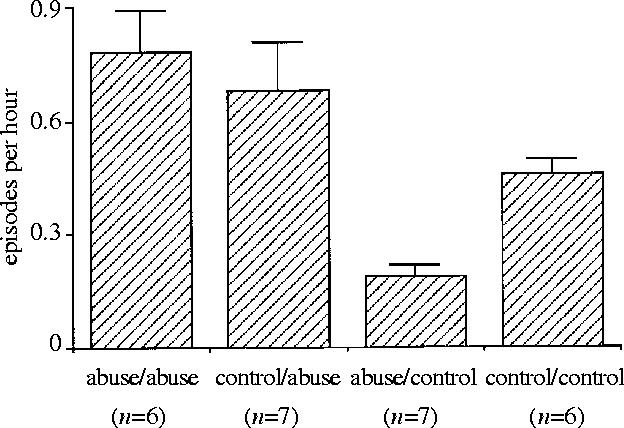
Mean (+ s.e.m.) number of infant‐handling episodes by juvenile females born to abusive mothers and reared by them (abuse/abuse), females born to control mothers and reared by abusive mothers (control/abuse), females born to abusive mothers and reared by control mothers (abuse/control), and females born and reared by control mothers (control/control). Average scores across the first three years of life are presented.
Infant‐handling rates during the first three years of life were significantly correlated with the rate of abuse experienced in the first three months of life (r=0.47, n=25; p=0.01) as well as with the composite measure of maternal rejection (r=0.51, n=25; p<0.01). Thus, females that were abused more or rejected more by their mothers in the first three months of life had higher rates of infant handling in the first three years. Rejection and abuse were not significantly correlated.
(c) Early experience, age at first conception and interest in infants
Age at first conception did not differ significantly between abused (196.43±5.95 weeks, n=14) and non-abused (196.92±7.49 weeks, n=13) females (t=−0.05, n.s.). There was no significant correlation between the females' age at first conception and the age at first conception of their biological mothers (n=27, r=0.01, n.s.), and no significant difference in age at first conception among the females in the abuse/abuse (190.33±7.75), control/abuse (201.00±8.73), abuse/control (197.57±6.62) and control/control groups (196.17±15.18; one-way ANOVA: F3,23=0.21, n.s.). No measure of maternal behaviour was a significant predictor of variation in female age at first conception. There was no significant correlation between female age at first conception and average infant‐handling rates in the first three years of life (r=0.06, n=26, n.s.).
The comparison between age at first conception between abused and non-abused females was re-run after adding data from subjects from previous studies. The analysis with this larger sample size (abused females, n=46, non-abused females, n=61) revealed that abused females had, on average, earlier age at first conception than controls (abused=189.85±3.94 weeks; controls=195.59±3.85 weeks), but this difference was not statistically significant (t=−1.02, p=0.30).
(d) Neuroendocrine correlates
Plasma cortisol levels under basal conditions, and in response to mild stress, CRH and ACTH were compared between the abused females (n=6) and the non-abused females (n=6) for which hormonal data were available. The six abused females had higher average cortisol responses to stress and to CRH across the first three years (figure 4a), but the differences only approached statistical significance (stress: t=1.80; p=0.1; CRH, t=1.91; p=0.08). There were no significant differences in cortisol basal levels (abuse: 24.19±1.69; control: 24.38±1.06; t=−0.09; n.s.) or in cortisol responses to ACTH (abuse: 12.98±2.62; control: 12.09±1.59; t=0.29; n.s.) between abused and non-abused females. The average female cortisol responses to stress across the three years were positively correlated with the average rates of infant handling across the three years (n=12, r=0.64, p=0.02; figure 4b). The correlation between the average female cortisol response to CRH and average rate of infant handling was also positive but not significant (n=12, r=0.24, n.s.). Female cortisol responses to stress or to CRH were not significantly correlated with female age at first conception.
Figure 4.
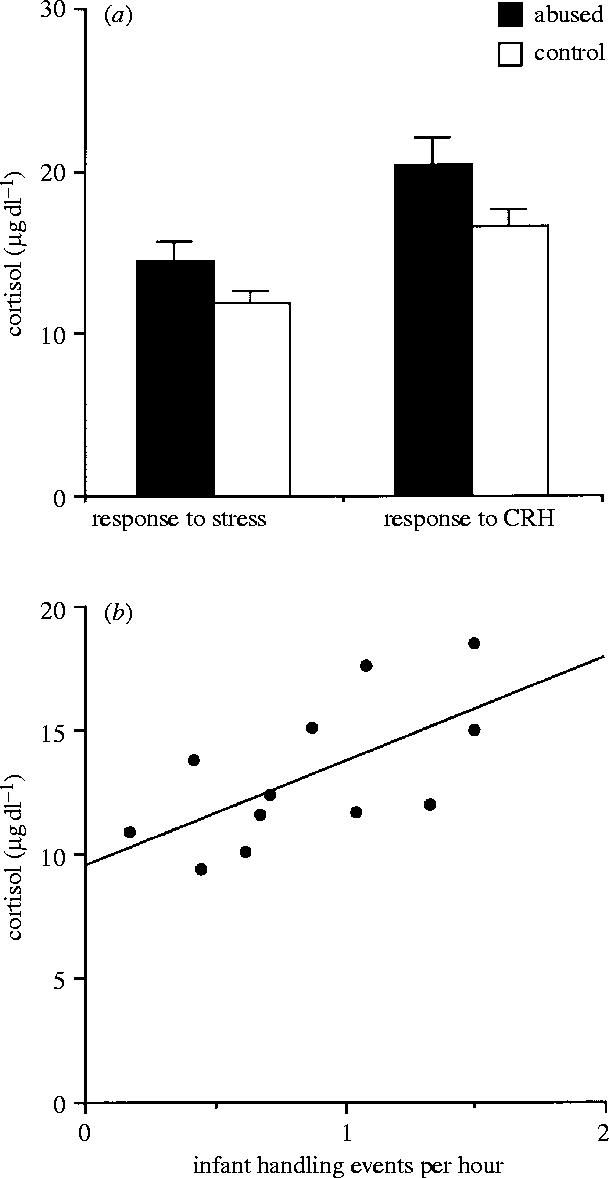
(a) Mean (+ s.e.m.) plasma concentrations of cortisol in response to mild stress and CRH in abused and control female juveniles. Average scores across the first three years of life are presented. (b) Correlation between cortisol responses to mild stress and average rates of infant handling by female juveniles in the first three years of life.
4. Discussion
Rhesus females initiated interactions with infants at much higher rates than males did in their first three years of life. Furthermore, the rate of female interactions with infants increased steadily in the first three years of life, whereas no such trend was observed for males. The sex difference in infant handling was not accounted for by differential availability of infant siblings or differential maternal behaviour towards sons and daughters in the first three years of life. Therefore, this sex difference suggests that young macaque females may be biologically predisposed to be attracted to infants relative to males. A similar predisposition may be present in humans as well (Maestripieri & Pelka 2002). Early experience, however, can be the source of considerable interindividual variation in female interest in infants. In this study, juveniles that were reared by abusive mothers exhibited significantly higher interest in infants than juveniles reared by non-abusive mothers, and this effect was greater for females than for males. The results from cross-fostered females indicated that the greater interest in infants among females reared by abusive mothers resulted from early experience with them or shared environment and not genetic inheritance. In particular, female infant‐handling rates in the first three years were predicted by the rates of infant abuse and maternal rejection experienced in the first three months of life. Since the controls were individually matched to the subjects for dominance rank, this measure of shared environment and its possible relation to access to infants were unlikely to account for the differences in infant handling between abused juveniles and their controls.
In a recent study, adolescent girls who grew up without a father exhibited both earlier menarche and greater interest in infants than girls who had their father at home (Maestripieri et al. 2004). The effects of father absence on these two variables, however, appeared to be independent of one another. In this study, females reared by abusive mothers did not exhibit earlier age at first conception than females reared by non-abusive mothers, and there was no significant correlation between age at first conception and interest in infants in the first three years. Larger sample sizes and measures of reproductive maturation other than age at first conception (e.g. timing of menarche or first ovulation) may be needed for a better understanding of the relation between exposure to harsh maternal care in infancy and the timing of female puberty in primates.
Although the sample size available for the cortisol data analysis was small, abused females tended to have higher cortisol responses to stress and to CRH than non-abused females. Furthermore, the average female cortisol responses to stress across the three years were positively correlated with the average rates of infant handling in the same period. Since there were no significant differences between abused and non-abused females in their cortisol responses to ACTH, the differences in HPA axis function between these groups of individuals are more likely to result from differences in pituitary function (e.g. upregulation of receptors for CRH in the pituitary gland) than in adrenal function. The correlation between cortisol responses to stress and rates of infant handling suggests that exposure to early infant abuse may result in HPA-mediated long-term changes in responsiveness to arousing stimuli. Data on behavioural responsiveness to stimuli other than infants in abused and non-abused females would be needed to test this hypothesis.
Overall, the reported effects of exposure to harsh maternal care during infancy and the development of female interest in infants are consistent with those of a number of human studies reporting a relation between early experience and reproductive maturation (see Ellis (2004), for a review). This study extends the research in this area by highlighting further parallels between the human and the non-human primate data, by demonstrating that the association between early exposure to harsh maternal care and earlier responsiveness to infants is due to an effect of experience and not genetic inheritance; and finally, by providing data on the intervening physiological mechanisms.
It must be emphasized that an association between exposure to poor maternal care and early female reproductive maturation does not imply that females who have such an experience will gain reproductive benefits compared with females who do not. Early puberty and reproduction may have negative consequences for the female's own growth and future reproduction, or for the health of her offspring. Similarly, females who develop responsiveness to infants earlier in life may not necessarily become better mothers, or produce a higher number of surviving offspring. Females on a fast reproductive track may simply be individuals who are making the best of a bad job. Exposure to adverse early experience may function as a cue of low probability of adult survival, and individuals with this experience may begin breeding earlier in life but end up with lower lifetime reproductive success. Alternatively, these may simply be individuals who are cutting their ‘childhood’ short (Ellis 2004).
It is also worth emphasizing that significant effects of early experience on social development, such as those reported in this study, do not necessarily imply the absence of genetic influences on behaviour. For example, previous research with some of the cross-fostered females used in this study indicated that general social tendencies for affiliation and aggression have a significant heritable component (Maestripieri 2003). While most aspects of macaque behaviour probably have both genetic and environmental components, the relative contribution of these components to explaining individual differences in behaviour is probably quite different for different behaviours. The present study suggests that, unlike general social tendencies for affiliation or aggression, the development of responsiveness to infants is an aspect of female behaviour that shows a strong and selective sensitivity to early experience, and in particular, to the quality of maternal behaviour experienced in infancy.
Acknowledgments
I thank Anne Graff, Kai McCormack, Nancy Megna and Richelle Scales for assistance with the experimental procedures and data collection. This work is supported by two grants from the Harry Frank Guggenheim Foundation, NIH grants R01-MH57249, R01-MH62577 and K02-MH63097 to the author, and NIH grant RR-00165 to the Yerkes Center. All experimental procedures in this study were conducted in accordance with the ASAB/ABS Guidelines for the Use of Animals in Research and approved by the Institutional Animal Care and Use Committees of Emory University and the University of Chicago. The Yerkes Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care.
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
References
- Ardito G. Check-list of the data on the gestation length of primates. J. Hum. Evol. 1976;5:213–222. [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: an evolutionary theory of socialization. Child Dev. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Chisholm J.S. Death, hope, and sex: life-history theory and the development of reproductive strategies. Curr. Anthropol. 1993;34:1–24. [Google Scholar]
- Ellis B.J. Timing of pubertal maturation in girls: an integrated life history approach. Psychol. Bull. 2004;130:920–958. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- Ellison P.T. Harvard University Press; Cambridge, MA: 2001. On fertile ground: a natural history of human reproduction. [Google Scholar]
- Graber J.A, Brooks-Gunn J, Warren M.P. The antecedents of menarcheal age: heredity, family environment, and early stressful life events. Child Dev. 1995;66:346–359. doi: 10.1111/j.1467-8624.1995.tb00875.x. [DOI] [PubMed] [Google Scholar]
- Jones B, Leeton J, McLeod I, Wood C. Factors influencing the age of menarche in a lower socio-economic group in Melbourne. Med. J. Aust. 1972;21:533–535. doi: 10.5694/j.1326-5377.1972.tb47460.x. [DOI] [PubMed] [Google Scholar]
- Kim K, Smith P.K, Palermiti A. Conflict in childhood and reproductive development. Evol. Hum. Behav. 1997;18:109–142. [Google Scholar]
- MacDonald K. An evolutionary perspective on human fertility. Popul. Environ. 1999;21:223–246. [Google Scholar]
- Maestripieri D. Parenting styles of abusive mothers in group-living rhesus macaques. Anim. Behav. 1998;55:1–11. doi: 10.1006/anbe.1997.0578. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Similarities in affiliation and aggression between cross-fostered rhesus macaque females and their biological mothers. Dev. Psychobiol. 2003;43:321–327. doi: 10.1002/dev.10143. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Pelka S. Sex differences in interest in infants across the lifespan: a biological adaptation for parenting? Hum. Nat. 2002;13:327–344. doi: 10.1007/s12110-002-1018-1. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Megna N.L, Jovanovic T. Adoption and maltreatment of foster infants by rhesus macaque abusive mothers. Dev. Sci. 2000;3:287–293. [Google Scholar]
- Maestripieri D, Roney J.R, DeBias N, Durante K.M, Spaepen G.M. Father absence, menarche, and interest in infants among adolescent girls. Dev. Sci. 2004;7:560–566. doi: 10.1111/j.1467-7687.2004.00380.x. [DOI] [PubMed] [Google Scholar]
- Moffitt T.E, Caspi A, Belsky J, Silva P.A. Childhood experience and the onset of menarche: a test of a sociobiological model. Child Dev. 1992;63:47–58. doi: 10.1111/j.1467-8624.1992.tb03594.x. [DOI] [PubMed] [Google Scholar]
- Pryce C.R. Socialization, hormones, and the regulation of maternal behavior in nonhuman primates. Adv. Stud. Behav. 1996;25:423–473. [Google Scholar]
- Quinlan R.J. Father absence, parental care, and female reproductive development. Evol. Hum. Behav. 2003;24:376–390. [Google Scholar]
- Roney J.R, Maestripieri D. Social development and affiliation. In: Maestripieri D, editor. Primate psychology. Harvard University Press; Cambridge, MA: 2003. pp. 171–204. [Google Scholar]
- Rowe D.C. On genetic variation in menarche and age at first sexual intercourse. A critique of the Belsky–Draper hypothesis. Evol. Hum. Behav. 2002;23:365–372. [Google Scholar]
- Schino G, D'Amato F.R, Troisi A. Mother–infant relationships in Japanese macaques: sources of interindividual variation. Anim. Behav. 1995;49:151–158. [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Wierson M, Long P.J, Forehand R.L. Toward a new understanding of early menarche. The role of environmental stress in pubertal timing. Adolescence. 1993;28:913–924. [PubMed] [Google Scholar]


