Abstract
Combination therapies have long been used to treat inflammation while reducing side effects. The present study was designed to evaluate the therapeutic potential of combination treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) and previously undescribed soluble epoxide hydrolase inhibitors (sEHIs) in lipopolysaccharide (LPS)-challenged mice. NSAIDs inhibit cyclooxygenase (COX) enzymes and thereby decrease production of metabolites that lead to pain and inflammation. The sEHIs, such as 12-(3-adamantan-1-yl-ureido)-dodecanoic acid butyl ester (AUDA-BE), stabilize anti-inflammatory epoxy-eicosatrienoic acids, which indirectly reduce the expression of COX-2 protein. Here we demonstrate that the combination therapy of NSAIDs and sEHIs produces significantly beneficial effects that are additive for alleviating pain and enhanced effects in reducing COX-2 protein expression and shifting oxylipin metabolomic profiles. When administered alone, AUDA-BE decreased protein expression of COX-2 to 73 ± 6% of control mice treated with LPS only without altering COX-1 expression and decreased PGE2 levels to 52 ± 8% compared with LPS-treated mice not receiving any therapeutic intervention. When AUDA-BE was used in combination with low doses of indomethacin, celecoxib, or rofecoxib, PGE2 concentrations dropped to 51 ± 7, 84 ± 9, and 91 ± 8%, respectively, versus LPS control, without disrupting prostacyclin and thromboxane levels. These data suggest that these drug combinations (NSAIDs and sEHIs) produce a valuable beneficial analgesic and anti-inflammatory effect while prospectively decreasing side effects such as cardiovascular toxicity.
Keywords: arachidonic acid, cyclooxygenase, epoxygenase, pain, linoleic acid
Coadministration of analgesic drugs is a popular therapeutic regimen for inflammatory diseases such as rheumatoid arthritis and osteoarthritis. An advantage of using combination therapy is that it can maximize the analgesic effects while minimizing the incidence of adverse side effects (1). This goal is accomplished if the combination of inhibitors offers analgesic synergism, which allows a reduction in required dosage and decreases the incidence of undesired side effects (2).
For example, a current combination therapy includes nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids, each of which has detrimental side effects. The mechanism of action of NSAIDs involves inhibition of prostaglandin (PG) synthesis, (3, 4) as well as direct central effects through modulation of neurotransmitter–receptor systems involved in pain transmission (5–7). It is believed that the inhibition of cyclooxygenase-2 (COX-2) by NSAIDs underlies at least part of their anti-inflammatory and antinociceptive properties (8). However, despite their ability to reduce inflammation and pain, NSAIDs cause a wide array of unwanted side effects such as renal failure, uncontrolled hypertension, aggravation of congestive heart failure, dyspepsia, and peptic ulceration hemorrhage and perforation (9–12).
Another approach to reduce PG formation is through suppression of COX-2 expression, which can be achieved by inhibiting NF-κB translocation with epoxyeicosatrienoic acids (EpETrEs or EETs). EETs are metabolites of arachidonic acid (AA) that undergo hydrolysis by soluble epoxide hydrolase (sEH) to generate dihydroxyeicosatrienoic acids (DiHETrEs or DHETs). EETs decrease nuclear translocation of NF-κB (13). sEH inhibitors (sEHIs) increase the concentrations of EETs and indirectly decrease the expression of COX-2 (14).
Therefore, combinations of sEHIs and NSAIDs could be used to control inflammation and pain while reducing NSAID side effects. The purpose of the present work was to investigate the anti-inflammatory/antinociceptive effect of two sEHIs, 12-(3-adamantan-1-yl-ureido)-dodecanoic acid butyl ester (AUDA-BE) and 1-adamantan-1-yl-3-{5-[2-(2-ethoxyethoxy)ethoxy]pentyl}urea (AEPU) [also known as IK-950, or 1-adamantan-3-(5-(2-(2-ethylethoxy)ethoxy)pentyl)urea)], and three NSAIDs, rofecoxib, celecoxib, or indomethacin, by administration alone or in combination. We assessed the antinociceptive effects of these drugs by measuring thermal hindpaw withdrawal latencies in a mouse model of lipopolysaccharide (LPS)-induced inflammatory hyperalgesia (15). We hypothesized that NSAIDs would have dose-dependent additive or enhanced (more than additive) interactions with a constant dose of sEHIs.
Results
Antinociceptive Effects of NSAIDs and/or sEHIs.
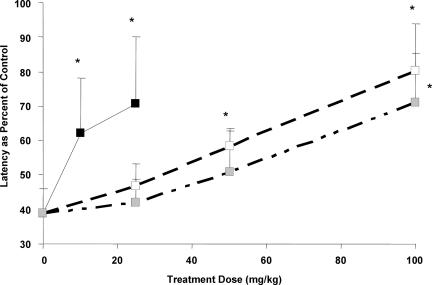
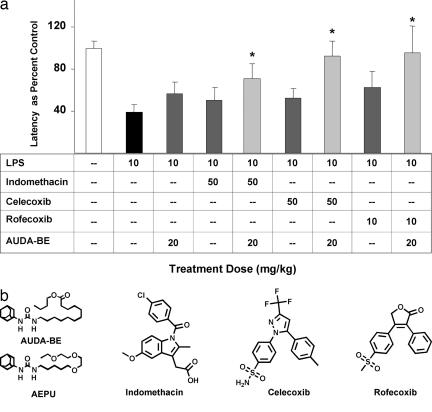
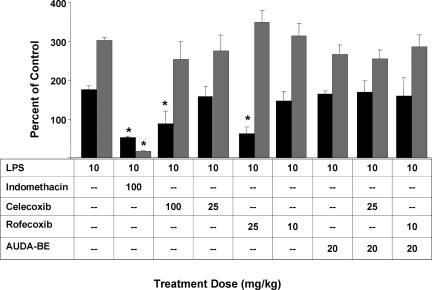
Nociception is the result of a complex cascade of events accomplished by diverse types of mechanisms, beginning with tissue damage in response to a noxious stimulus and subsequent release of pronociceptive and proinflammatory mediators. Alteration of these proinflammatory mediators is a current therapeutic approach to alleviate pain and inflammation. Here we compare the sEHIs with current drugs, including indomethacin, celecoxib, and rofecoxib. Mice (C57BL/6) injected with LPS (10 mg/kg; i.p.) develop hyperalgesia, indicated by a decrease in paw withdrawal latency to a noxious thermal stimulus. Upon LPS challenge, the latency for hindpaw withdrawal drops to 46 ± 7% as compared with mice administered saline vehicle. Prophylactic administration of rofecoxib (10 or 25 mg/kg), celecoxib (25, 50, or 100 mg/kg), or indomethacin (25, 50 or 100 mg/kg) produces a dose-dependent antihyperalgesic effect (Fig. 1). The sEHI, AUDA-BE (20 mg/kg), also elicits an antihyperalgesic effect equivalent to that of the low dose of rofecoxib (10 mg/kg; Fig. 2a). Coadministration of AUDA-BE (20 mg/kg) with low dose of rofecoxib (10 mg/kg), celecoxib (50 mg/kg), or indomethacin (50 mg/kg) has an additive effect in reducing thermal hyperalgesia (Fig. 2a). An additional sEHI, AEPU, that is structurally different, was also evaluated for its ability to reduce hyperalgesia and was used in combination with the lower doses of rofecoxib (10 mg/kg), celecoxib (50 mg/kg), or indomethacin (50 mg/kg) (see Fig. 2b for structures). Although similar trends were observed with this more polar sEHI, the results in the hindpaw withdrawal assay (data not shown) and oxylipin metabolite profiles were more variable, possibly due to the compound's pharmacokinetic parameters (see Fig. 7, which is published as supporting information on the PNAS web site).
Fig. 1.
Dose–response curves in a thermal hindpaw withdrawal latency model after pretreatment with various concentrations of COX inhibitors (rofecoxib, black; celecoxib, white; indomethacin, gray). The inhibitors reduce LPS-induced thermal hyperalgesia in a dose-dependent manner, indicated by an increase in withdrawal latency toward baseline. Thermal withdrawal latencies were assessed 6 h after LPS exposure. Data represent the average latency ± SD (n = 4) to paw withdrawal from a thermal stimulus. Mean latency values are normalized as percent of control mice receiving vehicle before LPS challenge. ∗, Significantly different from vehicle (P < 0.05) as determined by ANOVA followed by Dunnett's test. The dose is expressed in milligrams per kilogram in all figures.
Fig. 2.
Additive antinociception. (a) Coadministration of AUDA-BE and NSAIDs produces an additive effect in counteracting LPS-induced decreases in hindpaw withdrawal latency. Hindpaw withdrawal latency was assessed 6 h after LPS exposure. Data represent the latency ± SD (n = 4) to paw withdrawal from a thermal stimulus. The data are depicted as percentage of control mice receiving vehicle without LPS. Individual inhibitors alone are shown as dark gray bars. Coadministration of AUDA-BE with various COX-2 inhibitors are shown as light gray bars. ∗, Significantly different from NSAID alone (P < 0.05) as determined by ANOVA followed by Tukey's test. (b) Structures of the sEH and COX inhibitors. Names and synonyms are provided in the text.
Effects of NSAIDs or sEHIs on PG Synthesis.
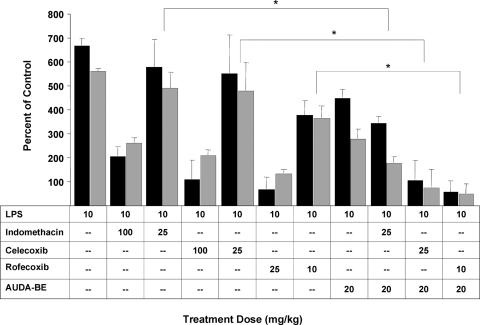
As expected, the NSAIDs reduced production of PGD2 and PGE2 in a dose-dependent manner. Previous work has shown that AUDA-BE indirectly reduced PGD2 and PGE2 in a dose-dependent manner (14). More specifically, a dose of AUDA-BE at 20 mg/kg reduces the levels of PGD2 and PGE2 by 31 ± 9% and 34 ± 6% compared with LPS, respectively, which is approximately equivalent to rofecoxib's antinociceptive efficacy at a dose of 10 mg/kg (Fig. 3).
Fig. 3.
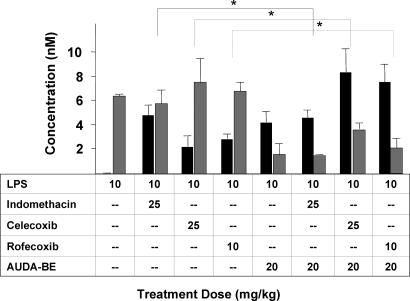
Coadministration of AUDA-BE and NSAIDs produces a synergistic decrease in PGD2 (gray bars) and PGE2 (black bars) 6 h after LPS exposure. As expected, all inhibitors individually decreased PGs in a dose-related manner. The data indicate that using a prophylactic dose of AUDA-BE with a nonoptimum therapeutic dose of COX inhibitor can further reduce the proinflammatory PGD2 and PGE2. The data represent average ± SD (n = 4) and are depicted as percentage of control mice receiving vehicle without LPS. Control values are PGD2, 1.1 (method detection limit), and PGE2, 2.6 ± 0.3 nM. ∗, Significantly different from NSAID alone (P < 0.05) as determined by ANOVA followed by Tukey's test.
When AUDA-BE (20 mg/kg) is administered in combination with low doses of NSAIDs, there is an additive or enhanced effect in reducing PGD2 and PGE2 concentrations. Specifically, coadministration of indomethacin (25 mg/kg) and AUDA-BE (20 mg/kg) reduces the PGD2 by 68 ± 6% and PGE2 by 51 ± 7% compared with LPS only. This decrease is comparable with an additive effect, which based on the sum of individual responses would be ≈41% and 48% respectively. For combination therapies using celecoxib (25 mg/kg) or rofecoxib (10 mg/kg) with AUDA-BE, the PGD2 was reduced by 88 ± 12% and 91 ± 7%, respectively, which shows a enhanced effect given that the additive effect would be ≈46% and 61%. This finding was also true for PGE2 with values of 84 ± 9% and 91 ± 8% compared with the calculated additive values of ≈53% and ≈76% for rofecoxib and celecoxib, respectively. Similar effects were seen when AEPU was used in combination with the lower doses of rofecoxib, celecoxib, or indomethacin (see Fig. 8, which is published as supporting information on the PNAS web site).
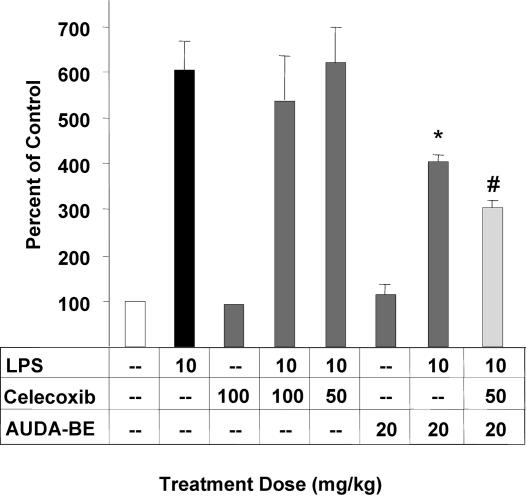
In addition, previous work has shown that the sEHIs suppress hepatic COX-2 protein (14). When a prophylactic dose of AUDA-BE is administered in combination with an intermediate dose of celecoxib (50 mg/kg), COX 2 induction is further decreased, by 14 ± 5% compared with AUDA-BE alone (Fig. 4). Although other researchers have shown that rofecoxib inhibits COX-2 and NF-κB in an in vitro preparation (16), to our knowledge celecoxib has not previously been shown to decrease COX-2 protein in vivo. This effect may be caused by COX inhibitors diverting AA from the COX to the P450 metabolic pathway, thus increasing the concentration of EETs.
Fig. 4.
A prophylactic dose of AUDA-BE reduces hepatic COX-2 protein expression 6 h after LPS exposure relative to mice receiving vehicle without LPS. Results from individual inhibitors at various doses are shown in dark gray bars. Coadministration of the sEHI and NSAID is depicted as a light gray bar, indicating that a prophylactic dose of AUDA-BE used in conjunction with a nonoptimum therapeutic dose of celecoxib (50 mg/kg) can further decrease COX-2 induction. Data represent the COX-2 protein levels ± SD (n = 3) in murine liver after exposure to LPS as determined by independent Western blots. ∗, Significantly different from vehicle (P < 0.05) as determined by ANOVA followed by Tukey's test. #, Significantly different from AUDA-BE alone (P < 0.05) as determined by ANOVA followed by Tukey's test. In addition, celecoxib at any dose did not statistically alter hepatic COX-1 or sEH protein levels compared with LPS-challenged mice (P < 0.05).
High concentrations of PGD2 and PGE2 alone can induce COX-2. Here we hypothesize that AUDA-BE decreases the amount of transcribed COX-2 and that celecoxib directly inhibits the enzyme producing less PGD2 and PGE2, thus depressing the positive feedback loop. In addition, the coadministration elevates the concentration of the EETs, which would further decrease COX-2 expression (Fig. 5; see also Fig. 9, which is published as supporting information on the PNAS web site).
Fig. 5.
The COX-2 inhibitors increase the flow of AA through the P450 pathway increasing EpETrEs [Σ (8(9), 11(12) and 14(15) EETs; black bars) and DiHTrEs (Σ 5,6; 8,9; 11,12; and 14,15 DHETs; gray bars) in murine plasma after exposure to LPS. The 5(6) EET data are excluded because of lactone formation during sample preparation. Although the regioisomers were measured separately, the data are presented with the regioisomers combined to simplify the tables. There is no statistical difference in the ratio of regioisomers of EETs or DHETs (P < 0.05). The data indicate that coadministration of AUDA-BE and COX inhibitors can further increase the anti-inflammatory EETs. The data are average ± SD (n = 4). ∗, Significantly different from NSAID alone (P < 0.05) as determined by ANOVA followed by Tukey's test.
Effects of NSAIDs or sEHIs on Epoxide Synthesis.
Approximately one-third of the AA carbon flow is metabolized by the COX enzymes, which is disrupted by administration of NSAIDs. This disruption shifts AA metabolism to the P450 and sEH or the lipoxygenase pathway and inevitably produces more EETs and DHETs or hydroxyeicosatetraenoic acids (HETEs). Fig. 5 depicts the shift in metabolism through the P450 and sEH pathways, as well as the effects of coadministration of AUDA-BE. In all cases the combination therapy with high doses of the NSAIDs doubled the concentration of EETs in the plasma after LPS challenge.
Potential Decrease in Side Effects.
Given that selective COX-2 inhibitors such as rofecoxib and celecoxib block the formation of vascular endothelial cell prostacyclin (PGI2, stable metabolite 6-keto-PGF1α), but not platelet COX-1 derived thromboxane A2 (TXA2) (stable metabolite TXB2) the large decrease in the PGI2-to-TXA2 ratio with COX-2 inhibitors may account for the increased incidence of thrombotic cardiovascular events. In contrast, when AUDA-BE is administered alone or in combination with COX-2 inhibitors, the relative ratio of PGI2 (6-keto-PGF1α) to TXA2 (TXB2) in plasma is not significantly altered from the ratio in LPS-challenged mice (Fig. 6; see also Fig. 10, which is published as supporting information on the PNAS web site). The data indicate that a combination therapy of sEHIs with low doses of rofecoxib or celecoxib results in the desired decrease in inflammatory eicosanoids like PGD2 and PGE2 (Figs. 3 and 8) without the changes in 6-keto-PGF1α-to-TXB2 ratio associated with undesirable cardiovascular risks. The mechanism by which 6-keto-PGF1α is elevated remains unknown. A potential hypothesis is that PG-I synthase, which produces PGI2, is up-regulated by the sEHIs or EETs. Certainly, if some NSAID side effects are compound related, a sEHI–NSAID combination permits a reduced NSAID dose and concomitant risk.
Fig. 6.
Coadministration of AUDA-BE and COX-2 inhibitors does not appear to create an imbalance in the PGI2 (stable metabolite 6-keto-PGF1α, black bars) and TXA2 (stable metabolite TXB2, gray bars) concentrations 6 h after LPS exposure. High doses of COX-2 inhibitors alone generate this disparity, leading to increased risk of thrombotic events. The data are average ± SD (n = 4) and are depicted as percentage of control mice receiving vehicle without LPS. Control values are 6-keto-PGF1α, 7.6 ± 0.4, and TXB2, 4.1 ± 0.4 nM. ∗, Significantly different from vehicle (P < 0.05) as determined by ANOVA followed by Tukey's test.
Conclusions
The goal of analgesic drug combinations is to optimize dose regimes that offer greater analgesic and anti-inflammatory effects, while at the same time decreasing detrimental side effects. Recently, much attention has focused on the potential of COX-2 inhibitors to increase myocardial infarction and stroke risk (17) and delay resolution of inflammation (18). Our previous work has shown that AUDA-BE has little effect on 6-keto-PGF1α and TXB2 imbalance associated with increased risk for thrombotic events and appears to promote the formation of proresolution anti-inflammatory mediators. Therefore, the combination of conventional/established NSAIDs and sEHIs appears to be a useful approach for decreasing pain and inflammation while avoiding unwanted side effects.
The dose of AUDA-BE (20 mg/kg) plus indomethacin (25 or 50 mg/kg) had an additive effect in reducing PGD2/PGE2 synthesis and hyperalgesia, respectively. The dose of AUDA-BE (20 mg/kg) plus rofecoxib (10 mg/kg) or celecoxib (25 mg/kg) had an enhanced effect in reducing PGD2 and PGE2 synthesis and an additive effect in reducing hyperalgesia without negatively impacting the 6-keto-PGF1α concentration in plasma.
The full mechanism underlying the interaction between the NSAIDs and sEHIs remains unknown. Inflammation and pain are controlled not only by oxylipins but also by multiple mediators including K+, ATP, substance P, bradykinin, cytokines, monoamines, lipid amides, and steroids. Because there are multiple inflammatory mediators that sensitize/activate nociceptors other than those affected by COX/sEH inhibition, it is not surprising that a combination therapy has only an additive effect in this model, while demonstrating enhanced effects where the two classes of inhibitors intersect. However, it is feasible that the mechanism of inhibiting COX-2 induction through sEHIs and then directly inhibiting the enzyme with NSAIDs would reduce proinflammatory mediators and pain. Alternatively, the mechanism could be due to different actions and sites of action of the inhibitor groups.
In summary, systemic coadministration of low-dose NSAIDs and sEHI has the following effects: (i) produces at least an additive antinociceptive effect in an inflammatory pain model; (ii) produces an enhanced effect in decreasing PGD2 and PGE2 levels that is greater than the sum of individual treatments; and (iii) does not result in the imbalance in 6-keto-PGF1α and TXB2 usually associated with COX-2 inhibitors. The data indicate that sEHIs and EETs can enhance the effects of NSAIDs and allow reduced doses of NSAIDs to be used for the same therapeutic effect. Similarly, low doses of aspirin should synergize the effect of sEHIs. These combinations should lead to more indomethacin-like than rofecoxib-like ratios of PGI2 to TXA2. These results demonstrate that sEHIs may have utility when combined with low doses of conventional NSAIDs as useful agents for the treatment of pain and inflammation. This observation may extend to other physiological processes associated with increased/decreased concentration of epoxy fatty acids.
Materials and Methods
Behavioral Nociceptive Tests and Treatments.
Behavioral nociceptive testing was conducted by assessing thermal hindpaw withdrawal latencies using a commercial Hargreaves apparatus (IITC, Woodland Hills, CA) according to procedures described by Woolfe and McDonald (19). Briefly, mice were placed in an acrylic experimental chamber with a glass surface. The temperature of this surface was maintained at 30°C. Before data collection, mice were acclimated to the experimental chambers in 30-min sessions daily for 3 days. On the 4th day, baseline readings were taken. After a 30-min period of acclimation, noxious radiant heat was applied to the plantar surface of the hindpaw. Five measurements per animal were taken in 2-min intervals, and these five values were averaged at each pre- and post-LPS measurement. The latency to withdraw the paw away from the thermal stimulus was recorded (seconds). Trioleate or 0.5% carboxymethylcellulose was then injected s.c. as vehicle controls for therapeutic agents in LPS-treated animals. Saline (0.9%) was injected i.p. as a vehicle control for the LPS injection. Measurements were taken 6 h later. Two days later, the same animals were s.c. injected with either NSAIDs or sEHIs, alone and in combination; 24 h later, they were i.p. challenged with LPS, immediately followed by another dose of the therapeutics. Six hours after LPS challenge, hindpaw withdrawal latency tests were conducted, and animals were killed for blood and tissue sampling. This dosing and sampling schedule was chosen based on previous work to obtain therapeutic levels as well as clear changes in hepatic COX-2 protein levels.
Dose Response.
The antinociceptive effects produced by rofecoxib, celecoxib, indomethacin, AUDA-BE, and AEPU were evaluated individually and in combination. First, each dose of rofecoxib [(COX2 IC50, 18.0 nM) 10 and 25 mg/kg], celecoxib [(COX2 IC50, 40.0 nM) 25, 50 and 100 mg/kg], or indomethacin [(COX1: COX2 IC50, 0.028: 1.68 μM) 25, 50 and 100 mg/kg], was given to four animals to obtain the corresponding dose–response. The doses were chosen in the therapeutic range from preliminary pharmacokinetic data, eicosanoid profiles, and current literature (20–23). For instance, celecoxib was administered at higher doses because it has a larger volume of distribution and is less efficacious when compared with rofecoxib.
The antinociceptive effects of AUDA-BE and AEPU were individually tested at 20 mg/kg. Previous work has indicated that 20 mg/kg is an effective dose at reducing acute inflammation (15). AUDA-BE and AEPU have quite different structures and physical properties. For example, their experimental water solubilities (5 and 120 μg/ml) and calculated logP values (4.19 and 1.86, respectively) are quite different. The fact that both sEHIs give similar results supports the hypothesis that enzyme inhibition is the common mechanism of action; however, they could interact with targets other than sEH. For example, as anticipated from the structure, AUDA-BE acts as a weak peroxisome proliferator-activated receptor (PPAR) α-agonist, whereas AEPU is unlikely to be an agonist. Both sEHIs have low-nanomolar IC50 values with AUDA-BE being slightly more potent. However, the higher efficacy of AUDA-BE in these studies is because of its longer in vivo half-life and volume of distribution (see Fig. 7).
Then the doses of rofecoxib, celecoxib, and indomethacin were each combined with a fixed dose of AUDA-BE and AEPU to analyze possible interactions. Mice were prophylactically administered s.c. the inhibitors 24 h before LPS (10 mg/kg) i.p. challenge. Immediately after the LPS exposure, another dose of the inhibitors was administered s.c. Trioleate and 0.5% carboxymethylcellulose were administered at the corresponding volume as a vehicle control in LPS-treated animals. At the end of the experiment, 6 h after the LPS challenge, the mice were killed, and blood, livers, spleens, and kidneys were collected for biochemical and Western analysis. Blood was collected by cardiac puncture with an EDTA-rinsed syringe. A combination of triphenylphosphine, butylated hydroxytoluene, and indomethacin (0.2% for each, wt/wt) was added to each collection tube. The samples were immediately spun; the plasma was separated and flash frozen in liquid nitrogen. All samples were stored at −80°C until analysis.
Data Analysis.
Results are expressed as average ± SD and are depicted as percentages of controls mice receiving vehicle without LPS. Statistical comparisons were analyzed by Student's t test and one-way ANOVA followed by Tukey or Dunnett's test for post hoc comparison. Statistical significance was considered to be achieved when P < 0.05.
Statistical significance between the theoretical additive and experimentally derived values were evaluated with Student's t test. An experimental value that was significantly lower than the theoretical additive value was considered to indicate an enhanced interaction between sEHI and NSAID (24). To determine additive or enhanced effect, a theoretical decrease in response (e.g., PGE2) was calculated by addition of percent decrease of the individual inhibitors. For example, indomethacin (10 mg/kg) decreased PGE2 by 11% as compared with LPS without treatment, and AUDA-BE decreased by 40%; therefore, the theoretical additive effect would be 51%. This theoretical value was then compared with the actual decrease found for coadministration of these inhibitors at the specified doses. If the actual value was greater than the theoretical value, then the combination therapy was considered to be enhanced.
For further details, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Environmental Health Sciences Grants R37 ES02710 and R01 ES013933; Superfund Basic Research Program Grant P42 ES04699; Center for Children's Environmental Health and Disease Prevention Grants P30 ES05707, P01 ES11269, and R01 HL59699-06A1; a University of California, Davis, Health Systems Research Award (to J.P.E.); the Paul F. Gulyassy Endowed Professorship (to J.P.E.); and University of California at Davis Department of Anesthesiology and Pain Medicine Grant R0I GM078167 (to S.L.J.).
Abbreviations
- NSAID
nonsteroidal anti-inflammatory drug
- PG
prostaglandin
- COX
cyclooxygenase
- LPS
lipopolysaccharide
- AA
arachidonic acid
- sEH
soluble epoxide hydrolase
- sEHI
sEH inhibitor
- DHET
dihydroxyeicosatrienoic acid
- EET
epoxyeicosatrienoic acid
- AUDA-BE
12-(3-adamantan-1-yl-ureido)-dodecanoic acid butyl ester
- AEPU
1-adamantan-1-yl-3-{5-[2-(2-ethoxyethoxy)ethoxy]pentyl}urea
- TX
thromboxane.
Footnotes
Conflict of interest statement: K.R.S., B.I., I.-H.K, and B.D.H. have filed patents for the University of California for sEH chemistry and inflammation and pain therapy. B.D.H. founded Arête Therapeutics to move sEH inhibitors into clinical trials.
References
- 1.Picard P, Bazin JE, Conio N, Ruiz F, Schoeffler P. Pain. 1997;73:401–406. doi: 10.1016/S0304-3959(97)00128-0. [DOI] [PubMed] [Google Scholar]
- 2.Pang W, Mok MS, Ku MC, Huang MH. Anesth Analg. 1999;89:995–998. doi: 10.1097/00000539-199910000-00032. [DOI] [PubMed] [Google Scholar]
- 3.Lorenzetti BB, Ferreira SH. Eur J Pharmacol. 1985;114:375–381. doi: 10.1016/0014-2999(85)90383-8. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira SH, Vane JR. Annu Rev Pharmacol. 1974;14:57–73. [Google Scholar]
- 5.McCormack K. Pain. 1994;59:9–43. doi: 10.1016/0304-3959(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 6.Bjorkman R. Acta Anaesthesiol Scand Suppl. 1995;103:1–44. [PubMed] [Google Scholar]
- 7.Miranda HF, Sierralta F, Pinardi G. Anesth Analg. 2001;93:430–435. doi: 10.1097/00000539-200108000-00039. [DOI] [PubMed] [Google Scholar]
- 8.Chan CC, Boyce S, Brideau C, Charleson S, Cromlish W, Ethier D, Evans J, Ford-Hutchinson AW, Forrest MJ, Gauthier JY, et al. J Pharmacol Exp Ther. 1999;290:551–560. [PubMed] [Google Scholar]
- 9.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, et al. N Engl J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 10.Brzozowski T, Konturek PC, Konturek SJ, Sliwowski Z, Drozdowicz D, Stachura J, Pajdo R, Hahn EG. Eur J Pharmacol. 1999;385:47–61. doi: 10.1016/s0014-2999(99)00681-0. [DOI] [PubMed] [Google Scholar]
- 11.Griffin MR. Am J Med. 1998;104:23S–29S. doi: 10.1016/s0002-9343(97)00207-6. and discussion 104: 41S–42S. [DOI] [PubMed] [Google Scholar]
- 12.Smith CJ, Zhang Y, Koboldt CM, Muhammad J, Zweifel BS, Shaffer A, Talley JJ, Masferrer JL, Seibert K, Isakson PC. Proc Natl Acad Sci USA. 1998;95:13313–13318. doi: 10.1073/pnas.95.22.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Proc Natl Acad Sci USA. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watkins LR, Wiertelak EP, Furness LE, Maier SF. Brain Res. 1994;664:17–24. doi: 10.1016/0006-8993(94)91948-8. [DOI] [PubMed] [Google Scholar]
- 16.Callejas NA, Fernandez-Martinez A, Castrillo A, Bosca L, Martin-Sanz P. Mol Pharmacol. 2003;63:671–677. doi: 10.1124/mol.63.3.671. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald GA. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 18.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 19.Woolfe G, McDonald A. J Pharmacol Exp Ther. 1944;80:300–307. [Google Scholar]
- 20.Laudanno OM, Cesolari JA, Esnarriaga J, Rista L, Piombo G, Maglione C, Aramberry L, Sambrano J, Godoy A, Rocaspana A. Dig Dis Sci. 2001;46:779–784. doi: 10.1023/a:1010748316889. [DOI] [PubMed] [Google Scholar]
- 21.Paulson SK, Zhang JY, Jessen SM, Lawal Y, Liu NW, Dudkowski CM, Wang YF, Chang M, Yang D, Findlay JW, et al. Xenobiotica. 2000;30:731–744. doi: 10.1080/00498250050078039. [DOI] [PubMed] [Google Scholar]
- 22.Raghoebar M, van den Berg WB, Huisman JA, van Ginneken CA. Agents Actions. 1987;22:314–323. doi: 10.1007/BF02009062. [DOI] [PubMed] [Google Scholar]
- 23.Halpin RA, Geer LA, Zhang KE, Marks TM, Dean DC, Jones AN, Melillo D, Doss G, Vyas KP. Drug Metab Dispos. 2000;28:1244–1254. [PubMed] [Google Scholar]
- 24.Tallarida R. Drug Synergism and Dose-Effect Data Analysis. New York: Chapman & Hall/CRC; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.