Abstract
Insulin-like growth factor (IGF-II) is overexpressed in a variety of human tumors and has both mitogenic and antiapoptotic activity. Although the mechanisms of IGF-II-induced proliferation have been well studied, the mechanisms underlying its survival signaling have been less well characterized. In this report, we investigated the role of IGF-II on cisplatin-induced apoptosis. We found that IGF-II overexpression was associated with an increase in p70 ribosomal protein S6 kinase (p70 S6K). Cisplatin treatment of C2C12 mouse myoblasts led to cell death associated with an inhibition of p70 S6K activity. Endogenous or exogenous IGF-II addition to C2C12 cells caused protection to cisplatin-induced apoptosis. This protection was associated in both cases with an increase in p70 S6K basal activity as well as resistance to cisplatin-induced decreased activity. Blockade of p70 S6K activation by rapamycin abrogated the IGF-II-mediated protection of cells to cisplatin-induced apoptosis. Furthermore, treatment of IGF-II-overexpressing Rh30 and CTR rhabdomyosarcoma cells with rapamycin restored sensitivity to cisplatin-induced apoptosis. These data together suggest that IGF-II-associated protection to cisplatin-induced apoptosis is mediated through an activation of the p70 S6K pathway. Thus, inhibition of the p70 S6 pathway may enhance chemotherapy-induced apoptosis in the treatment of IGF-II-overexpressing tumors.
Keywords: IGF-II, p70 S6K, cisplatin, apoptosis, survival
Introduction
Growth factors act through activation of specific transmembrane receptors to elicit regulation of cell growth and differentiation. This, in turn, triggers a variety of intracellular signaling pathways that ultimately control cell physiology. Insulin-like growth factor-II (IGF-II) is a paternally expressed, maternally imprinted, embryonic growth promoter and cell survival factor that plays a major role in cell growth, survival, differentiation, and development [1,2]. It is widely believed that IGF-II exerts its effects through the IGF-I receptor, a ligand-activated tyrosine protein kinase with high homology to the insulin receptor [3], and these effects are modulated by a family of specific IGF-binding proteins [4,5].
Elevated levels of IGF-II have been detected in many tumor types and likely promote cell proliferation through endocrine and paracrine/autocrine signaling [6–8].We have previously shown that forced overexpression of IGF-II in C2C12 myoblasts leads to transformed characteristics [9]. Furthermore, IGF-II overexpression was associated with a diminished G1 checkpoint after γ-irradiation and altered cell-cycle regulation in these cells [10]. IGF-II also plays an important role in maintaining cell survival during the transition from proliferating to terminally differentiating myoblasts [11]. Disruption of IGF-II expression in transgenic mice expressing T antigen in pancreatic cells causes a dramatic increase of β cell tumor apoptosis [12]. These results indicate that IGF-II acts as a survival factor. The signal transduction pathways involved in IGF-II-mediated cell survival have not been fully characterized.
Recent studies have shown that the p70 ribosomal protein S6 kinase (p70 S6K) plays a critical role in cell growth by modulating the translation of a family of mRNAs that contain an oligopyrimidine tract at their transcriptional start, which encodes components of the protein synthetic apparatus [13]. p70 S6K was first identified as an enzyme that catalyzes the phosphorylation of the S6 protein, a component of the 40S subunit of the eukaryotic ribosome [14]. Activation of p70 S6K is triggered by various growth factors. Inhibition of the kinase activation impedes cell growth and blocks cells in the G1 phase of the cell cycle [15,16]. Rapamycin blocks p70 S6K activation through inhibition of mammalian target of rapamycin (mTOR), a large-molecular-weight protein that is thought to serve as either a lipid or a protein kinase [17,18]. Activation of p70 S6K is accompanied by its phosphorylation on multiple serine and threonine residues [14,16]. Detailed analysis of the correlation between changes in the phosphorylation of individual sites in p70 S6K and its activity suggests that threonine 389 (Thr389) is particularly important [14]. The Thr389 Ala mutant is catalytically inactive. The Thr389 Glu mutant is largely insensitive to rapamycin [19]. Phosphorylation of Thr389 by mTOR has recently been demonstrated [20]. Taken together, Thr389 is a key target residue for the rapamycin-sensitive input, which leads to p70 SK activation.
Rapamycin-induced p70 S6K dephosphorylation and inactivation have been shown to be paralleled by dephosphorylation of the eukaryotic initiation factor 4E (elF4E) binding protein (4E-BP1) [21,22], showing that it is also downstream of mTOR. Increased phosphorylation of 4E-BP1 leads to its release from elF4E, allowing the initiation factor to then interact with the elF4G subunit of the mRNA cap-binding protein complex [21]. In contrast, dephosphorylated 4E-BP1 interacts with elF4E and thereby inhibits cap structure-dependent protein synthesis and cell growth [23].
In this study, we examined the signal transduction pathways involved in IGF-II-mediated skeletal muscle cell survival. We treated the IGF-II-overexpressing C2C12-2.7 cells and IGF-II-pretreated C2C12 myoblasts with cisplatin and compared the effects to vector control C2C12-1.1 and parental C2C12 cells. The control C2C12-1.1 and parental C2C12 cells undergo apoptosis after cisplatin treatment, whereas the IGF-II overexpressing C2C12-2.7 and IGF-II-pretreated C2C12 cells are relatively resistant to cisplatin-induced apoptosis. The IGF-II-mediated protection to cisplatin-induced apoptosis was associated with increased p70 S6 kinase. Treatment of IGF-II overexpressing mouse myoblast cells (C2C12-2.7) as well as rhabdomyosarcoma cells (Rh30 and CTR) with rapamycin, an inhibitor of p70 S6K, restores sensitivity to cisplatin. Our data imply that IGF-II-mediated antiapoptotic signaling may be mediated through the p70 S6K pathway.
Materials and Methods
Cell Cultures and Treatment
The mouse myoblast cells lines, C2C12-1.1 (vector control) and C2C12-2.7 (IGF-II overexpressing), were generated from the C2C12 cell line by C. P. Minniti and have previously been described [9]. Rh30 and CTR rhabdomyosarcoma cells have been described [24]. These cells were seeded in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, l-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 U/ml). Where indicated, cells were treated with 25 µM cisplatin (Sigma, St. Louis, MO) at indicated times.
Flow Cytometry
Flow cytometry analysis was performed as has previously been described in detail [10]. Briefly, confluent cells were treated as indicated in Figures 1 and 3, washed in PBS, and fixed in ice-cold 70% ethanol for at least 1 hour at 4°C. After washing in PBS containing 0.1% glucose, cells were treated with PBS staining buffer containing RNase A (1 mg/ml), propidium iodide (50 µg/ml), and 0.1% glucose in the dark at 4°C for 30 minutes A total of 10,000 cells were analyzed with a FACScan (Becton, Dickinson, San Diego, CA).
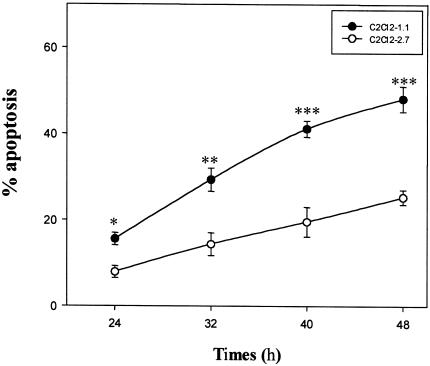
Figure 1.
Prevention of cell death by IGF-II overexpression. Confluent C2C12-1.1 and -2.7 cells were treated with or without cisplatin (25 µM) in 10% FBS medium at indicated time periods. Attached and floating cells were collected and analyzed on a FACScan flow cytometer for relative DNA content. The means±SE of triplicate determinations are shown. *P < 0.025; **P <0.01; ***P <0.001.
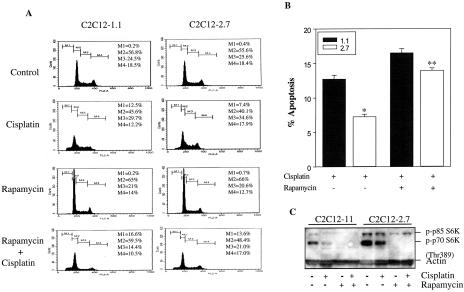
Figure 3.
Rapamycin abrogates the IGF-II-mediated protection of cells to cisplatin-induced apoptosis. (A) C2C12-1.1 and -2.7 cells were preincubated with rapamycin (100 nM) for 3 hours and then treated with or without cisplatin (25 µM) for 24 hours in 10% FBS medium. Cells were stained with propidium iodide and then analyzed by flow cytometry as described in Materials and Methods. The area labeled M1 is an apoptotic population, M2 is G1, M3 is S, and M4 is G2/M phase population. (B) The means±SE of triplicate determinations are shown. *P <0.005; **P>0.05. (C) Cells were preincubated with rapamycin (100 nM) for 3 hours and then treated with or without cisplatin (25 µM) for 24 hours in 10% FBS medium. Cell lysates were subjected to Western blotting with antibodies against phospho-p70 S6K and actin.
Western Blotting
Cells were treated as indicated in the figures. Cisplatin was purchased from Sigma. Rapamycin was obtained from Calbiochem (La Jolla, CA). IGF-II was supplied from R&D Systems, (Minneapolis, MN). These cells were lysed in lysis buffer (20 mM Tris-HCl, pH 7.5; 150 mM sodium chloride; 1 mM EDTA; 1 mM EGTA; 1% triton; 2.5 mM sodium pryophosphate; 1 mM β-glycerolphosphate; 1 mM sodium orthovanadate; 0.5 mM phenylmethylsulfonyl fluoride; 1 µg/ml leupeptin). Protein lysates (20–50 µg/lane), as determined by a Bio-Rad protein assay, were separated in 10–12% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) and then transferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech, Piscataway, NJ). Membranes were blocked with 5% nonfat dried milk in TBS-T (20 mM Tris-HCl, pH 7.5; 8 g/l of sodium chloride; 0.1% Tween 20) and then incubated with primary antibodies against phospho-p70 S6K (Thr389), p70 S6K, phospho-4E-BP1 (Ser65) (Cell Signaling Technology, Beverly, MA) and Actin (Amersham Pharmacia Biotech). Horseradish peroxidase conjugated anti-rabbit Ig G (Cell Signaling Technology) was used as a secondary antibody. Protein was visualized using an ECL system (Amersham Pharmacia Biotech).
p70 S6 Kinase Assay
C2C12-1.1 and C2C12-2.7 cells were treated with 100 nM rapamycin, or 25 µM cisplatin, or both for 16 hours and then lysed in lysis buffer as described above. Protein concentrations in cell extracts were determined by a Bio-Rad protein assay, and an equal amount of protein was used for each assay. Proteins were immunoprecipitated with anti-p70 S6K antibody (Cell Signaling Technology) overnight at 4°C. The immunocomplex was captured with 20 µl of protein-A agarose (Santa Cruz Biotechnology) for 1 hour at 4°C. The immunoprecipitates were washed three times in lysis buffer and once in kinase assay buffer (20 mM MOPS, pH 7.2, 25 mM β-glycerol phosphate, 5 mM EGTA, 1 mM sodium orthovanadate, 1 mM dithiothreitol). After washing, the p70 S6 kinase activity was measured with a p70 S6 kinase assay kit (Upstate Biotechnology, Lake Placid, NY) by using an S6 kinase peptide (AKRRRLSSLRA) as the substrate in accordance with the manufacturer's instructions.
MTT assay
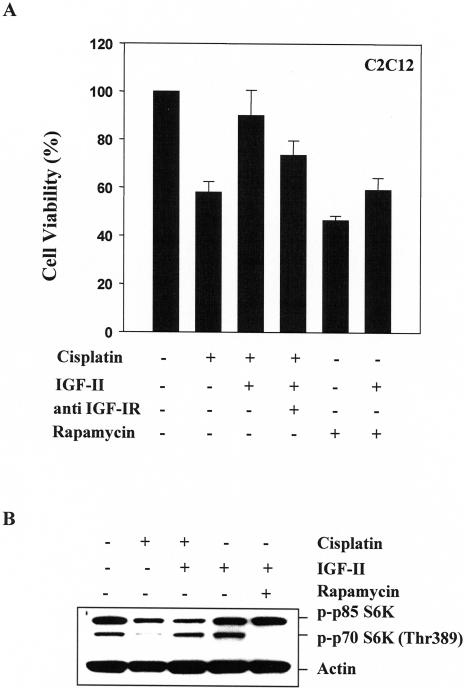
Log-phase cells were harvested and seeded in 96-well plates (1 x 104 cells/well). C2C12 cells were pretreated overnight with a monoclonal antibody against the IGF-I receptor (αIR3) at 1 µg/ml (Oncogene Science, Cambridge, MA), incubated with or without 25 ng/ml IGF-II for 1 hour, and then treated with 25 µM cisplatin or 100 nM rapamycin for 24 hours. Cell survival was determined using the MTT assay according to the manufacturer's instructions (Promega, Madison, WI).
Immunoprecipitation
C2C12 cells were pretreated with 25 ng/ml IGF-II for 1 hour followed by treatment with 25 µM cisplatin or 100 nM rapamycin for 24 hours and then lysed in lysis buffer as described above. Protein concentrations in cell extracts were determined by a Bio-Rad protein assay. Proteins (300 µg) were immunoprecipitated with an anti-phoshho4E-BP1 (Ser65) antibody (Cell Signaling Technology) for 4 hours at 4°C. The immunocomplex was captured with 20 µl of protein-A agarose overnight at 4°C. The immunoprecipitates were washed three times in a lysis buffer, resolved on 4–20% SDS-PAGE, and then transferred to a PVDF membrane. The membrane was blocked with 5% nonfat dried milk in TBS-T and then incubated with the primary antibody against phospho4E-BP1 (Ser65). Horseradish peroxidase conjugated anti-rabbit Ig G was used as a secondary antibody. Antigen-antibody complexes were visualized using ECL system (Amersham Pharmacia Biotech).
Statistical Analysis
Data are presented as mean±standard error (SE). Statistical significance was determined using a two-sided Student's t test. A P value of <0.05 was considered significant.
Results
IGF-II Overexpression Protected Cells from Apoptosis
We have previously demonstrated that IGF-II-overexpressing C2C12-2.7 cells have a diminished G1 checkpoint following DNA damage compared to control C2C12-1.1 cells. [10] To analyze in more detail the role of IGF-II as a survival factor, we exposed confluent C2C12-1.1 and C2C12-2.7 cells to cisplatin, an apoptosis-inducing agent, for varying times. As shown in Figure 1, treatment of C2C12-1.1 cells with 25 µM cisplatin significantly induced cell death in a time-dependent fashion as determined by propidium iodide staining and flow cytometry analysis. By contrast, C2C12-2.7 cells showed a lower incidence of cisplatin-induced apoptosis. After treatment with 25 µM cisplatin at all time points, there is a clear difference between the percentage of apoptotic cells of IGF-II-transfected cells and nontransfected cells (P<0.025 at 24 hours; P<0.01 at 32 hours; P<0.001 at 40 and 48 hours). We treated additional IGF-II-transfected C2C12 myoblast cell lines, and similar results were obtained by MTT assay (data not shown). These data indicate IGF-II overexpression protects these cells from apoptosis induced by cisplatin.
Inhibition of p70 S6K Induced by Cisplatin Was Reversed by IGF-II Overexpression
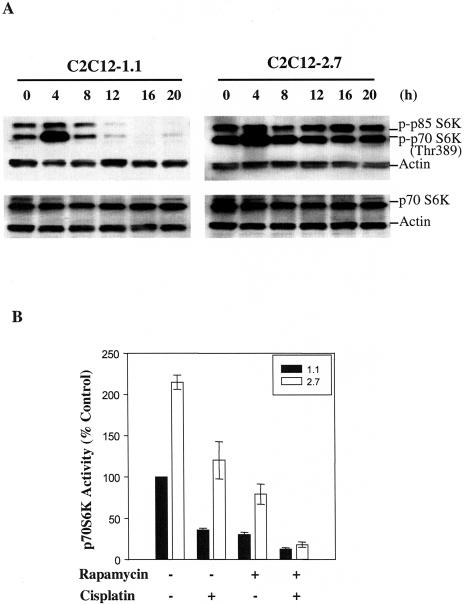
The results demonstrated in Figure 1 prompted us to evaluate the signal transduction cascades involved in IGF-II-regulated skeletal muscle cell survival. To determine whether p70 S6K activation was necessary for IGF-II-mediated survival signaling, we examined p70 S6K phosphorylation and expression in C2C12-1.1 and C2C12-2.7 cells treated with cisplatin by Western blotting analysis. As shown in Figure 2A, p70 S6K phosphorylation was significantly inhibited by cisplatin after 12 hours treatment in C2C12-1.1 cells. However, no significant change in the level of p70 S6K expression was detected in these cells. This cisplatin effect was not seen in IGF-II overexpressing C2C12-2.7 cells as well other IGF-II-transfected myoblast cell lines (data not shown). In contrast to the inhibition of p70 S6 kinase, cisplatin had a limited effect on inhibiting Akt phosphorylation in C2C12-1.1 or 2.7 cells (data not shown). In addition, p70 S6K phosphorylation was transiently increased by cisplatin treatment at 4 hours in both cells, which may be induced by direct cisplatin-mediated cell toxicity.
Figure 2.
Cisplatin-induced inhibition of p70 S6K phosphorylation is reversed by IGF-II overexpression. (A) Confluent C2C12-1.1 and -2.7 cells were treated with or without 25 µM cisplatin in 10% FBS medium at indicated times and then lysed in lysis buffer as described under Materials and Methods. Protein extracts (50 µg/lane) were separated on 10% SDS-PAGE gels, and subjected to Western blot analysis. Blotting with antibody against actin was used to demonstrate equal protein loading in each lane. Similar results were achieved in three separate experiments. (B) Confluent C2C12-1.1 and -2.7 cells were exposed to rapamycin (100 nM), or cisplatin (25 µM), or both for 16 hours in 10% FBS medium. p70 S6 kinase activity was measured using a peptide substrate (AKRRRLSSLRA) as described in detail in Materials and Methods. The p70 S6K activity in C2C12-1.1 cells was set at 100% as a control. Data are mean±SE (n=5).
Stimulation of p70 S6K Activity by IGF-II Overexpression
As demonstrated in Figure 2A, inhibition of p70 S6K phosphorylation by cisplatin in C2C12-1.1 cells was reversed by IGF-II overexpression. Transfection with IGF-II strongly induces p70 S6K activation compared with control cells (data not shown). Furthermore, similar results were obtained for p70 S6K enzymatic activity, as evaluated by an in vitro kinase assay. p70 S6K activity was induced more than two-fold in C2C12-2.7 cells compared to C2C12-1.1 cells (Figure 2B). Cisplatin and rapamycin were shown to inhibit p70 S6K activity, respectively, in both cell lines. However, the activity of p70 S6K was higher in C2C12-2.7 cells compared to C2C12-1.1 cells after treatment with cisplatin and rapamycin (Figure 2B). Thus, resistance to cisplatin-induced apoptosis may reflect a marked increase in p70 S6K activity stimulated by IGF-II overexpression. Preincubation of both cell lines with rapamycin (100 nM) followed by treatment with cisplatin (25 µM) resulted in abolishing the difference on the p70 S6K activity between C2C12-1.1 and C2C12-2.7 cells (Figure 2B). Together, these results show that IGF-II overexpression induces a significant activation of p70 S6 kinase. Furthermore, rapamycin prevents activation of p70 S6K associated with IGF-II overexpression.
The Effect of IGF-II Overexpression on Promoting Cell Survival Was Blocked by Rapamycin
To further evaluate the role of the p70 S6 kinase in IGF-II-mediated protection from apoptosis induced by cisplatin, confluent C2C12-1.1 cells and C2C12-2.7 cells were treated with rapamycin (100 nM) for 3 hours, followed by a 24-hour incubation with or without cisplatin (25 µM). Cells were stained with propidium iodide and then analyzed by flow cytometry. As seen in Figure 3A, the area labeled M1 represents the apoptotic population and the area of the peak labeled M2 represents the G1 phase population. Pretreatment of both control and C2C12-2.7 cells with rapamycin followed by treatment of cisplatin enhanced the effect of cisplatin-induced apoptosis compared to treatment with cisplatin alone in both cell lines (16.6% vs 12.5% in C2C12-1.1 control cells and 13.6% vs 7.4% in C2C12-2.7 cells). IGF-II-mediated protection to cisplatin in IGF-II-transfected cells (Figure 2B, P<0.025) was reversed by pretreatment of these cells with rapamycin (Figure 2B, P> 0.05). Similar data was obtained by MTT assay (data not shown). Thus, blockade of the p70 S6K pathway abolished IGF-II-mediated protection to cisplatin-induced apoptosis. In addition, the activation of p70 S6 kinase was completely inhibited by rapamycin in both cell lines (Figure 3C). These results support the role of p70 S6 kinase in IGF-II-mediated antiapoptotic signaling.
Rapamycin Restored Sensitivity of Rhabdomyosarcoma Cells to Cisplatin
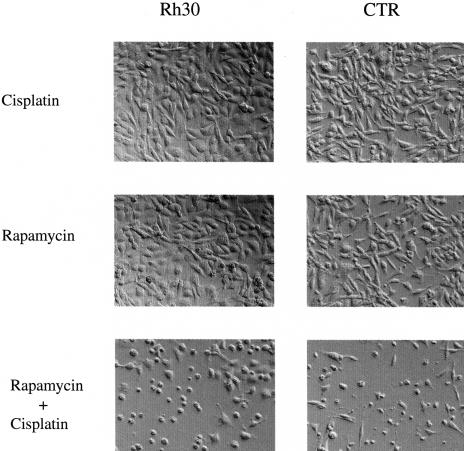
To determine whether rapamycin reversal of IGF-II-mediated protection from cisplatin seen in mouse myoblasts would be seen in tumors of skeletal muscle origin, we examined the rapamycin effect in IGF-II-overexpressing Rh30 and CTR rhabdomyosarcoma cells. Both Rh30 and CTR cells were resistant to cisplatin-induced apoptosis. Pretreatment of these cells with rapamycin for 3 hours followed by treatment with both rapamycin and cisplatin for 48 hours could abolish the protection of both cell lines to cisplatin-induced apoptosis (Figure 4). These results indicate that blockade of the p70 S6K pathway by rapamycin can enhance chemotherapy-induced apoptosis in the treatment of IGF-II-overexpressing rhabdomyosarcomas.
Figure 4.
Rapamycin abolishes the resistance of Rh30 and CTR rhabdomyosarcoma cells to cisplatin-induced apoptosis. Rh30 and CTR cells were pretreated with rapamycin (100 nM) for 3 hours and then treated with cisplatin (25 µM) for 48 hours in 10% FBS medium. Cells were examined and photographed under light microscope at 200x. The results were representative of three independent experiments.
Exogenous IGF-II Rescues C2C12 Cells from Cisplatin-Induced Apoptosis
To further examine the ability of IGF-II to reverse cisplatin-induced apoptosis, parental C2C212 cells were exposed to IGF-II (25 ng/ml) for 1 hour and then treated with 25 µM cisplatin for 24 hours. As demonstrated in Figure 5A, cells pretreated with IGF-II were relatively resistant to cisplatin-induced apoptosis compared with IGF-II-nontreated cells (viability: 90% vs 60%, respectively ). In addition, pretreatment with IGF-II partially protected cells from rapamycin-induced apoptosis. Similar results were observed in IGF-I-treated cells (data not shown). Neutralizing the antibody against IGF-IR (αIR3) partially abrogated IGF-II protection against cisplatin-induced apoptosis (Figure 5A). Thus, protection by IGF-II appears to be predominantly mediated through the type I IGF receptor. Furthermore, in cells treated with IGF-II for 24 hours, IGF-II-stimulated phosphorylation of p70 S6K was completely inhibited by rapamycin (Figure 5B). Inhibition of p70 S6K phosphorylation induced by cisplatin was reversed by IGF-II pretreatment in C2C12 cells (Figure 5B). Thus, the data in IGF-II-treated cells are consistent with that in IGF-II-overexpressing cells.
Figure 5.
IGF-II rescues C2C12 cells from cisplatin-induced apoptosis and reverses inhibition of p70 S6K phosphorylation induced by cisplatin. (A) C2C12 cells were treated with or without antibody against the IGF-I receptor (1 µg/ml) overnight, and incubated with or without IGF-II (25 ng/ml) for 1 hour, and then treated with cisplatin (25 µM) or rapamycin (100 nM) for 24 hours. Cell viability was determined by MTT assay. Data are mean±SE (n=6). (B) C2C12 cells were treated with or without IGF-II (25 ng/ml) for 1 hour and then treated with cisplatin (25 µM) or rapamycin (100 nM) for 24 hours. Protein extracts (40 µg/lane) were separated on 10% SDS-PAGE, and subjected to Western blot analysis. Blotting with antibody against actin was used to demonstrate equal protein loading in each lane. Similar results were achieved in two separate experiments.
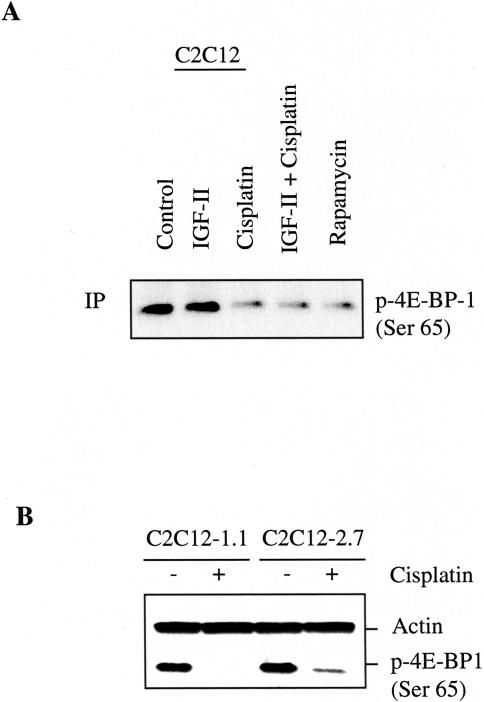
Effect of Cisplatin on Inhibiting 4E-BP1 Phosphorylation
Because p70 S6K and elF4E-binding protein 1 (4E-BP1) both lie downstream of mTOR, we examined the effect of cisplatin on regulating 4E-BP1 phosphorylation. C2C12 cells were exposed to IGF-II (25 ng) for 1 hour and then treated with cisplatin (25 µM) or rapamycin (100 nM) for 24 hours and then harvested for immunoprecipitation and Western blotting analysis using an anti-phospho4E-BP1 (Ser 65). As shown in Figure 6A, phosphorylation of 4E-BP1 was significantly diminished after 24 hours of cisplatin treatment. As expected, rapamycin almost completely inhibited 4EBP1 phosphorylation. However, 4E-BP1 phosphorylation did not increase on 24 hours of IGF-II treatment. This may reflect a loss of IGF-II's ability to stimulate 4E-BP1 phosphorylation over extended periods. We further tested the effect of cisplatin on inhibiting 4E-BP1 phosphorylation by Western blotting analysis in IGF-II-transfected and nontransfected cells. Figure 6B shows that IGF-II-transfected cells have a slight increase in the basal level of 4E-BP1 phosphorylation compared to IGF-II-nontransfected cells. Phosphorylation of 4E-BP1 was completely inhibited by cisplatin on 24-hour treatment in C2C12-1.1 cells. However, the phosphorylation of 4E-BP1 was still detected in C2C12-2.7 cells. This result suggests that IGF-II overexpression correlates with resistance to cisplatin-induced 4E-BP1 hypophosphorylation.
Figure 6.
Effect of cisplatin on inhibiting 4E-BP1 phosphorylation. (A) Confluent C2C12 cells were treated with IGF-II (25 ng/ml), cisplatin (25 µM), IGF-II together with cisplatin, and rapamycin (100 nM) for 24 hours. A total of 300 µg of cell lysates were immunoprecipitated with phospho-4E-BP1 (Ser65) antibody followed by Western blot analysis as described in Materials and Methods. (B) Confluent C2C12-1.1 and C2C12-2.7 cells were treated with cisplatin (25 µM) for 24 hours. Protein extracts (100 µg/lane) were separated on 10% SDS-PAGE, and subjected to Western blot analysis. Blotting with antibody against actin was used to demonstrate equal protein loading in each lane. Similar results were achieved in two separate experiments.
Discussion
Induction of apoptosis is widely believed to be the predominant mechanism by which chemotherapy and radiation kill cancer cells. Thus, there is considerable interest in understanding the cellular mechanisms that regulate the sensitivity of cells to therapy-induced apoptosis. In this study, we have demonstrated that C2C12 myoblasts transfected with human IGF-II cDNA or treated with IGF-II can become resistant to cisplatin-induced apoptosis compared with IGF-II nontransfected or untreated cells. Namely, IGF-II protects skeletal muscle cells from apoptosis induced by cisplatin.
Because survival and death likely represent a balance between antiapoptotic and proapoptotic signaling, we considered the possibility that IGF-II mediated cell survival by increasing antiapoptotic signaling, by decreasing proapoptotic signaling, or both. The data presented in this report reveal that p70 S6K activity was strongly induced by IGF-II overexpression. Thus, cisplatin-induced inhibition of p70 S6K phosphorylation in C2C12-1.1 and C2C12 cells was inhibited in IGF-II-transfected C2C12-2.7 and IGF-II-treated cells. Furthermore, pretreatment of cells with rapamycin increased the sensitivity of these cells to cisplatin-induced apoptosis. Blockade of the p70 S6K pathway by rapamycin resulted in abrogating the effect of IGF-II-mediated protection in C2C12-2.7 cells. These data suggest that the mechanism of IGF-II-mediated myoblast survival signaling may be mediated through regulation of p70 S6 kinase activity.
Because most effects of IGF-II are mediated by the IGFIR, we evaluated whether blockade of IGF-IR by neutralizing the antibody against IGF-1R (αIR3) could block IGF-II-induced p70 S6K activation. However, no change in p70 S6K activity was noted (data not shown). This is not surprising because this antibody at best inhibits proliferation in autocrine cells by 50% [25]. Perhaps this is due to partial agonist activity of the antibody, or due to other factors such as receptor occupancy.
Recent studies have shown that p70 S6 kinase participates in the translation of mRNAs, which contain an oligopyrimidine tract at their transcriptional start site. This family of mRNAs encodes many of the components of the translational apparatus, including ribosomal proteins and elongation factors. Translation plays an important role in control of cell growth [26,27]. Rates of protein synthesis are also coupled to cell-cycle progression because inhibition of translation leads to arrest of cell growth, generally at the G1 phase [11]. Inhibition of translation in response to DNA damage could contribute to cell-cycle arrest. Rapamycin has been shown to inhibit translation of IGF-II [28]. In our study, rapamycin completely inhibits phosphorylation of p70 S6 kinase in IGF-II-transfected, nontransfected and IGF-II-treated cells and inhibits about 70% of p70 S6K activity. Our results are consistent with a recent report demonstrating that phosphorylation of p70 S6K was inhibited during cisplatin-induced apoptosis and 60–70% of p70 S6K activity was inhibited by rapamycin [29]. In addition, rapamycin not only increased the sensitivity of cells to cisplatin-induced apoptosis but also diminished the IGF-II-related protective effect from cisplatin-induced apoptosis. Our previous study found that cells with IGF-II overexpression had a diminished G1 checkpoint arrest following DNA damage [10]. Inhibition of p70 S6K activation has been shown to induce G1 arrest [15,16]. It has been recently reported that rapamycin induces G1 arrest and apoptosis in Rh1 and Rh30 rhabdomyosarcoma cell lines and expression of a rapamycin-resistant mTOR prevents G1 arrest and apoptosis induced by rapamycin [30]. Our data show that treatment of cells with rapamycin suppressed p70 S6K activation and induced G1 arrest (Figure 3). These data suggest that rapamycin-induced G1 arrest and apoptosis are mediated through its inhibitory effect on mTOR/p70 S6 kinase activity. Activation of p70 S6 kinase appears to exert its function by regulating translation and protein biosynthesis to control cell cycle and survival.
In addition to cisplatin, other DNA-damaging agents, including etoposide and mitomycin-C, have been recently reported to inhibit phosphorylation of p70 S6K and 4E-BP1 in Swiss 3T3 and RAT-1 cells [29]. Our data are in agreement with these findings. Treatment of cells with cisplatin also led to inhibition of 4E-BP1 phosphorylation, and 4E-BP1 is a major regulator of elF-4E-mediated 5′ cap mRNA translation [31]. Thus, inhibition of 4E-BP1 phosphorylation inhibits cap-dependent translation and is considered permissive for apoptosis [32]. However, IGF-II-overexpressing cells are relatively resistant to cisplatin-induced inhibition of 4E-BP1.
Cisplatin is a widely used chemotherapeutic agent for the treatment of various malignant tumors [33,34]. Cisplatin reacts with DNA to form intrastrand and/or interstrand cross-links of platinum adducts [35]. These lesions are believed to be essential for the cellular toxicity of cisplatin to prevent DNA replication and transcription [36]. However, apoptosis induction by cisplatin should not be related exclusively to the inhibition in DNA synthesis but additional mechanisms might trigger induction of this process. Sanchez-Perez [37] has reported that exposure of cells to cisplatin induces a persistent activation of c-Jun N-terminal kinase (JNK), but not extracellular signal-related kinase (ERK), indicating that the JNK signaling pathway may be involved in the cellular response to cisplatin. In addition, other genes of interest that have been recently linked to cisplatin cellular biology include: p21, an inhibitor of the cyclin-dependent kinases, which can protect some cell types against DNA damage induced by cisplatin [38]; telomerase, which is inhibited by cisplatin [39]; NFκB, a transcription factor whose levels are increased after cisplatin treatment [40]. Understanding the molecular basis of cisplatin-mediated apoptosis could lead to more effective use of this compound in cancer therapy.
To our knowledge, this study is the first to link an increase of p70 S6K activity to IGF-II overexpression. Although the molecular mechanisms of cisplatin-induced p70 S6K inhibition and IGF-II induced p70 S6K activity are unknown, these data are of potential clinical importance. IGF-II is widely expressed in human malignant tumors. Inappropriate overexpression of IGF-II is a common theme in tumor biology and may be particularly important to pediatric embryonic tumors [8]. We examined the effect of cisplatin on IGF-II-overexpressing Rh30 and CTR rhabdomyosarcoma cells. As we expected, both cells were resistant to cisplatin-induced apoptosis. However, pretreatment with rapamycin aimed at inhibiting p70 S6 kinase activation enhances the efficacy of cisplatin in the treatment of IGF-II-overexpressing rhabdomyosarcoma cells (Figure 4). Although cisplatin is widely used in the treatment of various solid tumors, one of the major limitations to the use of the drug is the acquisition of resistance by initially responsive tumors [41]. In general, drug-induced apoptosis is dependent on the balance between cell-cycle checkpoints and DNA-repair mechanisms [42]. Thus, inhibition of the p70 S6k pathway may be an appropriate target for strategies to overcome the resistance to chemotherapeutic DNA-damaging drugs and to provide a therapeutic benefit for the treatment of IGF-II-overexpressing tumors.
Abbreviations
- IGF-II
insulin-like growth factor
- p70 S6K
p70 ribosomal protein S6 kinase
- mTOR
mammalian target of rapamycin
- Thr389
threonine 389
- elF4E
eukaryotic initiation factor 4E
- 4E-BP1
elF4E-binding protein
- Ser65
serine 65
References
- 1.Stewart CE, Rotwein P. Growth, differentiation and survival: multiple physiological functions for insulin-like growth factors. Physiol Rev. 1996;76:1005–1026. doi: 10.1152/physrev.1996.76.4.1005. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen FC. The molecular and cellular biology of insulin-like growth factor II. Prog Growth Factor Res. 1992;4:257–290. doi: 10.1016/0955-2235(92)90023-b. [DOI] [PubMed] [Google Scholar]
- 3.Ullrich A, Gray A, Tam AW, Yang-Fen T, Tsubokawa M, Collins C, Henzel W, Le Bon T, Kathuria S, Chen E, Jacobs S, Prancke U, Ramachandran J, Fyjita-Yamaguchi Y. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986;5:2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collett-Solberg PF, Cohen P. The role of the insulin-like growth factor binding proteins and the IGFBP proteases in modulating IGF action. Endocrinol Metab Clin North Am. 1996;25:591–614. doi: 10.1016/s0889-8529(05)70342-x. [DOI] [PubMed] [Google Scholar]
- 5.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 6.Reeve AE, Eccles MR, Wilkins RJ, Bell GI, Millow LJ. Expression of insulin-like growth factor-II transcriptions in Wilms' tumour. Nature. 1985;317:258–260. doi: 10.1038/317258a0. [DOI] [PubMed] [Google Scholar]
- 7.El-Badry OM, Helman LJ, Chatten J, Steinberg SM, Evans AE, Israel MA. Insulin-like growth factor II-mediated proliferation of human neuroblastoma. J Clin Invest. 1991;87:648–657. doi: 10.1172/JCI115042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toretsky JA, Helman LJ. Involvement of IGF-II in human cancer. J Endocrinol. 1996;149:367–372. doi: 10.1677/joe.0.1490367. [DOI] [PubMed] [Google Scholar]
- 9.Minniti CP, Luan D, O'Grady C, Rosenfeld RG, Oh Y, Helman LJ. Insulin-like growth factor overexpression in myoblasts induces phenotypic changes typical of the malignant phenotype. Cell Growth Differ. 1995;6:263–269. [PubMed] [Google Scholar]
- 10.Zhang L, Kim M, Choi YH, Goemans B, Yeung C, Hu Z, Zhan S, Seth P, Helman LJ. Diminished G1 checkpoint after γ-irradiation and altered cell cycle regulation by insulin-like growth factor II overexpression. J Biol Chem. 1999;274:13118–13126. doi: 10.1074/jbc.274.19.13118. [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D. Heritable formation of pancreatic beta-cell tumors in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 12.Christofori G, Naik P, Hanahan D. A second signal supplied by insulin-like growth factor II in oncogene-induced tumorigenesis. Nature. 1994;369:414–418. doi: 10.1038/369414a0. [DOI] [PubMed] [Google Scholar]
- 13.Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;16:3693–3704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Proud CG. p70 S6 kinase: an enigma with variations. Trends Biochem Sci. 1996;21:181–185. [PubMed] [Google Scholar]
- 15.Chou MM, Blenis J. The 70 kDa S6 kinase: regulation of a kinase with multiple roles in mitogenic signaling. Curr Opin Cell Biol. 1995;7:806–814. doi: 10.1016/0955-0674(95)80064-6. [DOI] [PubMed] [Google Scholar]
- 16.Pullen N, Thomas G. The molecular phosphorylation and activation of p70s6k. FEBS Lett. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- 17.Thomas G, Hall MN. TOR signaling and control of cell growth. Curr Opin Cell Biol. 1997;9:782–787. doi: 10.1016/s0955-0674(97)80078-6. [DOI] [PubMed] [Google Scholar]
- 18.Peterson RT, Schreiber SL. Translation control: connecting mitogens and the ribosome. Curr Biol. 1998;8:R248–R250. doi: 10.1016/s0960-9822(98)70152-6. [DOI] [PubMed] [Google Scholar]
- 19.Pearson RB, Dennis PB, Han JW, Willimson NA, Kozma SC, Wettenhall RE, Thomas G. EMBO J. 1995;14:5279–5287. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. Proc Natl Acad Sci USA. 1998;95:1432–1437. [Google Scholar]
- 21.Beretta L, Gingras A-C, Svitkin YV, Hall MN, Sonenberg N. Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J. 1996;15:658–664. [PMC free article] [PubMed] [Google Scholar]
- 22.Von Manteuffel SR, Gingras A-C, Ming X-F, Sonenberg N, Thomas G. 4E-BP1 phosphorylation is mediated by the FRAP-p70s6k pathway and is independent of mitogen-activated protein kinase. Proc Natl Acad Sci USA. 1996;93:4076–4080. doi: 10.1073/pnas.93.9.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rousseau D, Gingras A-C, Pause A, Sonenberg N. The eIF4E-binding proteins 1 and 2 are negative regulators of cell growth. Oncogene. 1996;13:2415–2420. [PubMed] [Google Scholar]
- 24.Felix CA, Kappel CC, Mitsudomi T, Nau MM, Tsokos M, Crouch GD, Nisen PD, Winick NJ, Helman LJ. Frequency and diversity of p53 mutations in childhood rhabdomyosarcoma. Cancer Res. 1992;52:2243–2247. [PubMed] [Google Scholar]
- 25.El-Badry OM, Minniti C, Kohn EC, Houghton PJ, Daughaday WH, Helman LJ. Insulin-like growth factor II acts as an autocrine growth and motility factor in human rhabdomyosarcoma tumors. Cell Growth Differ. 1990;7:325–331. [PubMed] [Google Scholar]
- 26.Conlon I, Raff M. Size control in animal development. Cell. 1999;96:235–244. doi: 10.1016/s0092-8674(00)80563-2. [DOI] [PubMed] [Google Scholar]
- 27.Norbury C, Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen FC, Ostergaard L, Nielsen J, Christiansen J. Growth-dependent translation of IGF-II mRNA by a repamycine-sensitive pathway. Nature. 1995;377:358–362. doi: 10.1038/377358a0. [DOI] [PubMed] [Google Scholar]
- 29.Tee AR, Proud CG. DNA-damaging agents cause inactivation of translational regulators linked to mTOR signaling. Oncogene. 2000;19:3021–3031. doi: 10.1038/sj.onc.1203622. [DOI] [PubMed] [Google Scholar]
- 30.Hosoi H, Dilling MB, Shikata T, Liu LN, Shu L, Ashmun RA, Germain GS, Abraham RT, Houghton PJ. Rapamycin causes poorly reversible inhibition of mTOR and induces p53-independent apoptosis in human rhabdomyosarcoma cells. Cancer Res. 1999;59:886–894. [PubMed] [Google Scholar]
- 31.Sonenberg N, Gingras AC. The mRNA 5′ cap-binding protein eIF4E and control of cell growth. Curr Opin Cell Biol. 1998;10:268–275. doi: 10.1016/s0955-0674(98)80150-6. [DOI] [PubMed] [Google Scholar]
- 32.Polunovsky VA, Gingras A-C, Sonenberg N, Peterson M, Tan Annie, Rubins JB, Manivel JC, Bitterman PB. Translational control of the antiapoptotic function of Ras. J Biol Chem. 2000;275:24776–24780. doi: 10.1074/jbc.M001938200. [DOI] [PubMed] [Google Scholar]
- 33.Loehrer PJ, Einhorn LH. Drugs five years later. Ann Intern Med. 1984;100:704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- 34.Reed E. In: Cancer Chemotherapy. Dabbolkar M, Chabner BA, editors. Philadelphia, PA: Lippincott-Raven Publishers; 1996. pp. 357–3578. [Google Scholar]
- 35.Zamble DB, Lippard SJ. Cisplatin and DNA Repair in Cancer Chemotherapy. Trends Biochem Sci. 1995;20:435–439. doi: 10.1016/s0968-0004(00)89095-7. [DOI] [PubMed] [Google Scholar]
- 36.Reed E. In: Cancer Principle and Practice of Oncology. DeVita VT Jr, Hellman S, Rosenberg SA, editors. Philadelphia, PA: JB Lippincott; 1993. pp. 390–400. [Google Scholar]
- 37.Sanchez-Perez I, Murguia JR, Perona R. Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene. 1998;16:533–540. doi: 10.1038/sj.onc.1201578. [DOI] [PubMed] [Google Scholar]
- 38.Fan S, Chang JK, Smith ML, Duba D, Fornace AJ Jr, O'Connor PM. Cells lacking CIP1/WAF1 genes exhibit preferential sensitivity to cisplatin and nitrogen mustard. Oncogene. 1997;14:2127–2136. doi: 10.1038/sj.onc.1201052. [DOI] [PubMed] [Google Scholar]
- 39.Burger AM, Double J, Newell DR. Inhibition of telomerase activity by cisplatin in human testicular cancer cells. Eur J Cancer. 1997;33:638–644. doi: 10.1016/s0959-8049(96)00521-7. [DOI] [PubMed] [Google Scholar]
- 40.Maldonado V, Melendez-Zajgla J, Ortega A. Modulation of NF-kappa B, and Bcl-2 in apoptosis induced by cisplatin in HeLa cells. Mutat Res. 1997;381:67–75. doi: 10.1016/s0027-5107(97)00150-4. [DOI] [PubMed] [Google Scholar]
- 41.Ozols RF. Ovarian cancer: Part II. Treatment. Curr Probl Cancer. 1992;16:61–126. [PubMed] [Google Scholar]
- 42.Alaoui-Jamali MA, Paterson J, Al Moustafa AE, Yen L. The role of ErbB-2 tyrosine kinase receptor in cellular intrinsic chemoresistance: mechanisms and implications. Biochem Cell Biol. 1997;75:315–329. [PubMed] [Google Scholar]