Abstract
Habitat fragmentation may change local climatic conditions leading to altered selection regimes for life-history traits in small ectotherms, including several insects. We investigated temperature-related performance in terms of fitness among populations of the woodland butterfly Pararge aegeria (L.) originating from populations of a closed, continuous woodland landscape versus populations of an open, highly fragmented agricultural landscape in central Belgium. Female fecundity and longevity were evaluated in a temperature-gradient experiment. As predicted, females of woodland landscape origin reached higher maximum daily fecundity and lifetime number of eggs than did agricultural landscape females at low ambient temperatures, but this reversed at high ambient temperature. Egg weight decreased with temperature, and eggs of woodland butterflies were smaller. Contrary to what is generally assumed, remaining thorax mass was a better predictor of lifetime reproductive output than was abdomen mass. Since we used the F2 generation from wild-caught females reared under common garden conditions, the observed effects are likely to rely on intrinsic, heritable variation. Our results suggest that differential selection regimes associated with different landscapes intervene by intraspecific variation in the response of a butterfly to variation in ambient temperature, and may thus be helpful when making predictions of future impacts on how wild populations respond to environmental conditions under a global change scenario, with increasing temperatures and fragmented landscapes.
Keywords: oviposition, fecundity, habitat fragmentation, microclimate, temperature, butterflies
1. Introduction
As patterns of resource distribution change with the process of habitat fragmentation, consequent selective changes in dispersal have been the major focus of evolutionary studies within this field (e.g. Travis & Dytham 1999; Thomas 2000; Merckx et al. 2003). However, altered environmental conditions in fragments compared with continuous habitat (Matlack 1993; Malcolm 1998) may also change life-history traits other than dispersal. For insects, temperature and humidity are among the most important factors affecting insect evolutionary strategies (Partridge et al. 1995; Nevo et al. 1998). Variation in life-history traits among differently fragmented landscapes may result from adaptation, but also from constraints when organisms can no longer buffer traits against environmental changes. Therefore, it is not enough to compare survival and fecundity in different landscapes (e.g. Johannessen et al. 2003), but one needs to study heritable life-history traits in controlled, common environments. To the best of our knowledge, this has only rarely been done.
For flying heliothermous insects, changes in thermal profiles with fragmentation may have important consequences for several aspects of their biology, including longevity and reproductive strategies and capacities. Microclimatic edge effects are also likely to affect host plant quality (Jones 1992), which may in turn affect herbivorous insects. Few studies, however, have linked animal responses to microclimate changes within the context of habitat fragmentation (Meyer & Sisk 2001). We use Pararge aegeria (L.) as a model to test for differences in key life-history traits—female lifespan, egg number and egg size—among populations from differently fragmented landscapes. Throughout Europe, it is primarily a woodland butterfly (Tolman & Lewington 1997), but also occurs in more open, fragmented landscapes with some woodland aspect. We compare egg-laying patterns and longevity between females from woodland versus agricultural landscapes (cf. Merckx et al. 2003) in an experimental common environment consisting of a gradient of ambient temperatures in the laboratory. We used naive butterflies reared under controlled conditions from females collected in both types of landscape. Hence, this experiment addresses the question of whether, and to what extent, woodland and agricultural speckled woods vary intrinsically—relying on heritable variation—in the way that they deal with different temperatures in terms of reproductive output and survival.
Our temperature-gradient experiment aims at testing two predictions on longevity and fecundity, and at exploring variation in egg size and body mass allocation patterns between the different landscapes. The first prediction is: longevity decreases with increasing ambient temperature, and—irrespective of temperature—woodland butterflies survive better than do agricultural landscape butterflies. When fully active in the field, thoracic temperature is within 32 to 34 °C (Shreeve 1984; Van Dyck & Matthysen 1998). When body temperature is permanently at this range, reserves will be sooner exhausted—as metabolic rate is likely to be near the optimum (or at least high). Assuming a higher cost of flight in terms of flight apparatus investment and ‘fuel’ in open landscape where resources are much more scattered than in continuous woodland, we predict agricultural butterflies to have a reduced lifespan as they allocate more resources into their flight morphology and reserves. In a breeding experiment, P. aegeria females had relatively larger thoraxes (i.e. more flight muscles) in agricultural landscape than in woodland (Merckx & Van Dyck, unpublished data). The second prediction is: woodland butterflies lay more eggs at low ambient temperature, whereas agricultural butterflies lay more eggs at higher ambient temperature. Deciduous woodlands are typically relatively cool, but thermally better buffered, environments compared with more open landscapes (Klein 1989; Noss & Csuti 1997). We expect woodland butterflies to be more specialized in producing eggs under cooler conditions, whereas agricultural butterflies are expected to be generalists in terms of temperature-related fecundity. Hence, at high temperature, the latter will perform relatively better than the former. So, we predict a significant interaction effect between temperature regime and landscape of origin for number of eggs laid. Next, we also explore variation in egg size between both landscape groups relative to ambient temperature and interpret the results in relation to desiccation risks on the one hand and to differential time-budget trade-offs, which in turn affect a trade-off between number and size of eggs, on the other hand. Finally, we test for differences in allocation pattern with landscape by comparing thorax and abdomen mass loss. Mostly, studies on allocation in flying insects assume thorax mass to be an indicator of flight investment and abdomen mass an indicator of reproductive potential (e.g. Hill et al. 1999).
2. Methods
(a) Study species
Speckled woods Pararge aegeria (L.) are temperate-zone butterflies that lay their eggs singly on different species of grasses, including Poa annua (Shreeve 1986). Eggs are deposited on grasses surrounded by bare ground at rather shaded, wet sites (Wiklund & Persson 1983). In agricultural landscape, females oviposit on grasses growing under hedgerows (Dover & Sparks 2000). Karlsson & Wiklund (1985) showed that egg mortality increased with decreasing humidity, but was independent of size. At eclosion, females have no (Karlsson 1987), or only a few (8–24; Bink 1992), mature oocytes. Under laboratory conditions (25 °C with the lights on—20 °C at night), females lay on average 167±10 eggs and have an average lifespan of 19–23 days (Karlsson & Wickman 1990; Gotthard et al. 2000). Without access to sucrose solution but only to water, lifespan is less than half (9.5 days; Karlsson & Wickman 1990).
(b) Sampled landscapes and breeding
We collected females in two different landscapes in central Belgium: five females from deciduous woodland landscape (Meerdaalwoud—50°48′ N and 4°40′ E) and four females from agricultural landscape (80% intensively used fields and pastures, 5% commercial orchards, 10% houses and farms) with hedgerows and tiny deciduous woodlots (Rillaar—50°58′ N 4°54′ E; cf. Merckx et al. 2003). Females were captured at several sites with a distance between 500 m and 5 km to avoid the possibility of sampling relatives. Parallel measurements in the period May to July 2003 indicate that temperature profiles of sites used by P. aegeria differ significantly between the landscapes: both average and maximal day temperatures are higher in agricultural than in woodland landscape (average: 17.0±0.2 versus 16.1±0.2 °C—max: 23.8±0.5 versus 21.3±0.5 °C), but mean wind speed was significantly higher in the former landscape (T. Merckx & H. Van Dyck, unpublished data).
In the laboratory, the collected females were allowed to lay eggs. Newly hatched larvae were placed on tufts of the grass Poa annua growing in 400 ml transparent jars. Tufts were changed several times to supply an ample supply of fresh food. Larvae were reared under a direct development regime until eclosion in a climate room (20 °C, LD 22 : 2). Animals from this F1 generation were then mated (cross family matings) and F1 females were allowed to lay eggs in the laboratory. F2 larvae were raised under the same conditions as above. On the day of eclosion, females were weighed on a Sauter AR 1014 balance (accuracy: 0.1 mg) and placed in a climate room at 8 °C. Female mass at eclosion did not differ among the two groups (F1,73=2.38, p>0.12—overall mean fresh body mass: 83.8±1.1 mg). On their second day, females were mated in a 0.5×0.5×0.5 m flight cage in the laboratory with access to 20% sucrose solution as a feeding source.
(c) Oviposition experiment
After mating, females from the F2 generation were immediately and individually transferred to 1 l transparent plastic jars for egg laying. Thirty-seven females were from the Meerdaalwoud population and thirty-eight females were from the Rillaar population. Each jar contained a tuft of the grass Poa annua, which is one of the natural host grasses (Shreeve 1986). The top of each jar was covered with a fine net. A piece of cotton wool soaked in 20% sucrose solution was placed on top of the net to allow females to take in liquid and carbon. The cotton was moistened every day and replaced every third day. Jars were assigned to one of seven environmental treatments that all had the same photoperiodic regime (LD 12 : 12) but differed in constant ambient temperature: 20, 22.5, 25, 27.5, 30, 32.5 and 35 °C, respectively. The number of females in each combination of temperature–landscape of origin was five, except for some combinations: for Meerdaalwoud it was four at 35 °C, six at 22 °C and seven at 25 °C, and for Rilaar, six at 27.5, at 30 and at 35 °C. As many original lineages as possible of available butterflies were used in each combination. The number of eggs laid was counted daily for each female. The eggs laid on the first day of the oviposition experiment by a female were used as a measure of mean egg mass. Eggs were weighed on a Cahn 28 automatic electrobalance (accuracy: 0.001 mg). Fertility of eggs was checked to ensure that only properly inseminated females were included in the analyses.
Besides the total number of eggs laid by a female, we also analysed the number of eggs laid on the first day of oviposition and the maximum number of eggs laid on a single day. These additional measures of fecundity are relevant for interpretations under field conditions because butterflies seldom (if ever) lay their full complement of available eggs and environmental conditions (like poor weather) may reduce fecundity either directly or indirectly by an increased mortality (Warren 1992). Hence, we also test whether fecundity also differs at shorter time-frames than their entire life in captivity.
(d) Analyses and statistics
The Wilk–Shapiro test was used to test for normality, and three variables—lifespan, total dry mass and abdomen mass—were log-transformed to improve normality. The different fecundity measures were analysed in relation to landscape of origin (woodland or agricultural landscape), ambient temperature and their interaction effect using a general linear model. If the interaction term was non-significant it was removed from the model. In these analyses, fresh body mass at eclosion was always used as a covariate. We refer to the experimental group of speckled woods that originated from woodland landscape as ‘woodland butterflies’, and similarly to ‘agricultural butterflies’ for those that originated from the agricultural landscape with hedgerows and some woodlots.
3. Results
(a) Longevity
Females had a mean adult longevity of 14.0±0.7 days (range: 4–37 days). Lifespan declined with increasing temperature; at 35 °C, longevity was reduced by approximately 58% compared with 20 °C (figure 1). Lifespan did not differ between the two groups at the extreme conditions of the ambient temperature gradient, but woodland butterflies lived for longer than agricultural butterflies at intermediate temperatures (figure 1). However, overall lifespan along the temperature gradient was on average about 2 days longer in woodland butterflies compared with agricultural butterflies (model, F8,66=4.75, p=0.0001; factor landscape, F1=4.37, p=0.040; factor temperature, F6=4.88, p=0.0003; interaction factor, not significant (n.s.), removed; covariate fresh body mass (log), F1=0.98, p=0.32).
Figure 1.
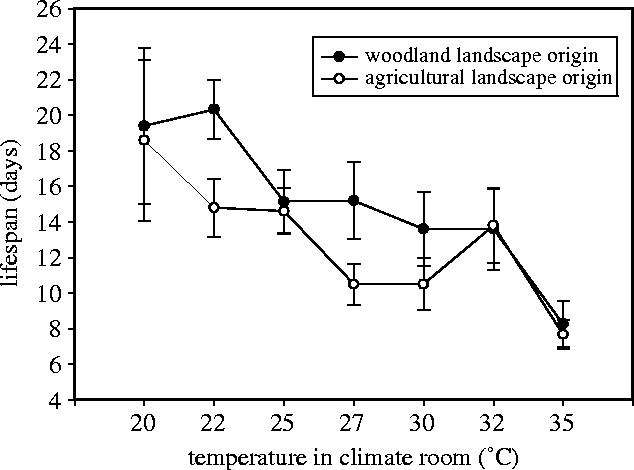
Lifespan of females (F2) originating from butterflies living in open agricultural landscape or closed woodland landscape under seven temperature treatments. Mean values±s.e.
(b) Egg-laying patterns
Females laid on average 176.2±9.8 eggs, but the number of eggs varied with landscape of origin and temperature regime. Lifetime egg number decreased with temperature, but at low temperature, woodland butterflies laid more eggs during their lifetime than agricultural butterflies, while the reverse was true at high temperatures (figure 2a). Therefore, the analysis of lifetime egg number shows a significant landscape of origin×temperature interaction, besides the main effects of both factors (table 1a).
Figure 2.
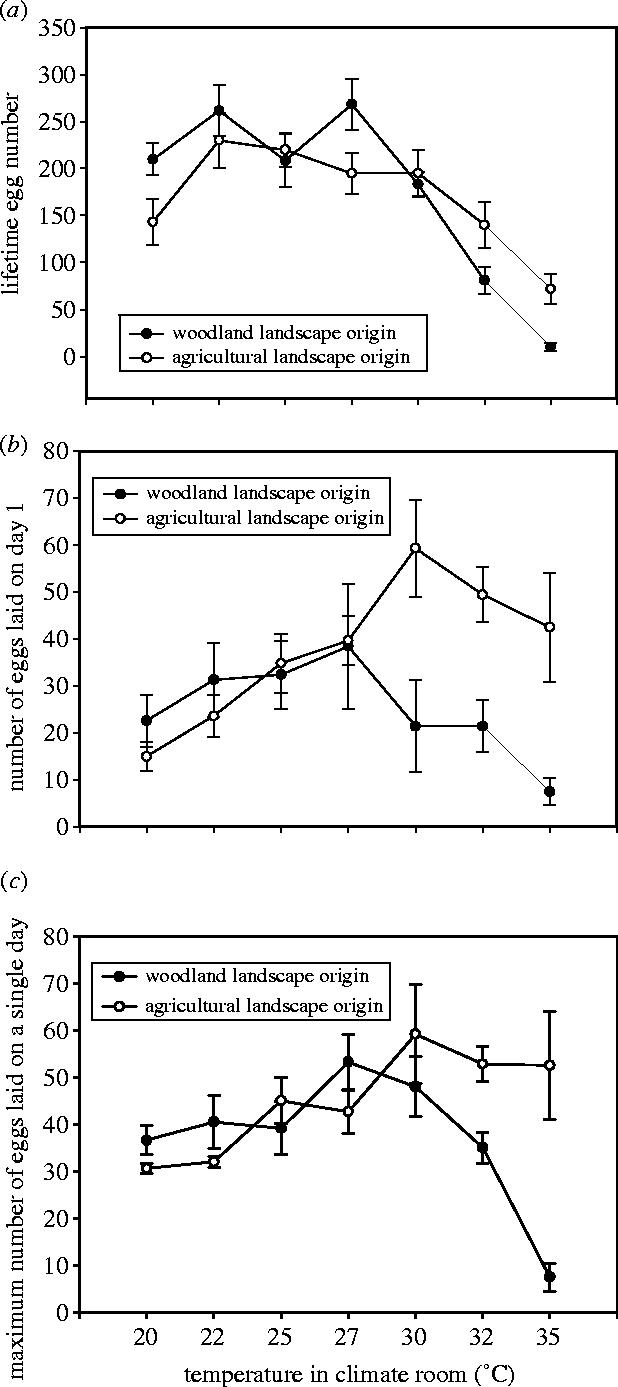
Total lifetime egg production (a), number of eggs laid on the first day of egg laying (b), and the maximum number of eggs laid during one single day (c) of females (F2) originating from butterflies living in open agricultural landscape or closed woodland landscape under seven temperature treatments. Mean values±s.e.
Table 1.
Analyses of different fecundity measures in relation to landscape of origin and temperature treatment (ANCOVAs with general linear model) with fresh body mass (log) as covariate.
| (a) lifetime egg number | ||||
| model | F14,60=9.73, p=0.0001 | landscape | F1=0.54 | p=0.465 |
| temperature | F6=14.88 | p=0.0001 | ||
| landscape×temperature | F6=2.41 | p=0.038 | ||
| log body mass | F1=3.24 | p=0.077 | ||
| (b) number of eggs on first day of oviposition | ||||
| model | F14,60=2.64, p=0.0047 | landscape | F1=7.45 | p=0.0080 |
| temperature | F6=1.64 | p=0.153 | ||
| landscape×temperature | F6=2.85 | p=0.017 | ||
| log body mass | F1=1.87 | p=0.667 | ||
| (c) maximum number of eggs laid in one day | ||||
| model | F14,60=3.57, p=0.0003 | landscape | F1=3.74 | p=0.058 |
| temperature | F6=2.59 | p=0.027 | ||
| landscape×temperature | F6=3.58 | p=0.0042 | ||
| log body mass | F1=1.76 | p=0.189 |
The number of eggs laid on the first day of oviposition was overall lower in butterflies of woodland landscape origin compared with agricultural butterflies, but there was a strong interaction effect with ambient temperature as egg numbers diverged above 27 °C to high levels in agricultural butterflies and to low levels in woodland butterflies (figure 2b and table 1b).
The maximum number of eggs laid in a single day increased gradually with temperature in agricultural butterflies, whereas in woodland butterflies this number decreased strongly after an optimum at 27 °C (figure 2c). Thus, at high temperatures, agricultural butterflies had higher values for the maximum number of eggs laid in one day than woodland butterflies. Therefore, the analysis showed a strong landscape of origin×temperature interaction effect besides a strong main effect of ambient temperature (table 1c).
(c) Egg size
The eggs of agricultural butterflies were significantly heavier than the eggs of woodland butterflies (0.431±0.005 versus 0.409±0.008 mg, respectively). Eggs tended to be smaller with increasing temperature (model, F8,66=4.80, p=0.00011; factor landscape, F1=6.23, p=0.015; factor temperature, F6=3.30, p=0.066; interaction factor, n.s., removed; covariate fresh body mass (log), F1=3.63, p=0.061).
(d) Use of body resources
To control for artefacts and hence to avoid bias in mass variation of the females used in the experimental treatments, fresh body mass did not differ with landscape of origin (cf. §2). Interestingly, total body dry mass after death tended to be smaller in woodland butterflies compared with agricultural butterflies (model, F8,66=4.34, p=0.0003; factor landscape, F6=3.35, p=0.072; factor temperature, F6=0.0011; interaction factor, n.s., removed; covariate fresh body mass (log), F1=3.66, p=0.06). If the insignificant covariate term is also removed from the model, the effect of landscape on final body dry mass is significant (model, F7=4.26, p=0.0006; factor landscape, F1=4.85, p=0.031; factor temperature: F6=3.78, p=0.0026).
Next, we focused separately on the major body parts (using log fresh body mass as the covariate). Dry abdomen mass after death was lower in the woodland butterflies than in the agricultural butterflies (figure 3; model, F8,66=2.51, p=0.019; factor landscape, F1=4.11, p=0.047; factor temperature, F6=2.29, p=0.046; interaction factor, n.s., removed; covariate fresh body mass (log), F1=0.28, p=0.60). Dry thorax mass after death increased with experienced ambient temperature and again there was a landscape effect with a generally lower mass of thorax remaining after death in the woodland butterflies (model, F8,66=10.19, p=0.00001; factor temperature, F6=9.44, p=0.00001; factor landscape, F1=5.14, p=0.027; interaction factor, n.s., removed; covariate fresh body mass (log), F1=19.48, p=0.0001).
Figure 3.
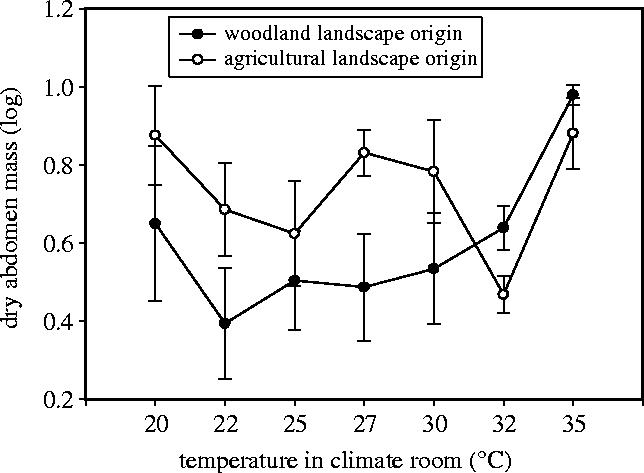
Remaining abdomen mass (mg) after death in females (F2) originating from butterflies living in open agricultural landscape or closed woodland landscape under seven temperature treatments. Mean values±s.e.
Finally, we regressed the total number of eggs as the dependent variable in relation to either log abdomen mass (and separately log thorax mass) and log fresh body mass as independent variables to evaluate the extent to which resources left after death correlate with egg output. Both dry abdomen and thorax correlated significantly with egg output (R2=0.36 and R2=0.67, respectively), but thorax mass after death was a much better predictor of how many eggs a female had laid during her life (figure 4).
Figure 4.
Total number of eggs laid in relation to remaining mass in the thorax after death (controlling for total body mass). Values for total number of eggs are plotted as the partial residual from the regression of total number of eggs on log female body mass and log thorax mass remaining after death. The line is the regression of the partial residual on log thorax mass: yres=−580.9(log(thorax mass)+0.00001), r=−0.793.
4. Discussion
We found that P. aegeria females originating from a continuous woodland landscape differed in a number of life-history traits estimated in a common environment compared with females from a highly fragmented agricultural landscape. In line with our predictions, woodland females reached a higher lifetime number of eggs than did agricultural females at low ambient temperatures, but this reversed as the number of eggs laid by woodland females dropped strongly at high ambient temperature. Because we used offspring from wild-caught females reared under common garden laboratory conditions, the differences with landscape of origin suggest heritable variation among the populations in the way they intrinsically deal with their thermal environment. However, two notes need to be made here. First, maternal effects could in principle be involved, but we are not aware of known maternal effects that would explain the differences. Moreover, we used the F2 generation to avoid eventual maternal effects from the wild-caught females (Zera & Rankin 1989). Second, owing to practical limitations there was not a replication of the landscape effect in the current analyses (Fahrig 2003), which seems to be a more general limitation of evolutionary ecological studies making comparisons among types of landscape (e.g. Partecke et al. 2004). Hence, at this stage our results are highly suggestive, but not definitively conclusive for the landscape structure effect per se.
Both groups originated from very different landscapes, but the geographical distance among the sites is only approximately 20 km. According to Hill et al. (2003), the estimated maximum dispersal rate of P. aegeria is 2.7 km yr−1 in Britain and 1.03 km yr−1 in Denmark (i.e. estimated rate of expansion during the twentieth century without correcting for geometric distribution of potential new areas for colonization). The dispersal range of the average individual is likely to be considerably less than such values of an invasion front (Hill et al. 1999). However, in Drosophila there are cases where microclimatic natural selection overrides the importance of migration at even much smaller spatial scale as multiple adaptive differences between D. melanogaster isofemale lines derived from two opposite slopes of a canyon that contrast sharply in physical factors have been shown (Nevo et al. 1998). Hence, our results point at a contribution to the diverse and highly significant effects that habitat fragmentation may have on invertebrate life history through changed thermal profiles of landscapes.
At high ambient temperature, fecundity differences between the two landscape groups were particularly pronounced for the number of eggs laid on the first day (figure 2b) and for the maximum number of eggs laid on a single day (figure 2c). So, the influence on oviposition has an instant effect from the first day. This most probably relies on the fact that P. aegeria has only very few mature eggs at emergence. Whether this means that species with more mature oocytes at emergence are better buffered against variation in temperature, remains to be examined. Besides differences in total fecundity, the two fecundity measures for shorter time-frames are particularly relevant for extrapolation under field conditions where longevity is likely to be reduced compared with our predation-free experiments. They also provide additional insight in the temporal pattern to attain a particular level of lifetime fecundity. At average temperature (25–27 °C), females of both landscape groups lay approximately 35 eggs on the first day, which represents approximately 15% of lifetime fecundity. At high temperature (32–35 °C), this number increased in the agricultural group (ca. 45 eggs) and decreased in the woodland group (ca. 10 eggs). But the proportional importance of the first day differs as well, since these numbers represent 45% and 22%, respectively, of lifetime fecundity.
Our results on egg size showed that egg mass decreased with increasing ambient temperature. Fischer et al. (2003) have recently shown that the relationship of cooler butterflies laying larger eggs could be induced by either developmental temperature (developmental plasticity) or ovipositing temperature (acclimation). In our experiment, only ovipositing temperature was varied, but Fischer et al. (2003) found that effects of developmental environment could be largely reversed by acclimation in the adult stage. Further experiments are required to evaluate whether this plastic response of egg size to thermal change is adaptive or rather results from non-adaptive physiological constraints.
Wiklund & Persson (1983) showed that P. aegeria egg weight was not correlated with hatchability, mortality, larval growth rate or pupal weight under standard laboratory conditions (with constant levels of humidity). In addition to the effect of ovipositing temperature, eggs of females from agricultural landscape were heavier (i.e. lower surface to volume ratio). Two, not mutually exclusive, adaptive hypotheses provide an explanation. The first hypothesis refers to adaptive differences in egg size in terms of juvenile survival relative to environmental conditions. Desiccation risks for both eggs and host grasses are expected to be higher along hedgerows and in small woodlots experiencing higher levels of desiccating wind and higher ambient temperature compared with continuous woodland having a more humid, cool and thermally buffered microclimate. Pararge aegeria abundance decreases significantly with warm, dry weather in the previous summer, and the species is susceptible to desiccation of host plants through drought (Pollard 1988). Using females from Swedish woodland, egg size had no effect in a breeding experiment with constant temperature and humidity on several fitness variables of the offspring, including egg and larval survival and pupal weight (Wiklund & Persson 1983). Owing to a higher surface/volume ratio, larger eggs would in principle be better to cope with desiccation. Wiklund & Persson (1983) argued that relative humidity is unlikely to be a selective factor in this respect, since females almost invariantly deposit eggs in wet parts of the forest. However, in agricultural landscape the places used for oviposition are typically much drier and warmer (T. Merckx & H. Van Dyck, unpublished data). The same host grasses will more frequently contain lower water and nutrient content—hence they are a tougher feeding source—in an agricultural landscape compared with woodland landscape (e.g. Mika et al. 1998). This needs to be validated by field data. As larger eggs produce young caterpillars with larger mandibles, they are better capable of feeding tough material (Braby 1994).
A second hypothesis refers to differential time allocation for oviposition with landscape. It is in line with the hypothesis of Wiklund & Persson (1983) on a general trade-off between egg-laying and time spent searching for suitable oviposition sites. In an agricultural landscape, females have to move from hedgerow to hedgerow (or woodlot) to spread the eggs on suitable host plants. So, average distance between oviposition events during the track of life will be much larger compared with females of continuous woodland. Translated in terms of time, agricultural butterflies will be able to lay fewer eggs per time unit. Assuming that egg number is traded off against egg size (e.g. Fischer & Fiedler 2001), woodland females are expected to lay smaller eggs, which corresponds with our results. The real extent of such a trade-off may be partly hidden when butterflies cannot fly large distances in their experimental cages. Hence, differences under field conditions can be larger if the differential cost of flight between both types of landscape is operating.
Total fresh mass was equal among groups before the experiment, but dry mass at death differed significantly for total mass, thorax mass and abdomen mass. This suggests heritable differences in resource-use associated with landscape of origin. Woodland butterflies used overall more of their body resources compared with agricultural ones. However, patterns of resource allocation and use are not only a function of fecundity and longevity, but also of other ecological functions like dispersal. Because we know that there are differences in movements and behaviour at boundaries between females from woodland versus agricultural landscape (Merckx et al. 2003), a comparison of temperature-related variation in female fecundity and longevity may indeed be affected or confounded by life-history trade-offs with mobility or dispersal. It has recently been shown that P. aegeria had an increased dispersal ability—measured indirectly by thorax size—associated with reduced fecundity at the front of a distributional expansion compared with the centre of the range within the UK (Hughes et al. 2003). This difference in fecundity was not reflected in abdomen mass (Hill et al. 1999). Observations by Karlsson (1987) indicate that wild females do not maintain constant reproductive reserves by adult feeding. As reproductive reserves decline with number of eggs laid, egg weight and egg production decrease. The use of body resources is largely mirrored by the output of eggs (figure 2 versus figure 3). There is circumstantial evidence that resources derived from flight muscles by histolysis are used for egg production as females age (Karlsson 1994). This may explain, in contrast to what is widely assumed, why loss of thorax mass was a much better predictor of realized egg output than abdomen mass loss. Knowing that the relationship with thorax mass explained approximately 67% of the variation in egg output, the measure may have a great value as a surrogate estimate of realized fecundity in other experiments.
The observed relationships between fecundity and ambient temperature among populations living in different thermal biotopes show interesting parallels to patterns at the interspecific level. In a similar oviposition experiment along a temperature gradient, Karlsson & Wiklund (2005) found that lifetime fecundity peaked at higher ambient temperatures in satyrine butterfly species confined to dry and warm open biotopes compared with shade-dwelling satyrine butterflies associated with woodland, including P. aegeria.
Analyses of current effects of temperature are essential for prediction of future impacts on natural populations under global warming scenarios (Parmesan 2003; Thomas et al. 2004). Butterflies have been called suitable bio-indicators in this respect, and the evidence for climate-related changes in occurrence over recent decades has accumulated considerably for both spatial (Parmesan et al. 1999; Hill et al. 2003) and temporal patterns (Roy & Sparks 2000). Our results suggest that differential selection regimes associated with different landscapes may intervene by intraspecific variation in the response of a butterfly to variation in ambient temperature. This study does obviously not provide insight on the generality of the importance of landscape-related evolutionary responses on climate change, but it provides an intriguing scope for further mechanistic studies to understand life-history variation and changes in changing environments, including climate change (Boggs 2003).
Acknowledgments
Thanks are due to Thomas Merckx for assistance with collecting butterflies. This research was funded by the University of Antwerp (GOA 15R/3942) to H.V.D. (and colleagues), by a grant from the Swedish Research Council for Environment, Agricultural Sciences and spatial planning to B.K., and by a grant from Magn. Bergvall foundation to B.K.
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
References
- Bink F.A. Schuyt & Co.; Haarlem, The Netherlands: 1992. Ecologische atlas van de dagvlinders van Noordwest-Europa. [Google Scholar]
- Boggs C.L. Environmental variation, life histories, and allocation. In: Boggs C.L, Watt W.B, Ehrlich P.R, editors. Butterflies: ecology and evolution taking flight. University of Chicago Press; Chicago: 2003. pp. 185–206. [Google Scholar]
- Braby M.F. The significance of egg size variation in butterflies in relation to host plant quality. Oikos. 1994;71:119–129. [Google Scholar]
- Dover J, Sparks T. A review of the ecology of butterflies in British hedgerows. J. Environ. Magnmt. 2000;60:51–63. [Google Scholar]
- Fahrig L. Effects of habitat fragmentation on biodiversity. Ann. Rev. Ecol. Evol. Syst. 2003;34:487–515. [Google Scholar]
- Fischer K, Fiedler K. Egg weight variation in the butterfly Lycaena hippothoe: more small or fewer large eggs? Popul. Ecol. 2001;43:105–109. [Google Scholar]
- Fischer K, Eenhhoorn E, Bot A.N.M, Brakefield P.M, Zwaan B.J. Cooler butterflies lay larger eggs: developmental plasticity versus acclimation. Proc. R. Soc. B. 2003;270:2051–2056. doi: 10.1098/rspb.2003.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotthard K, Nylin S, Wiklund C. Mating opportunity and the evolution of sex-specific mortality rates in a butterfly. Oecologia. 2000;122:36–43. doi: 10.1007/PL00008833. [DOI] [PubMed] [Google Scholar]
- Hill J.K, Thomas C.D, Blakeley D.S. Evolution of flight morphology in a butterfly that has recently expanded its geographic range. Oecologia. 1999;121:165–170. doi: 10.1007/s004420050918. [DOI] [PubMed] [Google Scholar]
- Hill J.K, Thomas C.D, Huntley B. Modeling present and potential future ranges of European butterflies using climate response surfaces. In: Boggs C.L, Watt W.B, Ehrlich P.R, editors. Butterflies: ecology and evolution taking flight. University of Chicago Press; Chicago: 2003. pp. 149–167. [Google Scholar]
- Hughes C.L, Hill J.K, Dytham C. Evolutionary trade-offs between reproduction and dispersal in populations at expanding range boundaries. Proc. R. Soc. B. 2003;270(Suppl. 2):S147–S150. doi: 10.1098/rsbl.2003.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen E, Aars J, Andreassen H.P, Ims R.A. A demographic analysis of vole population responses to fragmentation and destruction of habitat. Popul. Ecol. 2003;45:47–58. [Google Scholar]
- Jones H. Cambridge University Press; New York: 1992. Plants and microclimate: a quantitative approach to environmental plant physiology. [Google Scholar]
- Karlsson B. Variation in egg weight, oviposition rate and reproductive reserves with female age in a natural population of the speckled wood butterfly, Pararge aegeria. Ecol. Entomol. 1987;12:473–476. [Google Scholar]
- Karlsson B. Feeding-habits and change of body-composition with age in 3 nymphalid butterfly species. Oikos. 1994;69:224–230. [Google Scholar]
- Karlsson B, Wickman P.-O. Increase in reproductive effort as explained by body size and resource allocation in the speckled wood butterfly Pararge aegeria (L.) Funct. Ecol. 1990;4:609–617. [Google Scholar]
- Karlsson B, Wiklund C. Egg weight variation in relation to egg mortality and starvation endurance of newly hatched larvae in some satyrid butterflies. Ecol. Entomol. 1985;10:205–211. [Google Scholar]
- Karlsson B, Wiklund C. Butterfly life-history and temperature adaptations: dry open habitats select for increased fecundity and longevity. J. Anim. Ecol. 2005;(74):99–104. [Google Scholar]
- Klein B.C. Effects of forest fragmentation on dung and carrion beetle communities in central Amazonia. Ecology. 1989;70:1715–1725. [Google Scholar]
- Malcolm J.R. A model of conductive heat flow in forest edges and fragmented landscapes. Clim. Change. 1998;39:487–502. [Google Scholar]
- Matlack G.R. Microenvironment variation within and among forest edge sites in the eastern United States. Biol. Conserv. 1993;66:185–194. [Google Scholar]
- Merckx T, Van Dyck H, Karlsson B, Leimar O. The evolution of movements and behaviour at boundaries in different landscapes: a common arena experiment with butterflies. Proc. R. Soc. B. 2003;270:1815–1821. doi: 10.1098/rspb.2003.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C.L, Sisk T.D. Butterfly response to microclimatic conditions following Ponderosa pine restoration. Restorat. Ecol. 2001;9:453–461. [Google Scholar]
- Mika V, Kohoutek A, Beran S. Grass quality at shading of the stand. Rostlinna Vyroba. 1998;44:431–436. [Google Scholar]
- Nevo E, Rashkovetsky E, Pavlicek T, Korol A. A complex adaptive syndrome in Drosophila caused by microclimatic contrasts. Heredity. 1998;80:9–16. doi: 10.1046/j.1365-2540.1998.00274.x. [DOI] [PubMed] [Google Scholar]
- Noss R.F, Csuti B. Habitat fragmentation. In: Meffe G.K, Carroll C.R, editors. Principles of conservation biology. 2nd edn. Sinauer Associates, Inc; Sunderland, MA: 1997. pp. 269–304. [Google Scholar]
- Parmesan C. Butterflies as bioindicators for climate change effects. In: Boggs C.L, Watt W.B, Ehrlich P.R, editors. Butterflies: ecology and evolution taking flight. The University of Chicago Press; Chicago: 2003. pp. 541–560. [Google Scholar]
- Parmesan C, et al. Polewards shifts in geographic ranges of butterfly species associated with regional warming. Nature. 1999;399:579–583. [Google Scholar]
- Partecke J, Van't Hof T, Gwinner E. Differences in the timing of reproduction between urban and forest European blackbirds (Turdus merula): result of phenotypic flexibility or genetic differences? Proc. R. Soc. B. 2004;271:1995–2001. doi: 10.1098/rspb.2004.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Barrie B, Barton N.H, Fowler K, French V. Rapid laboratory evolution of adult life-history traits in Drosophila melanogaster in response to temperature. Evolution. 1995;49:538–544. doi: 10.1111/j.1558-5646.1995.tb02285.x. [DOI] [PubMed] [Google Scholar]
- Pollard E. Temperature, rainfall and butterfly numbers. J. Appl. Ecol. 1988;25:819–828. [Google Scholar]
- Roy D.B, Sparks T.H. Phenology of British butterflies and climate change. Global Change Biol. 2000;6:407–416. [Google Scholar]
- Shreeve T.G. Habitat selection, mate-location, and micro-climatic constraints on the activity of the speckled wood butterfly Pararge aegeria. Oikos. 1984;42:371–377. [Google Scholar]
- Shreeve T.G. Egg-laying by the speckled wood (Pararge aegeria): the role of female behaviour, host plant abundance and temperature. Ecol. Entomol. 1986;11:229–236. [Google Scholar]
- Thomas C.D. Dispersal and extinction in fragmented landscapes. Proc. R. Soc. B. 2000;267:139–145. doi: 10.1098/rspb.2000.0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.D, Cameron A, Green R.E, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- Tolman T, Lewington R. HarperCollins; London: 1997. Butterflies of Britain and Europe. [Google Scholar]
- Travis J.M.J, Dytham C. Habitat persistence, habitat availability and the evolution of dispersal. Proc. R. Soc. B. 1999;266:723–728. [Google Scholar]
- Van Dyck H, Matthysen E. Thermoregulatory differences between phenotypes in the speckled wood butterfly: hot perchers and cold patrollers? Oecologia. 1998;114:326–334. doi: 10.1007/s004420050454. [DOI] [PubMed] [Google Scholar]
- Warren M.S. Butterfly populations. In: Dennis R.L.H, editor. The ecology of butterflies in Britain. Oxford University Press; Oxford: 1992. pp. 73–92. [Google Scholar]
- Wiklund C, Persson A. Fecundity, and the relation of egg weight variation to offspring fitness in the speckled wood butterfly Pararge aegeria, or why don't butterfly females lay more eggs? Oikos. 1983;40:53–63. [Google Scholar]
- Zera A.J, Rankin M.A. Wing dimorphism in Gryllus rubens: genetic basis of morph determination and fertility differences between morphs. Oecologia. 1989;80:249–255. doi: 10.1007/BF00380159. [DOI] [PubMed] [Google Scholar]



