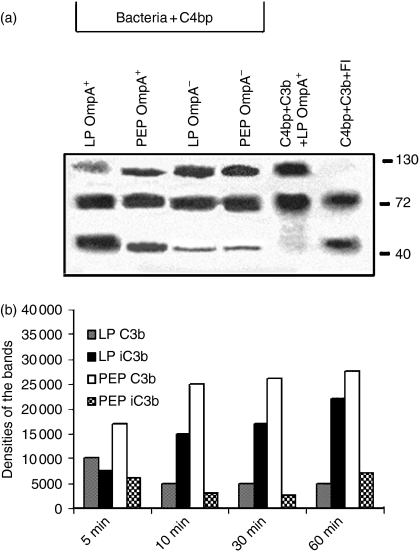
Figure 6.
C4bp bound to OmpA+Escherichia coli exhibits cofactor activity to FI in cleavage of C3b. (a)Either OmpA+ or OmpA–E. coli grown to LP and PEP were incubated with purified C4bp for 30 min at 37°. The bacteria were then collected by centrifugation, washed, and incubated with C3b, and FI for 1 hr at 37°. The bacteria were then pelleted and a portion of the supernatant was dissolved in SDS buffer, and subjected to immunoblotting with anti-C3 antibody (which recognizes α- and β-chains and the cleavage products). The experiments were carried out at least twice with similar results. (b)In separate experiments, LP and PEP OmpA+E. coli were incubated with 40% C8-deficient serum for various periods, centrifuged, washed, the bacterial pellets were treated with hydroxylamine and mixed with SDS sample buffer containing β-mercaptoethanol. The proteins were then subjected to Western blotting with anti-C3 antibody, the bands on the blot were scanned and their intensities were normalized to total bacteria added and plotted on graphs.