Abstract
Previous studies from this laboratory have shown that ultraviolet A (UVA) light can bleach the yellow advanced glycation end products (AGEs) of aged and cataractous human lenses. The AGEs OP-lysine and argpyrimidine are two UVA-absorbing posttranslational modifications that are abundant in the eye lens. The purpose of this study was to outline the changes in these two AGEs due to UVA irradiation. The changes of OP-lysine, OP-phenethylamine (a phenethylamine analogue of OP-lysine), and argpyrimidine due to irradiation with UVA light in the presence or absence of air and ascorbic acid were followed by different spectral methods. Aged human lenses were similarly irradiated in artificial aqueous humor. The amounts of OP-lysine in the irradiated lenses and in the corresponding dark controls were determined by HPLC. Both OP-lysine and argpyrimidine decreased 20% when irradiated with UVA light in the absence of ascorbic acid. Under the same conditions, OP-lysine was bleached 80% in the presence of ascorbic acid during irradiation experiments. In contrast, argpyrimidine UVA light bleaching was not affected by the presence of ascorbic acid. Interestingly the major product of OP-phenethylamine after UVA irradiation in the presence of ascorbic acid was phenethylamine, which indicates that the entire heterocycle of this AGE was cleaved and the initial amino group was restored. Some AGEs in the human eye lens can be transformed by UVA light.
Keywords: ascorbic acid, OP-lysine, UVA light, eye lens, glycation
INTRODUCTION
The yellowing of the human eye lens due to posttranslational modifications of proteins is a typical process during aging and in some types of cataract. There is strong evidence that the coloration is due to the formation of advanced glycation end products (AGEs) on lens proteins.1,2 Alternatively, it has been proposed that the yellowing color could be due to the covalent binding of so-called filter compounds3 or to the photooxidation of lens protein.4
Previous studies from this laboratory demonstrated that the anaerobic irradiation of aged and cataractous lenses with ultraviolet A (UVA) light causes significant bleaching of their coloration and that ascorbic acid is an important participant in this process.5,6 While the chemical nature of the coloration of aged human lenses is still unknown, it was interesting to study the photochemical properties of AGEs existing in the lens. The two major UVA-absorbing AGEs in the human lens are OP-lysine 1 (2-ammonio-6-(3-oxidopyridinium-1-yl)hexanoate)7 and argpyrimidine 2 (N-delta-(5-hydroxy-4,6-dimethylpyrimidine-2-yl)-L-ornithine).8 The purpose of the present study was to investigate the photochemical changes of OP-lysine 1 and argpyrimidine 2 (Fig. 1) due to irradiation with UVA light. A synthetic analogue of OPlysine — OP-phenethylamine (1-(2-phenylethyl)pyridinium-3-olate) 3—was employed to identify bleaching products (Fig. 1).
Figure 1.
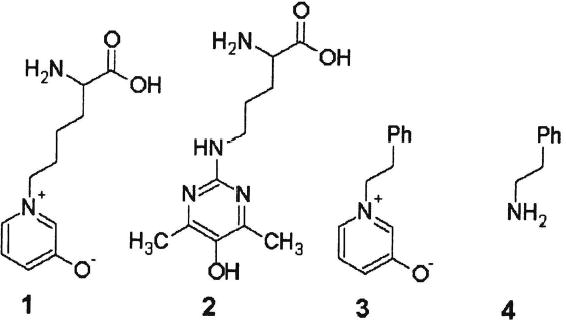
Structures of OP-lysine 1, argpyrimidine 2, OP-phenethylamine 3, and phenethylamine 4.
EXPERIMENTAL METHODS
Materials
OP-lysine 17 and argpyrimidine 29 were prepared as described above. All of the other reagents, including Chelex resin, were purchased from Sigma-Aldrich (St. Louis, MO). The artificial aqueous humor was prepared as described previously10 except that 1 mM ascorbic acid was added to the solution prior to lens addition and irradiation. The value of 1 mM ascorbic acid in the human aqueous humor is in agreement with data published previously.11,12
Irradiation Experiments
All irradiations were carried out using a 1000 W Hg/Xe lamp (Oriel Corp., Stratford, CT) with a 338-nm cutoff filter, producing 900 J h−1 cm−2 of UVA light.
Bleaching of OP-Lysine and Argpyrimidine in Vitro
Solutions of OP-lysine 1 and argpyrimidine 2 at an absorbance of 0.05 at 320 nm were prepared in 50 mM phosphate buffer, 0.1 mM DTPA, pH 7.0, and in the presence and absence of 0.1 mM ascorbic acid, The headspace of the cuvette was subjected to a stream of O2-scrubbed argon for 15 min with stirring. The samples were irradiated with UVA light. The bleaching of the starting compounds was followed by the changes of the absorbance of the reaction mixtures at 320 nm.
Bleaching of OP-Lysine in Human Lenses
The bleaching of OP-lysine was studied using three aged human lens pairs (63, 70, and 70 years old). One of the lenses from each pair was assigned as a dark control, and the other one was irradiated with UVA light in the presence of artificial aqueous humor under argon. After the irradiation, each lens was homogenized, extensively dialyzed, and lyophilized, and the proteins were hydrolyzed with 6 N HCl at 110°C. The protein concentrations were measured using the bicinchoninic acid (BCA) method as described by the manufacturer (Pierce, Rockford, IL). The amount of OP-lysine in hydrolysates was measured by analytical HPLC using the absorbance of OP-lysine at 289 nm at pH 2 and fluorescence at ex/em = 290/390 nm. Fluorescence-based HPLC profiles, typical for argpyrimidine at ex/em = 320/380, were recorded as well.
Synthesis of OP-Phenethylamine
(2-Iodoethyl)benzene (510.6 mg, 318 mL, 2.2 mmols) and 3-hydroxypyridine (190 mg, 2 mmols) were refluxed in 10 mL of 1-propanol under argon for 3 h (Fig. 2). The solvent was evaporated under reduced pressure, and the residue was recrystallized from ethanol; yield, 94 mg. Then 86.2 mg (0.252 mmols) of the product was dissolved in a mixture of 5 mL water and 5 mL acetonitrile. Silver oxide (32 mg, 0.139 mmols, 1.1 equiv.) was added, and the sample was sonicated for two minutes. The solid was separated by centrifugation, and the supernatant was concentrated under reduced pressure to give a dark brown oil. This was dissolved in a mixture of 0.7 mL methanol and 0.7 mL water. Chelex resin (170 mg) was added, and the sample was shaken for five minutes. The mixture was centrifuged, and the supernatant was concentrated under reduced pressure to give the final product; yield, 38.7 mg. ESI MS: M+1 = 200 Th. 1H NMR (CD3OD): 7.60–7.10 (9H, m, aromatic protons), 4.52 (2H, t, CH2-N+), 3.2 (2H, t, CH2-Ph), ppm. 13C NMR (CD3OD): 169.4, 137.5, 135.1, 134.7, 139.9, 129.8, 128.3, 128.2, 127.5, 63.1, 38.2 ppm.
Figure 2.
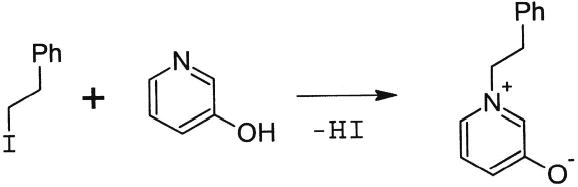
Synthesis of OP-phenethylamine from phenethyliodide and 3-hydroxypyridine.
Bleaching of OP-Phenethylamine
A reaction mixture (2.0 mL) containing OP-phenethylamine (11.8 mg, 0.059 mmols), ascorbic acid (32 mg, 0.18 mmols), 100 mM phosphate buffer, and 1 mM DTPA pH 7 was bubbled with argon for 10 minutes in a screw-cap cuvette and irradiated for 6 h with UVA light. A minor fraction of a white precipitate appeared as a result of the irradiation and was eliminated by centrifugation. The products were analyzed by preparative RP HPLC composed of a Waters Delta 600 pump, a 600 controller, and a 996 Photo Diode Array Detector. The fluorescence of the column eluate was monitored by a Linear Instruments LC 305 fluorescence detector. A Prodigy ODS 250 · 21.2 mm was eluted with solvent A, 0.1% HFBA in water, and solvent B, 50% acetonitrile, in 0.1% HFBA. The gradient employed was: 0–10 min, isocratic 10% B; 10–50 min, linear gradient 10–100% B; 50–100 min, isocratic 100% B at a flow rate of 10 mL/min. The peak fractions absorbing at 211 nm were individually collected and analyzed.
RESULTS
Both OP-lysine and argpyrimidine exhibit an absorption maximum around 320 nm at pH 7 (Fig. 3). The data obtained during the irradiation of model mixtures containing OP-lysine and argpyrimidine are presented in Figure 4. Three major experimental conditions were employed: (i) irradiation in the absence of both ascorbic acid and air; (ii) irradiation in the presence of ascorbic acid under argon, which mimics the conditions in a healthy lens; and (iii) dark control in the presence of ascorbic acid under argon. We found that the compounds of interest 1 and 2 were both stable in the absence of UVA light (Fig. 4). The stability of argpyrimidine toward UVA irradiation was practically the same in the presence or absence of ascorbic acid under argon. In contrast the bleaching of OP-lysine at 320 nm under anaerobic conditions was significantly accelerated in the presence of ascorbic acid.
Figure 3.
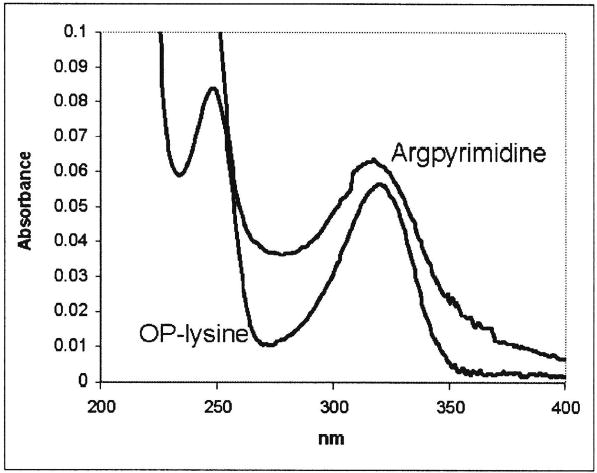
UV spectra of OP-lysine and argpyrimidine in 50 mM phosphate buffer, pH 7.0.
Figure 4.
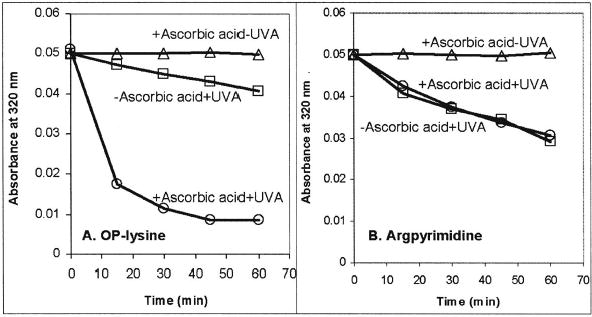
Changes in the absorbance at 320 nm due to UVA light irradiation of OPlysine solutions (left panel) and argpyrimidine solutions (right panel) at pH 7.0. Circles indicate irradiation in the presence of ascorbic acid; squares, irradiation in the absence of ascorbic acid; triangles, dark control in the presence of ascorbic acid.
The UVA irradiation of aged human lenses under anaerobic conditions resulted in significant but not complete bleaching of their yellow color (data not shown). The amount of OP-lysine in the irradiated lenses and the corresponding dark controls was measured by RP HPLC after homogenization, dialysis, and acid hydrolysis. In all three lens pairs we studied, there was significant decrease of the amount of OPlysine due to UVA irradiation (Fig. 5). At the same time the HPLC profiles of the hydrolysates recorded by fluorescence at ex/em = 320/380 showed no significant difference between the irradiated lenses and the corresponding dark controls in the regions where a synthetic standard of argpyrimidine eluted (data not shown). Thus, the data obtained from irradiated lenses were consistent with those from in vitro irradiations of OP-lysine and argpyrimidine: in both cases OP-lysine was much more sensitive to UVA irradiation in the presence of ascorbic acid compared to argpyrimidine.
Figure 5.
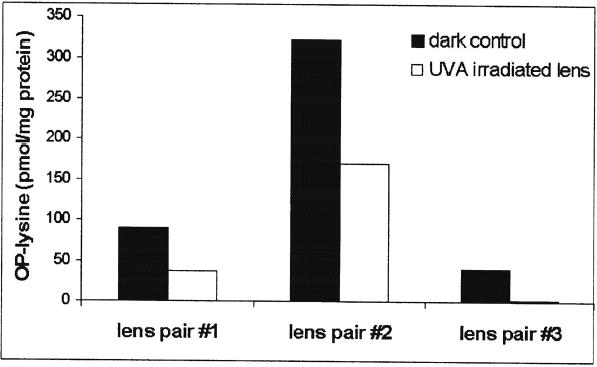
Amounts of OP-lysine measured in UVA-irradiated lenses and in the corresponding dark controls. The irradiation experiments were done under anaerobic conditions in artificial aqueous humor containing 0.1 mM ascorbic acid.
The structural studies on the photochemical changes of the heterocyclic chromophore of OP-lysine 1 were done using a model substance: OP-phenethylamine 3. Compound 3 as well as its potential degradation products exhibits a strong absorbance at 211 and 256 nm due to the benzene ring. The irradiation of OP-phenethylamine 3 in the presence of ascorbic acid led to the formation of a minor fraction of water-insoluble components, indicating that some of the bleaching products were more hydrophobic than the original compound. The HPLC profile of the predominant water-soluble product is given in Figure 6. The peak with retention time at γ↔46 min was identical to the starting OP-phenethylamine due to the same retention time, the same UV spectrum, the same proton 1H-NMR spectrum, and the same molecular mass measured by ESI MS (data not shown). The major peak with retention time at γ↔40 min was collected and analyzed by UV, NMR spectroscopy, and mass-spectrometry. All the data obtained, including comparisons with an authentic sample, confirmed that the structure of the major degradation product of OP-phenethylamine 3 is phenethylamine 4. The UV-spectrum of the peak showed the typical multiplet band of benzene ring at 256 nm. The proton 1H-NMR spectrum in CD3OD showed signals at: 7.4–7.2 (5H, m, benzene ring protons), 3.2 (2H, t, CH2-N), 2.9 (2H, t, CH2-Ph) ppm. The 13C NMR spectrum of the same sample showed signals at: 137.8, 130.0, 129.7, 128.3, 41.9, and 34.6 ppm. The ESI-MS spectrum showed that [M+1] = 122 Th, which is in agreement with structure 4. In this way the major result due to UVA irradiation of OP-phenethylamine 3 was a complete cleavage of the heterocyclic ring.
Figure 6.
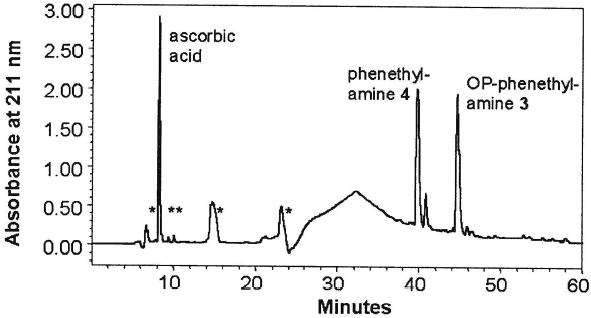
HPLC profile of the products obtained after UVA irradiation of OPphenethylamine in the presence of ascorbic acid. The peak marked as ascorbic acid had the retention time and UV spectrum of ascorbic acid. The peaks marked with an asterisk did not show the presence of a benzene ring in their UV spectra and probably are due to ascorbic acid degradation products.
DISCUSSION
Both OP-lysine and argpyrimidine decreased 20% when irradiated with UVA light. In vitro experiments of OP-lysine and argpyrimidine in the presence of ascorbic acid, however, show that different AGEs can have markedly different stability toward UVA irradiation under anaerobic conditions. This is in agreement with the previous data showing that the aged human lens proteins are not completely bleached by UVA light.
The significant decrease of OP-lysine observed after UVA irradiation of lenses in artificial aqueous humor is the first example of bleaching of a specific posttranslational modification in the lens. The formation of more hydrophobic products as a result of UVA irradiation of OP-phenethylamine could contribute to the decrease of solubility of lens proteins seen during aging in vivo. On the other hand, the complete recovery of the amino group of OP-phenethylamine by irradiation shows that some posttranslational modifications of lens proteins due to glycation can be reversed by UVA light.
Acknowledgments
This work was supported in part by NIH Grants EY 07070 and EY02035 and in part by a departmental grant from Research to Prevent Blindnes, Inc.
References
- 1.Cheng R, Lin B, Lee KW, Ortwerth BJ. Similarity of the yellow chromophores isolated from human cataracts with those from ascorbic acid–modified calf lens proteins: evidence for ascorbic acid glycation during cataract formation. Biochim Biophys Acta. 2001;1537:14–26. doi: 10.1016/s0925-4439(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 2.Kamei A, Kato M. Contribution of glycation to human lens coloration. Chem Pharm Bull. 1991;39:1272–1276. doi: 10.1248/cpb.39.1272. [DOI] [PubMed] [Google Scholar]
- 3.Garner B, Shaw DC, Lindner RA, et al. Non-oxidative modification of lens crystallins by kynurenine: a novel post-translational protein modification with possible relevance to aging and cataract. Biochim Biophys Acta. 2000;1476:265–278. doi: 10.1016/s0167-4838(99)00234-4. [DOI] [PubMed] [Google Scholar]
- 4.Dhir P, Akhtar NJ, Sun TX, Liang JJ. Photooxidized products of recombinant alpha A-crystallin and W9F mutant. Photochem Photobiol. 1999;69:329–335. doi: 10.1562/0031-8655(1999)069<0329:pporca>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Ortwerth BJ, Chemoganskiy V, Olesen PR. Studies on singlet oxygen formation and UVA light-mediated photobleaching of the yellow chromophores in human lenses. Exp Eye Res. 2002;74:217–229. doi: 10.1006/exer.2001.1114. [DOI] [PubMed] [Google Scholar]
- 6.Ortwerth BJ, Chemoganskiy V, Mossine VV, Olesen PR. The effect of UVA light on the anaerobic oxidation of ascorbic acid and the glycation of lens proteins. Invest Ophthalmol Vis Sci. 2003;44:3094–3102. doi: 10.1167/iovs.02-0857. [DOI] [PubMed] [Google Scholar]
- 7.Argirov OK, Lin B, Ortwerth BJ. 2-Ammonio-6-(3-oxidopyridinium-1-yl)hexanoate (OP-lysine) is a newly identified advanced glycation end-product in cataractous and aged human lenses. J Biol Chem. 2004;279:6487–6495. doi: 10.1074/jbc.M309090200. [DOI] [PubMed] [Google Scholar]
- 8.Wilker SC, Chellan P, Arnold BM, Nagaraj RH. Chromatographic quantification of argpyrimidine, a methylglyoxal-derived product in tissue proteins: comparison with pentosidine. Ann Biochem. 2001;290:353–358. doi: 10.1006/abio.2001.4992. [DOI] [PubMed] [Google Scholar]
- 9.Shipanova IN, Glomb MA, Nagaraj RH. Arch Biochem Biophys. 1997;344:29–36. doi: 10.1006/abbi.1997.0195. [DOI] [PubMed] [Google Scholar]
- 10.Duncan G, Jacob TJ. Influence of external calcium and glucose on internal total and ionized calcium in the rat lens. J Physiol. 1984;357:485–493. doi: 10.1113/jphysiol.1984.sp015512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee P, Lam KW, Lai M. Aqueous humor ascorbate concentration and openangle glaucoma. Arch Ophthalmol. 1977;95:308–310. doi: 10.1001/archopht.1977.04450020109018. [DOI] [PubMed] [Google Scholar]
- 12.Koliakos GG, Konstas GPA, Schlotzer-Schrehardt U, et al. Ascorbic acid concentration is reduced in the aqueous humor of patients with exfoliation syndrome. Am J Ophthalmol. 2002;134:879–883. doi: 10.1016/s0002-9394(02)01797-x. [DOI] [PubMed] [Google Scholar]


