Abstract
The underlying basis of major histocompatibility complex (MHC) restriction is unclear. Nevertheless, current data suggest that a common thermodynamic signature dictates αβ T cell receptor (TcR) ligation. To evaluate whether this thermodynamic signature defines MHC restriction, we have examined the thermodynamic basis of a highly characterized immunodominant TcR interacting with its cognate peptide–MHC-I ligand. Surprisingly, we observed this interaction to be governed by favorable enthalpic and entropic forces, which is in contrast to the prevailing generality, namely, enthalpically driven interactions combined with markedly unfavorable entropic forces. We conclude that extrinsic molecular factors, such as coreceptor ligation, conformational adjustments involved in TcR signaling, or constraints dictated by higher-order arrangement of ligated TcRs, might play a greater role in guiding MHC restriction than appreciated previously.
Keywords: immunodominance, energetics, cytotoxic T lymphocyte, microcalorimetry, Epstein–Barr virus
The cytotoxic T cell response is directed toward class I major histocompatibility complex (MHC-I) molecules complexed to peptide antigens (pMHC-I). These complexes are expressed on the surface of antigen-presenting cells and are specifically recognized by clonally distributed αβ T cell receptors (TcRs). How the TcR engages the pMHC-I has been revealed by a number of structural and biochemical investigations (1–4). This exquisite TcR–pMHC-I corecognition event means that T cells are highly specific and genetically restricted to recognizing MHC-I molecules of the individual from whom they were derived.
Despite the increasing number of available ternary TcR–pMHC-I crystal structures, the underlying structural basis for MHC-I restriction remains elusive, with surprisingly few structural properties common to the observed interfaces. Nevertheless, several generalizations have been suggested regarding the nature of the TcR–pMHC-I interaction. First, at least some TcRs engage the pMHC-I in an approximately diagonal orientation by a two-step process (5, 6). Second, the complementarity-determining region (CDR) 1 and CDR2 loops of some TcRs contribute predominantly to the MHC-driven interactions, whereas the CDR3 loops contribute primarily to the peptide-mediated contacts and hence govern the antigen specificity in any given TcR–pMHC-I interaction (2). Furthermore, it has been predicted that pMHC-I-restricted TcRs have a minimal footprint of residues 65, 69, and 155 on the MHC-I heavy chain (7). On the other hand, only a subtle bias is observed in TcR αβ variable (V) region usage in MHC class I- vs. class II-restricted TcRs, and this bias has not identified a conserved footprint, such as CDR1/CDR2 sequences, which guide TcR docking on pMHC complexes. Moreover, MHC class I- and II-restricted T cells select their receptors from a common TcR repertoire. In other words, the same Vα or Vβ regions can be used in recognizing either class I or class II molecules, further implying an intrinsic promiscuity of TcRs rather than an innate bias toward recognizing particular MHC molecules. Accordingly, it is important to consider whether other biochemical aspects of the TcR–pMHC interaction reveal a signature of MHC restriction.
Structural comparisons of TcRs in the nonliganded and liganded states have revealed significant conformational changes, mainly in the CDR3 loops, which occur during pMHC-I ligation (8–10). This event is characterized by a slow association rate and fast dissociation rate, resulting in a low-affinity (≈10 μM) interaction (11). The conformational plasticity of TcRs is consistent with a potentially distinctive thermodynamic signature of TcR–pMHC-I interactions, which is characterized by favorable enthalpy and unfavorable entropy of interaction (12–14). An unfavorable entropic term, which represents an increase in the ordered state, is anticipated from the formation of one kinetically independent object but is also consistent with the ordering of CDR loops on pMHC-I ligation, whereas the enthalpic driving forces are likely to arise from intermolecular contacts formed at the extensive TcR–pMHC-I interface. Evidence suggests that only a small energetic window governed by these thermodynamic and kinetic parameters distinguishes productive signaling outcomes on TcR engagement, indicating a crucial role for thermodynamic parameters in TcR–pMHC recognition (1, 11, 13, 15). It is not yet clear, however, whether all TcRs conform to this emerging structural and thermodynamic paradigm of pMHC-I recognition and to what extent this paradigm might reflect a guiding principle in MHC restriction of TcR recognition.
To address these issues we have studied the thermodynamics of a well characterized TCR–pMHC interaction in unrelated HLA-B8+ (B*0801) individuals. These donors share immunodominant cytotoxic T cell responses to the peptide FLRGRAYGL (FLR) derived from the latent Epstein–Barr virus nuclear antigen 3A (16). We reasoned that such immunodominant TcRs might provide strong clues to the basis of MHC restriction, especially given that the detailed structural properties for this TcR (LC13) bound to FLR–HLA-B8 have been established (10, 17, 18).
In this study we demonstrate that the LC13 TcR–FLR–HLA-B8 is entropically and enthalpically driven and therefore does not conform to the expected thermodynamic profile for a TcR–pMHC interaction. Structural analyses of this complex reveal that the CDR loops are well ordered in the unliganded state and undergo a conformational isomerization to adopt the liganded state, with the simultaneous expulsion of water molecules from this interface. These results suggest that the underlying thermodynamic basis of MHC restriction is unpredictable and varies from one TcR to another. We conclude that other molecular factors, such as CD8/CD4 coreceptor ligation, must play a crucial role in guiding MHC restriction.
Results
Thermodynamic Measurements.
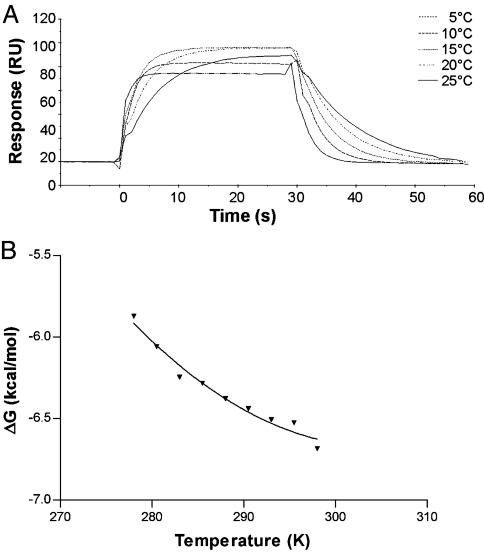
The affinity of the interaction of LC13 with FLR–HLA-B8 was determined by using surface plasmon resonance (SPR) over the temperature range of 5–25°C. Within this temperature range the affinity of the LC13 TcR for the FLR–HLA-B8 complex undergoes a moderate increase (≈2-fold) as the temperature increases because of the association and dissociation rates increasing 8- and 4-fold, respectively (Fig. 1A). The temperature was plotted against the ΔG values calculated from the affinity (Fig. 1B). Nonlinear regression was used to fit the three-parameter equation to the curve and thus calculate the thermodynamic parameters ΔH, ΔS, and ΔCp (Fig. 1B and Table 1). The interaction shows an enthalpic contribution of −2.4 ± 2.8 kcal/mol and entropic contribution (TΔS) of +4.2 ± 2.6 kcal/mol with a ΔCp of −0.62 ± 0.27 kcal/mol at 25°C.
Fig. 1.
Thermodynamic analysis of the interaction between LC13 TcR and FLR–HLA-B8. (A) Temperature dependence of the LC13 TcR-binding FLR–HLA-B8 detected by SPR. RU, resonance units. (B) Free energy (ΔG) values over the temperature range of 5–25°C. Each data point is the mean of at least two independent SPR experiments; nonlinear regression was used to fit the three-parameter equation to calculate ΔH, ΔS, and ΔCp.
Table 1.
Thermodynamic parameters
| Parameter | LC13–FLR–HLA-B8 | JM22–flu–HLA-A2* | 2C–dEV8–H2-Kb† |
|---|---|---|---|
| SPR-derived thermodynamic parameters ‡ | |||
| ΔG, kcal/mol §¶ | −6.7 ± 0.05 | −7.1 | −6.3 |
| ΔH, kcal/mol | −2.4 ± 2.8 | −23 | −22.7 |
| TΔS, kcal/mol ¶ | +4.2 ± 2.6 | −15.9 | −16.2 |
| ΔCp, kcal/mol·K | −0.62 ± 0.27 | — | −1.1 |
| ITC∥ | |||
| ΔG, kcal/mol¶ | −7.0 ± 0.2 | — | — |
| ΔH, kcal/mol | −3.6 ± 0.5 | −19.7 | — |
| TΔS, kcal/mol¶ | +3.4 ± 0.7 | — | — |
| Structural analysis | |||
| ΔCp, kcal/mol·K | −0.24 | −0.21 | −0.25 |
| BSA, ** Å2 | 2,220 | 1,570 | 1,876 |
| Interface H bonds | 13 | 8 | 12 |
| Interface water-mediated H bonds | 5 | 10 | 5 |
*Thermodynamic parameters are from ref. 14; structural analysis is from Protein Data Bank entry 1OGA (24).
†Thermodynamic parameters are from ref. 13; structural analysis is from Protein Data Bank entry 1TRC (23).
‡SPR data are reported ± the SE of curve fitting with the exception of the ΔG.
§ΔG = RT ln(Kd), mean of two experiments ± SEM, where R = gas constant and T = thermodynamic temperature.
¶Values are reported for 298 K.
∥Isothermal titration calorimetry (ITC) values are reported as the mean of three independent experiments ± the SE.
**BSA, buried surface area.
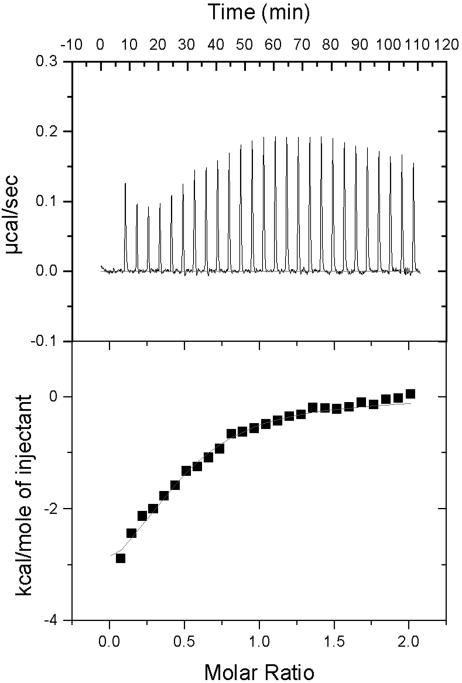
Discrepancies between enthalpies determined by indirect methods such as the SPR analysis and by direct analyses have been observed previously (19). To discount these discrepancies, we determined the ΔH of the interaction of LC13 with FLR–HLA-B8 in solution by using ITC. Fig. 2 shows the amount of heat absorbed as LC13 is titrated into FLR–HLA-B8. Fitting the integrated peak area vs. TcR concentration revealed that the interaction has an affinity of 8.1 ± 2.7 μM, an enthalpy of −3.6 ± 0.5 kcal/mol, and a favorable entropic contribution of 11.5 ± 2.2 cal/mol (TΔS = 3.4 ± 0.7 kcal/mol at 25°C). Thus, the parameters derived from fitting the three-parameter equation to the SPR data were in close agreement with the ITC measurements (Table 1) as noted previously in thermodynamic studies of TcR–pMHC-I interactions (13, 14).
Fig. 2.
The change in enthalpy at binding was measured directly by ITC. (Upper) Heat released over time for the titration of LC13 TcR into FLR–HLA-B8 at 25°C. (Lower) Baseline-subtracted data expressed as the molar ratio plotted against the area under the peak and fitted by a model for single-site binding.
Heat Capacity of the Interaction of LC13 and FLR-HLA-B8 Is Normal.
A change in heat capacity (ΔCp) has been used to indicate changes in exposure or burial of protein surface area on binding (20–22). A positive change in ΔCp equates to an increase in exposed surface area, whereas a negative ΔCp indicates a burial of surface area. In cases in which there is wide divergence between the calculated ΔCp (ΔCp,calc) from structural information and the observed ΔCp (ΔCp,obs), conformational change or flexibility has been inferred. Conformational ΔCp (ΔCp,conf), that is, the heat capacity change that cannot be attributed to the static structures and is believed to be a result of conformational change and/or change in flexibility, is determined by ΔCp,obs − ΔCp,calc (13). Computational assessment of the change in the polar and nonpolar accessible surface area on binding of LC13 TcR to FLR–HLA-B8 by using the crystal structures of the liganded and unliganded LC13 TcR (10, 17) yielded a ΔCp,calc of 0.25 kcal/mol·K and a ΔCp,conf of −0.37 kcal/mol·K (Table 1). This ΔCp,calc is consistent with that of other TcR–pMHC-I interactions.
Positive Enthalpic Forces.
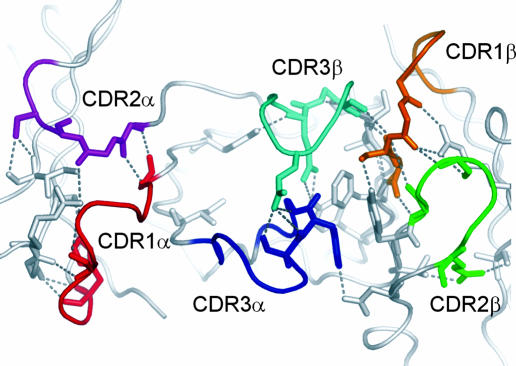
The small enthalpic contribution to the formation of the LC13 TcR–FLR–HLA-B8 complex is somewhat surprising given that the complex is stabilized by a similar number of hydrogen bonds (H bonds) and has a relatively large BSA of 2,220 Å2 compared with that of other solved TcR–pMHC-I structures, which have ranged from ≈1,200 to 2,000 Å2 (Table 1) (2, 10). To address why the enthalpic contribution of this complex is significantly less than that reported for other TcR–pMHC-I complexes we analyzed the bonding networks in the variable domains of the unliganded and liganded LC13 structures and compared the results with those of the mouse 2C TcR (8, 23). This structural analysis revealed that whereas the 2C TcR has a large net increase in the number of internal H bonds on complex formation (+59 H bonds), the LC13 TcR has very little change in the total number of H bonds, gaining only 4 additional internal H bonds in the liganded conformation. Furthermore, the H bonding network in the unliganded LC13 TcR is extensive (Fig. 3), suggesting that the unliganded conformation of the LC13 TcR does not have the inherent flexibility that has been reported for other systems.
Fig. 3.
The LC13 TcR forms an extensive hydrogen-bonding network stabilizing the CDR loops in the unliganded conformation. The binding interface of the unliganded TcR is depicted with the side chains of the residues that form inter-CDR contacts and the hydrogen-bonding network that they form (gray dashed lines).
Hydrophobic Forces and Entropy.
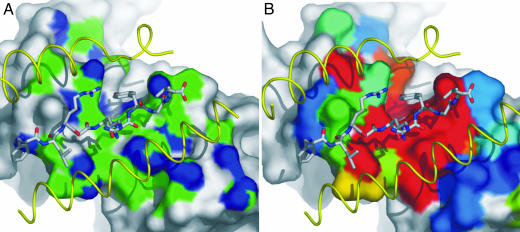
To assess whether the favorable entropic contribution could be attributed to hydrophobic interactions at the interface, the nature of each potential contacting atom was assessed in terms of the contribution to the BSA and assigned as either hydrophobic or hydrophilic in nature. This analysis was carried out for the LC13–FLR–HLA-B8 (10), 2C–dEV8–H2-Kb (8), and JM22–flu–HLA-A2 (24) complexes, which have TΔS values of 4.2, −16.2, and −15.9 kcal/mol (13), respectively. Although these complexes have significantly different TΔS values, the contribution of hydrophobic contacts to the interface is remarkably similar, making up 58%, 50%, and 59% of the contacting BSA for the LC13, 2C, and JM22 TcRs, respectively (Fig. 4A).
Fig. 4.
Hydrophobic regions of the binding interface correspond to the energetic hot spot of binding. (A) Surface representation of the LC13 TcR binding interface highlighting the hydrophobic (green) and hydrophilic (blue) atomic contacts with FLR–HLA-B8. The FLR peptide and HLA-B8 helices (yellow) have been overlaid to indicate the binding orientation. (B) Residues that interact with FLR–HLA-B8 have been colored according to the impact they have on binding as determined by alanine-scanning mutagenesis (18). Residues colored red have the most significant impact, followed by shades of yellow, green, and blue; dark blue residues have no effect on binding.
We recently defined an energetic hot spot for the LC13 TcR interaction with FLR–HLA-B8 through site-directed mutagenesis (Fig. 4B) (18). We hypothesized that there would be a correlation between this energetic hot spot and the hydrophobic interactions at the interface. The energetic contribution of LC13 TcR interface residues can be divided into a significant, moderate, and little or no effect on the affinity of TcR binding. Each of the atomic interactions at the interface was assessed for both its hydrophobic nature and impact of TcR binding to determine whether there is an inherent bias toward these entropically favorable interactions. This analysis showed that 64% of the BSA that forms hydrophobic interactions was attributed to residues that have a significant impact on TcR binding, whereas only 48% of the hydrophilic interacting BSA involved such energetically important residues. Furthermore, only 9% of the hydrophobic interacting BSA comprised residues that had no effect on TcR binding, whereas 30% of the hydrophilic interacting BSA involved these residues.
Water Molecules Are Excluded from the Binding Interface.
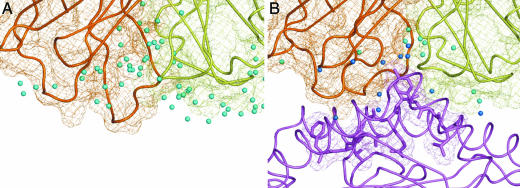
During ligation, the LC13 TcR undergoes a marked conformational change in four of the six CDR loops (10). This movement increases the surface complementarity between the LC13 TcR and FLR–HLA-B8 and results in the apparent release of water molecules, allowing the TcR to interact directly with the pMHC (Fig. 5). In the unliganded state a myriad of water molecules are associated with not only the CDR residues that comprise the binding interface but also with residues of adjacent CDR loops that form bridging water-mediated H bonds to help stabilize the unliganded state (Fig. 5A). Despite the difference in the resolution of the two structures, and thus the number of water molecules that can be visualized, it appears likely that during ligation these interactions are disrupted, and the ordered water molecules are dislodged. This disruption gives the TcR an opportunity to maximize the hydrophobic interactions at the interface and thus contributes favorably to the entropic forces driving this interaction (Fig. 5B).
Fig. 5.
During ligation the LC13 TcR expels solvent from the binding interface. (A) Surface representation (mesh) of the unliganded LC13 TcR. Water molecules associated with the residues that form the binding interface at ligation and those that form bridging H bonds between the α (orange) and β (yellow) chains of the TcR are shown as cyan spheres (17). (B) Ligated conformation of the LC13 TcR bound to the FLR–HLA-B8 complex (purple). Water molecules that form bridging H bonds between the TcR and FLR–HLA-B8 are shown in blue, whereas those that remain associated with only the TcR are depicted in cyan (10).
Discussion
One of the great challenges in structural immunology is to delineate the structural basis underlying MHC restriction. Although 11 distinct TcR–pMHC-I structures have been determined to date (7–10, 24–30), there is surprisingly little evidence of a unifying theme or conserved TcR–pMHC-I contact points. TcR–pMHC topologies can vary significantly (31), and the only clues to suggest a general mode of binding are the very approximate docking mode, the conserved restriction triad for TcR–pMHC-I interactions (7), and the suggestion in a TcR–pMHC-II complex of an anchor point in the Vβ ligation of the class II molecule (32). The difficulty in addressing this problem is compounded by TcR diversity, polymorphism within the classical MHC-I, and the variability of the bound antigenic peptide.
There is some evidence that MHC reactivity is inherent in the germ-line-encoded portions of the TcR and that there are subtle biases in V region usage for MHC class I- vs. class II-restricted T cell selection (33–35). Notably, Wilson and colleagues (36) observed that the CDR1 and 2 loops of the TcR dominated the landscape of pMHC-I recognition, and certain sequences in these loops enhance affinity for MHC-I. These observations fit the two-step mechanism of TcR recognition, which postulates that the TcR is guided initially to interact with the MHC heavy chain by its germ-line-encoded CDR1 and CDR2 loops followed by induced fit of the hypervariable CDR3 loops over the peptide (5). However, the arguments for an intrinsic tendency of TcRs to recognize MHC are inconclusive; for example, biases in TcR MHC class I restriction are surprisingly weak, and the same V regions that are biased toward class II recognition (33) can be restricted to MHC class I recognition (37). Moreover, despite the limited subset of available TcR–pMHC-I structures, divergences from the generalities on the role of different CDRs have already been observed, including that of the HLA-A2 system, the BM3.3, the xenoreactive murine Ahiii 12.2, and LC13 TcRs (18, 24, 27, 29). These data do not rule out the notion that MHC restriction is guided by docking onto a mixture of conserved and polymorphic residues at the pMHC-I interface (38); however, it appears just as possible that MHC restriction is not intrinsically “hardwired” in the structure of the TcR but instead may depend on extrinsic factors. Therefore, it is considered important to look for alternative clues such as the thermodynamics of the interaction, which might indicate a general mechanism underpinning MHC restriction.
T cell immunodominance represents a potential opportunity to understand the basis of MHC restriction. An immunodominant TcR, which is selected uniquely to interact with a specific pMHC-I complex, limits the parameters that structural biologists have to consider at the TcR–pMHC-I interface. We determined previously the crystal structure of an immunodominant TcR (termed LC13) in complex with FLR–HLA-B8 (10). This study revealed that the CDR3α loops were not restricted to recognizing the peptide, but instead played a major role in recognizing the HLA molecule. Furthermore, we dissected the energetic landscape of pMHC-I recognition, revealing that the CDR3 loops dictate the energetics of pMHC-I recognition, whereas the CDR1 and CDR2 loops play only supporting energetic roles (18). Collectively, these studies argue against the germ-line-encoded regions of the LC13 TcR possessing an inherent bias for HLA-B8.
We now also show that the thermodynamic basis of the LC13 TcR interaction does not conform to the generally accepted model of an enthalpically driven interaction brought about by the stabilization of flexible TcR loops. We show that the interaction of LC13 with FLR–HLA-B8 is favored entropically, in contrast to the majority of systems investigated previously. The thermodynamics of LC13–FLR–HLA-B8 ligation is not the only exception in that studies of the Tax–HLA-A2 system showed that the A6 TcR bound with entropically favorable forces, whereas the B7 TcR, which binds the same pMHC complex, conforms to the general entropically disfavored energetic profile (39). Together these data suggest that the current thermodynamic model for the TcR–pMHC interaction applies only to a subset of TcR ligation events, and the complexity of forces guiding the docking is yet to be unraveled fully.
The favorable entropic force for the LC13 TcR was unexpected given the magnitude of conformational change at this interface. Close examination of the CDR loops in the nonliganded state of the LC13 TcR reveals that the loops are unlikely to be inherently flexible. Instead, apart from the extreme tip of the CDR3α loop, they appear to be held in a fixed conformation by a network of direct and water-mediated inter-CDR loop contacts. Thus, at the time of ligation it appears that the CDR loops move, in a concerted action, from one defined conformation to another. To make these conformational changes, bonds must be broken before new favorable intermolecular bonds are formed. This process is consistent with the low contribution of enthalpy to this interaction and the favorable entropic forces. If the loops of the LC13 TcR became more ordered at ligation, this process would be more likely to lead to unfavorable entropic forces. Furthermore, the favorable entropy of the interaction of LC13 with FLR–HLA-B8 is also consistent with the observation that water molecules are displaced from the interface, unlike previously determined TcR–pMHC-I interactions (24, 27, 40). Although the interface between the two proteins is vast, an analysis of the relative contributions that side chains make to complex formation (18) suggests that most contributions to binding energetics come from hydrophobic contacts, thereby explaining the expulsion of water molecules from the interface. It appears that the interface of LC13 and FLR–HLA-B8 is more reminiscent of a typical protein–protein subunit interface (41).
Collectively, our data indicate that there is no single consensus describing the modus operandi of the TcR: no conserved TcR–pMHC-I contact points, no conserved TcR energetic landscape, and as shown here, disparate rather than conserved thermodynamic parameters governing TcR–pMHC-I interactions. The variable diagonal footprint is not conserved in all TcR–class I interactions and has even been observed in the structure of an HLA-A1-restricted, peptide-specific Fab bound to the pMHC complex. Thus, the approximate diagonal binding of pMHC-specific mAbs and TcRs may reflect an accommodation of the ridges posed by the MHC helices rather than a signature of MHC restriction. Our overall findings suggest that the thermodynamic principles underlying MHC restriction do not reside solely at the TcR–pMHC-I interface. We suggest that other molecular factors, such as coreceptor ligation, conformational adjustments involved in TcR signaling, or constraints dictated by higher-order arrangement of ligated TcRs might play a greater role in guiding MHC restriction than appreciated previously. It will be important to test this idea in future experiments.
Materials and Methods
Protein Production.
Recombinant protein was expressed, refolded, and purified as described in refs. 42 and 43. LC13 TcR chains α and β, HLA-B*0801 heavy chain, and human β2-microglobulin were expressed separately in BL21(DE3) Escherichia coli. Inclusion bodies were isolated, washed, and solubilized in 8 M urea, and the protein complexes were then refolded by rapid dilution into a buffer containing 400 mM l-arginine·HCl, 100 mM Tris·HCl (pH 8.0), 2 mM NaEDTA, 5 mM reduced glutathione, and 0.5 mM oxidized glutathione. The HLA-B8 class I complex was refolded in the presence of a molar excess of FLR peptide. Refolded proteins were purified to homogeneity by anion-exchange and size-exclusion chromatography.
SPR.
The affinity measurements for the interaction of LC13 with FLR–HLA-B8 were made by using a Biacore 3000 instrument as described in ref. 18. Briefly, W6/32 antibody was immobilized onto the surface of a Biacore CM5 chip by using standard amine coupling. Immediately before each injection of the LC13 TcR, 300–400 resonance units of FLR-HLA-B8 were captured onto the W6/32 surface. Data sets were collected at temperatures ranging from 5°C to 25°C in 2.5°C intervals. The affinity constant (Kd) for each data set was calculated by simultaneous global fitting of the kinetic association (ka) and dissociation (kd) rate constants (Kd = kd/ka) by using a modified version of the 1:1 Langmuir binding model, allowing for a drifting baseline and local fitting of the binding maxima, to accommodate the capture system.
Thermodynamic Calculations.
The thermodynamic parameters were calculated from SPR Kd values calculated over 5–25°C. The temperature in K was plotted against the affinity constants expressed as ΔG values (Eq. 1). The changes in ΔH, ΔS, and heat capacity (ΔCp) were calculated simultaneously by nonlinear regression to fit the three-parameter equation (Eq. 2) by using prism version 4.0 (GraphPad, San Diego). The mean of at least two independent experiments was used for each affinity measurement; results are reported ± SE of the curve fit,
where R is the gas constant, 1.987 × 10−3 kcal/mol·K, and T is the temperature in K.
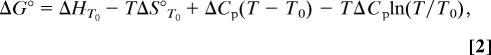 |
where T0 is an arbitrary reference temperature (298 K), ΔG° is the standard free energy of binding at T (kcal/mol), ΔHT0 is the enthalpy change at the time of binding at T0 (kcal/mol), and ΔCp is the specific heat capacity (kcal/mol·K).
Accessible surface area calculations were made by using naccess Version 2.1.1 (44), and the ΔCp,calc values were calculated by using
where ΔAnp and ΔAp are the changes in water-accessible nonpolar and polar surface area, respectively (22).
Microcalorimetry.
ITC studies were carried out at 25°C by using a VP-ITC titration calorimeter (Microcal, Amherst, MA). Proteins were dialyzed into PBS (pH 7.4) and degassed before the experiment. After an initial 1-μl injection, 391 μM LC13 was titrated into 39 μM FLR–HLA-B8 or buffer alone as a control by using a series of 10-μl injections 4 min apart until the reaction was saturated. As a further control, buffer was titrated into 39 μM FLR–HLA-B8. The titration of LC13 into buffer was subtracted from each data set, and the first data point was removed. origin Version 5.0 software (Microcal Software, Northampton, MA) was used to fit a single-site binding model by nonlinear regression to the binding isotherms. Results are reported as the mean of three experiments ± SEM.
Acknowledgments
We thank F. Carbone for a critical reading of the manuscript and N. Borg for assistance with figure generation. This work was supported by the National Health and Medical Research Council, the Australian Research Council, and the Roche Organ Transplantation Research Foundation. J.R. was supported by an Australian Research Council Professorial Fellowship, L.K.E. by a National Health and Medical Research Council CJ Martin Fellowship, T.B. by a National Health and Medical Research Council Peter Doherty Fellowship, and C.S.C. by a Monash University Research Fellowship. J.M.M. is a Charles and Sylvia Viertel Research Fellow, and A.W.P. is a Russell Grimwade Fellow of the Department of Biochemistry and Molecular Biology at the University of Melbourne.
Abbreviations
- BSA
buried surface area
- CDR
complementarity-determining region
- Cp
heat capacity
- FLR
FLRGRAYGL
- HLA
human leukocyte antigen
- ITC
isothermal titration calorimetry
- MHC-I
MHC class I
- pMHC
peptide-MHC
- SPR
surface plasmon resonance
- TcR
αβ T cell receptor
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.van der Merwe P. A., Davis S. J. Annu. Rev. Immunol. 2003;21:659–684. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- 2.Rudolph M. G., Wilson I. A. Curr. Opin. Immunol. 2002;14:52–65. doi: 10.1016/s0952-7915(01)00298-9. [DOI] [PubMed] [Google Scholar]
- 3.Ely L. K., Kjer-Nielsen L., McCluskey J., Rossjohn J. IUBMB Life. 2005;57:575–582. doi: 10.1080/15216540500215556. [DOI] [PubMed] [Google Scholar]
- 4.Garcia K. C., Adams E. J. Cell. 2005;122:333–336. doi: 10.1016/j.cell.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Wu L. C., Tuot D. S., Lyons D. S., Garcia K. C., Davis M. M. Nature. 2002;418:552–556. doi: 10.1038/nature00920. [DOI] [PubMed] [Google Scholar]
- 6.Krogsgaard M., Davis M. M. Nat. Immunol. 2005;6:239–245. doi: 10.1038/ni1173. [DOI] [PubMed] [Google Scholar]
- 7.Tynan F. E., Burrows S. R., Buckle A. M., Clements C. S., Borg N. A., Miles J. J., Beddoe T., Whisstock J. C., Wilce M. C., Silins S. L., et al. Nat. Immunol. 2005;6:1114–1122. doi: 10.1038/ni1257. [DOI] [PubMed] [Google Scholar]
- 8.Garcia K. C., Degano M., Pease L. R., Huang M., Peterson P. A., Teyton L., Wilson I. A. Science. 1998;279:1166–1172. doi: 10.1126/science.279.5354.1166. [DOI] [PubMed] [Google Scholar]
- 9.Reiser J. B., Gregoire C., Darnault C., Mosser T., Guimezanes A., Schmitt-Verhulst A. M., Fontecilla-Camps J. C., Mazza G., Malissen B., Housset D. Immunity. 2002;16:345–354. doi: 10.1016/s1074-7613(02)00288-1. [DOI] [PubMed] [Google Scholar]
- 10.Kjer-Nielsen L., Clements C. S., Purcell A. W., Brooks A. G., Whisstock J. C., Burrows S. R., McCluskey J., Rossjohn J. Immunity. 2003;18:53–64. doi: 10.1016/s1074-7613(02)00513-7. [DOI] [PubMed] [Google Scholar]
- 11.Davis M. M., Boniface J. J., Reich Z., Lyons D., Hampl J., Arden B., Chien Y.-h. Annu. Rev. Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 12.Boniface J. J., Reich Z., Lyons D. S., Davis M. M. Proc. Natl. Acad. Sci. USA. 1999;96:11446–11451. doi: 10.1073/pnas.96.20.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krogsgaard M., Prado N., Adams E. J., He X. L., Chow D. C., Wilson D. B., Garcia K. C., Davis M. M. Mol. Cell. 2003;12:1367–1378. doi: 10.1016/s1097-2765(03)00474-x. [DOI] [PubMed] [Google Scholar]
- 14.Willcox B. E., Gao G. F., Wyer J. R., Ladbury J. E., Bell J. I., Jakobsen B. K., van der Merwe P. A. Immunity. 1999;10:357–365. doi: 10.1016/s1074-7613(00)80035-7. [DOI] [PubMed] [Google Scholar]
- 15.Ely L. K., Green K. J., Beddoe T., Clements C. S., Miles J. J., Bottomley S. P., Zernich D., Kjer-Nielsen L., Purcell A. W., McCluskey J., Rossjohn J., Burrows S. R. J. Immunol. 2005;174:5593–5601. doi: 10.4049/jimmunol.174.9.5593. [DOI] [PubMed] [Google Scholar]
- 16.Argaet V. P., Schmidt C. W., Burrows S. R., Silins S. L., Kurilla M. G., Doolan D. L., Suhrbier A., Moss D. J., Kieff E., Suclley T. B., et al. J. Exp. Med. 1994;180:2335–2340. doi: 10.1084/jem.180.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kjer-Nielsen L., Clements C. S., Brooks A. G., Purcell A. W., McCluskey J., Rossjohn J. Structure. 2002 doi: 10.1016/s0969-2126(02)00878-x. [DOI] [PubMed] [Google Scholar]
- 18.Borg N. A., Ely L. K., Beddoe T., Macdonald W. A., Reid H. H., Clements C. S., Purcell A. W., Kjer-Nielsen L., Miles J. J., Burrows S. R., McCluskey J., Rossjohn J. Nat. Immunol. 2005;6:171–180. doi: 10.1038/ni1155. [DOI] [PubMed] [Google Scholar]
- 19.Naghibi H., Tamura A., Sturtevant J. M. Proc. Natl. Acad. Sci. USA. 1995;92:5597–5599. doi: 10.1073/pnas.92.12.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myszka D. G. Methods Enzymol. 2000;323:325–340. doi: 10.1016/s0076-6879(00)23372-7. [DOI] [PubMed] [Google Scholar]
- 21.Privalov P. L., Jelesarov I., Read C. M., Dragan A. I., Crane-Robinson C. J. Mol. Biol. 1999;294:997–1013. doi: 10.1006/jmbi.1999.3285. [DOI] [PubMed] [Google Scholar]
- 22.Spolar R. S., Record M. T., Jr Science. 1994;263:777–784. doi: 10.1126/science.8303294. [DOI] [PubMed] [Google Scholar]
- 23.Garcia K. C., Degano M., Stanfield R. L., Brunmark A., Jackson M. R., Peterson P. A., Teyton L., Wilson I. A. Science. 1996;274:209–220. [PubMed] [Google Scholar]
- 24.Stewart-Jones G. B., McMichael A. J., Bell J. I., Stuart D. I., Jones E. Y. Nat. Immunol. 2003;4:657–663. doi: 10.1038/ni942. [DOI] [PubMed] [Google Scholar]
- 25.Garboczi D. N., Ghosh P., Utz U., Fan Q. R., Biddison W. E., Wiley D. C. Nature. 1996;384:134–141. [PubMed] [Google Scholar]
- 26.Ding Y. H., Smith K. J., Garboczi D. N., Utz U., Biddison W. E., Wiley D. C. Immunity. 1998;8:403–411. doi: 10.1016/s1074-7613(00)80546-4. [DOI] [PubMed] [Google Scholar]
- 27.Reiser J. B., Darnault C., Guimezanes A., Gregoire C., Mosser T., Schmitt-Verhulst A. M., Fontecilla-Camps J. C., Malissen B., Housset D., Mazza G. Nat. Immunol. 2000;1:291–297. doi: 10.1038/79728. [DOI] [PubMed] [Google Scholar]
- 28.Luz J. G., Huang M., Garcia K. C., Rudolph M. G., Apostolopoulos V., Teyton L., Wilson I. A. J. Exp. Med. 2002;195:1175–1186. doi: 10.1084/jem.20011644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buslepp J., Wang H., Biddison W. E., Appella E., Collins E. J. Immunity. 2003;19:595–606. doi: 10.1016/s1074-7613(03)00269-3. [DOI] [PubMed] [Google Scholar]
- 30.Chen J. L., Stewart-Jones G., Bossi G., Lissin N. M., Wooldridge L., Choi E. M., Held G., Dunbar P. R., Esnouf R. M., Sami M., et al. J. Exp. Med. 2005;201:1243–1255. doi: 10.1084/jem.20042323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahn M., Nicholson M. J., Pyrdol J., Wucherpfennig K. W. Nat. Immunol. 2005;6:490–496. doi: 10.1038/ni1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maynard J., Petersson K., Wilson D. H., Adams E. J., Blondelle S. E., Boulanger M. J., Wilson D. B., Garcia K. C. Immunity. 2005;22:81–92. doi: 10.1016/j.immuni.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Sim B.-C., Zerva L., Greene M. I., Gascoigne N. R. J. Science. 1996;273:963–966. doi: 10.1126/science.273.5277.963. [DOI] [PubMed] [Google Scholar]
- 34.Sim B. C., Wung J. L., Gascoigne N. R. J. Immunol. 1998;160:1204–1211. [PubMed] [Google Scholar]
- 35.Zerrahn J., Held W., Raulet D. H. Cell. 1997;88:627–636. doi: 10.1016/s0092-8674(00)81905-4. [DOI] [PubMed] [Google Scholar]
- 36.Manning T. C., Schlueter C. J., Brodnicki T. C., Parke E. A., Speir J. A., Garcia K. C., Teyton L., Wilson I. A., Kranz D. M. Immunity. 1998;8:413–425. doi: 10.1016/s1074-7613(00)80547-6. [DOI] [PubMed] [Google Scholar]
- 37.Sha W. C., Nelson C. A., Newberry R. D., Kranz D. M., Russell J. H., Loh D. Y. Nature. 1988;335:271–274. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- 38.Garboczi D. N., Biddison W. E. Immunity. 1999;10:1–7. doi: 10.1016/s1074-7613(00)80001-1. [DOI] [PubMed] [Google Scholar]
- 39.Davis-Harrison R. L., Armstrong K. M., Baker B. M. J. Mol. Biol. 2005;346:533–550. doi: 10.1016/j.jmb.2004.11.063. [DOI] [PubMed] [Google Scholar]
- 40.Anikeeva N., Lebedeva T., Krogsgaard M., Tetin S. Y., Martinez-Hackert E., Kalams S. A., Davis M. M., Sykulev Y. Biochemistry. 2003;42:4709–4716. doi: 10.1021/bi026864+. [DOI] [PubMed] [Google Scholar]
- 41.Stites W. E. Chem. Rev. 1997;97:1233–1250. doi: 10.1021/cr960387h. [DOI] [PubMed] [Google Scholar]
- 42.Clements C. S., Kjer-Nielsen L., MacDonald W. A., Brooks A. G., Purcell A. W., McCluskey J., Rossjohn J. Acta Crystallogr. D. 2002;58:2131–2134. doi: 10.1107/s0907444902015482. [DOI] [PubMed] [Google Scholar]
- 43.Kjer-Nielsen L., Clements C. S., Brooks A. G., Purcell A. W., Fontes M. R., McCluskey J., Rossjohn J. J. Immunol. 2002;169:5153–5160. doi: 10.4049/jimmunol.169.9.5153. [DOI] [PubMed] [Google Scholar]
- 44.Hubbard S. J., Thornton J. M. naccess. London: Department of Biochemistry and Molecular Biology, University College; 1993. Version 2.1.1. [Google Scholar]