Abstract
Observational studies of wild chimpanzees (Pan troglodytes) have revealed population-specific differences in behavior, thought to represent cultural variation. Field studies have also reported behaviors indicative of cultural learning, such as close observation of adult skills by infants, and the use of similar foraging techniques within a population over many generations. Although experimental studies have shown that chimpanzees are able to learn complex behaviors by observation, it is unclear how closely these studies simulate the learning environment found in the wild. In the present study we have used a diffusion chain paradigm, whereby a behavior is passed from one individual to the next in a linear sequence in an attempt to simulate intergenerational transmission of a foraging skill. Using a powerful three-group, two-action methodology, we found that alternative methods used to obtain food from a foraging device (“lift door” versus “slide door”) were accurately transmitted along two chains of six and five chimpanzees, respectively, such that the last chimpanzee in the chain used the same method as the original trained model. The fidelity of transmission within each chain is remarkable given that several individuals in the no-model control group were able to discover either method by individual exploration. A comparative study with human children revealed similar results. This study is the first to experimentally demonstrate the linear transmission of alternative foraging techniques by non-human primates. Our results show that chimpanzees have a capacity to sustain local traditions across multiple simulated generations.
Keywords: culture, diffusion chain, social learning, tradition
Long-term observational studies of wild chimpanzees (Pan troglodytes) across Africa have revealed a diverse range of behavioral differences between populations, thought to represent local traditions (1–3). The inference that the differences are socially learned is based on (i) patterns of distribution that appear incompatible with genetic or simple environmental explanations; (ii) records of close observation of adults by infants as well as matching of mother–offspring foraging styles (4–7); and (iii) studies of both wild (8–10) and captive chimpanzees (11–15) showing that social learning from conspecifics can affect the acquisition of tool-use skills. Each wild chimpanzee community exhibits a distinct profile defined by several different kinds of putative traditions that have been described as cultures. In recent years, complexities of these kinds have increasingly been reported in other wild primates [orangutans (16), capuchins (17, 18), and taxa such as cetaceans (19)].
However, the evidence from the wild is essentially circumstantial and correlational. Although some researchers have gone to great lengths to establish that the presence of a behavior pattern at one site but not another is not dictated by the availability of raw materials (20, 21), skeptics have emphasized that it is not possible to identify all relevant factors (22–24). Some behaviors of wild chimpanzees, such as termite fishing, have now been recorded over multiple generations (7) (E. Lonsdorf, personal communication). Yet, whether such behaviors are culturally transmitted requires evidence that chimpanzees can transmit behavior with sufficient fidelity for distinct behavioral variants to be maintained.
Controlled experiments would, in principle, be able to provide this evidence, but field experiments face obstacles that have yet to be surmounted. Matsuzawa and colleagues (8, 10, 25, 26) have pioneered an “outdoor laboratory” to document the transmission of nut-cracking behavior in the wild, but the logistics of such studies have limited the application of control conditions possible in captive experiments, in which some chimpanzees see no model, allowing a more conclusive establishment of the role of social learning.
About 30 controlled social learning experiments (27, 28) have been conducted with chimpanzees. The relevance of this work to the traditions of wild chimpanzees is limited, however. First, of the 22 studies tabulated in the most recent review (28), 14 used human models, not chimpanzee models, and it is unclear that such models are as effective as conspecifics. Second, 17 studies were restricted to dyadic learning between a model and an observer. However, what we really need to establish is whether chimpanzees learn from each other with sufficient fidelity for behavior to spread within a community. Thus far, only four experiments concern the spread of behavior at the group level (11, 15, 29, 30). None of these investigations used a no-model control group, however, which might have demonstrated that the spread of behavior was not due to a gradual rise in individual learning.
To remedy these limitations, we recently conducted an experiment with a powerful “three-group, two-action” design (31). One chimpanzee from each of two social groups was trained to solve a foraging task using one of two alternative techniques. Members of each group were then allowed to observe the trained model, resulting in the differential spread of each foraging technique within the two different groups. Members of a third control group who did not observe a model failed to solve the task. This finding is therefore of direct relevance to the question of culture in wild chimpanzees.
However, one significant limitation in our study can only be overcome by the complementary approach of the present experiment. Because the whole group had access to the device throughout the previous study, it was not possible to track precisely who learned from whom. At one extreme, all may have learned by watching the initial model; alternatively, each may have learned something from all, many, or just one of those who mastered the task before them. In the present study, we instead employ a “diffusion chain” paradigm that allows accurate tracking of exactly who learns from whom over a number of transmission events. Just one individual was allowed to watch the initial model, and then was allowed a solo attempt; once successful, they became the model for a third individual, with the original model excluded, and so on along a chain of transitions that simulate “cultural generations” for the behavior.
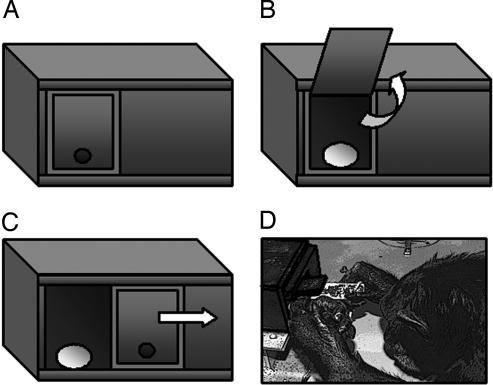
Diffusion chains were originally used by Bartlett (32) to study how narrative stories changed as they were transmitted between successive pairs of people, forming a chain. The essential idea was later used in other human studies (33) and in a small number of animal experiments addressing the transmission of predator avoidance in birds (34, 35), food preferences and foraging in rats (36–38), and foraging pathways in fish (39). This literature fails to cite what was arguably the first animal diffusion study (40), in which habituation to novel play objects was transmitted among 19 young chimpanzees. With the exception of ref. 38, diffusion studies have typically compared only one chain with the actions of a control group. It is therefore difficult to determine whether the transmission of behavior involved learning a new action or learning only that a desirable result could be attained by using an action the subjects were already predisposed to. By contrast, in the present study, we return to our three-group, two-action design, in which each of two diffusion chains begins with a model trained to tackle a foraging problem in a different way, while a third, control group sees no model. Any differential transmission in the chains then implies that the specific techniques have been maintained by social learning. The study was conducted with three groups of chimpanzees from Yerkes National Primate Research Center's Field Station: FS1 (chain 1), FS2 (chain 2), and FS3 (no-model control group). Each of the two chains was initiated by a model trained to either lift a door (FS1) or slide a door (FS2) to retrieve food from a foraging device (named the “Doorian fruit” after the door mechanism as a play on the durian fruit, a popular orangutan food; see Fig. 1A). In the lift door method, the door frame remained in place while the door was lifted up (Fig. 1 B and D). In the slide door method, the door remained closed while the frame slid to the right (Fig. 1C; and see Movies 1 and 2, which are published as supporting information on the PNAS web site).
Fig. 1.
Doorian Fruit apparatus. (A) The starting position with door closed. (B) Lift method. (C) Slide method. (D) Outlined photograph of model GG performing lift method.
In each chain, a naïve observer was allowed to watch the model retrieve food from the Doorian fruit. Once the observer had seen ≈10 successes, they were given an opportunity to interact with the Doorian fruit alone. Observers who successfully opened the Doorian fruit 10 times then became the model for the next chimpanzee in the chain. This methodology generated a chain of six chimpanzees from FS1 group who exclusively used the lift door method and a chain of five chimpanzees from FS2 group who used the slide door method. Chimpanzees were excluded from the chains and hence became “side branches” if they failed to observe at least one demonstration by the model or were unable to consistently succeed and therefore could not reliably act as a model for the next individual. Six control chimpanzees from FS3 group interacted with the Doorian fruit without observing a model. Three failed to open the door, two used the lift door method, and one used the slide door method. Building on the pioneering work of Menzel et al. (40), our study uses diffusion chains to systematically examine whether a non-human primate species is able to accurately transmit a foraging behavior across multiple simulated “cultural generations.”
Human culture provides an inevitable benchmark against which to evaluate animal studies. For an explicit comparison, we replicated our chimpanzee study as closely as possible with human children, focusing on 3-year-olds, following earlier research indicating that this age group represents a sensible cognitive comparison with chimpanzees (41–43). Moreover, it is well known that children of this age group readily learn from the cultural phenomena that surround them (43).
Results
Chimpanzee Study.
Behavior of the model.
We found that chimpanzees typically opened the door multiple times before retrieving a reward. Because the slide door method was spring-loaded, it often took several attempts to catch and keep open the door. When attempting to lift the door, chimpanzees often had to flick it up several times before it could be held open. Each such lift/slide was scored, because observers could potentially gain useful information every time the door was opened. We determined that observers must witness 10 reward retrievals before being allowed to interact with the Doorian fruit alone. However, it was possible to witness many more door openings. The number of door openings was not used as a criterion for sufficient observation because some models performed 10 or more door openings before retrieving the first reward.
Observation of the model.
In practice, observers sometimes displaced the model from the apparatus before they had witnessed 10 complete demonstrations. However, all chimpanzees who met the criteria for inclusion in the main chains witnessed at least one demonstration of door opening during their observation phase (median number of observations FS1 = 5, range 1–24: median FS2 = 8, range 1–17; see Table 1, which is published as supporting information on the PNAS web site). There was no significant difference between FS1 and FS2 in the number of demonstrations that were observed.
In both chains, two forms of co-action were observed between the models and observers: (i) door co-action (of lift or slide) occurred when the observer touched the door or the hand of the model while he/she opened the door; (ii) retrieval co-action occurred when the observer touched the hand of the model as he/she used their fingers to retrieve rewards from the opened Doorian fruit. In FS1, door co-action occurred once between RI and TA and once between MA and MS (both mother–daughter pairs). Retrieval co-action occurred once between TA and MA (unrelated) and twice between MA and MS (mother–daughter). In FS2, door co-action occurred once between ER and AM, once between ER and CY, and twice between BB and KE, and retrieval co-action occurred twice between CY and VV (all unrelated pairs). Even more remarkable, on two occasions, model ER slid the door and held it open while VV (unrelated) inserted her fingers to retrieve rewards.
Transmission of modeled behavior.
In FS1, model GG initiated the chain using the lift door technique, which was passed between a further five individuals in the diffusion chain, thus demonstrating five transmission events. During the observation phase, observers watched a mean of 65% of the model's lifting actions (range 25–100%). Except in the single instance when BO displaced model KT (see below), all chimpanzees in this chain performed the lift method exclusively both in their test phase and when acting as a model (Fig. 2), which was accordingly transmitted with 100% fidelity from GG, along the chain, to the sixth and final generation. At the end of the chain, MA would not participate without her daughter MS. Hence, when MS's turn came, she had observed both TA and MA before going on to a successful performance, and therefore her acquisition of the lift door technique cannot be considered as consecutive linear transmission from MA.
Fig. 2.
Diffusion chains starting with a model performing either the lift (Left) or slide (Right) method. The ID code of each chimpanzee is in bold (GG is the initial model performing lift; ER is the initial model performing slide). Dark shaded areas represent the percentage of door opening that involved lift, first when tested solo (T) and later when in the role of model (M) for the next, naïve, chimpanzee in the chain. Dark hatched areas represent the percentage of a model's lift actions watched by the naïve chimpanzee, and light hatched areas represent the same for slide actions. Dashed arrows indicate each individual's progression from the “T” (test) phase to the “M” (model) phase. Filled arrows mark pairings of each competent model with the naïve chimpanzee that followed them in the chain. Individuals excluded from chains were BO (did not observe the model), RN (failed model motivation check), AM (was not efficient at the task and withdrew), and CY (was aggressive toward the observer).
In FS2, model ER initiated the chain using the slide door technique, which was passed along a diffusion chain of five individuals. Observers watched a mean of 54% of the model's slide actions (range 3–100%). They exclusively performed the slide method, with the exception of BB, who performed one lift action when acting as a model for KE. KE observed this lift action as well as 78% of BB's much greater number of slide actions, then went on to perform slide actions exclusively herself (Fig. 2). Accordingly, the slide method was transmitted along this chain with 100% fidelity by the fifth and final generation.
Two chimpanzees in each group failed to meet the criteria for inclusion in the chains and therefore became side branches to the linear chains (Fig. 2). In FS1, BO did not observe the model she was paired with (KT), whom she was able to displace, and so attempted the task independently. She discovered the alternative method (slide) to that active in the existing chain (lift). Having failed to observe a single demonstration, she did not meet the criteria for subsequent inclusion as a model (note that a chimpanzee discovering the alternative method after watching the model would meet the criteria for becoming the next model). Second, RN acted successfully during her test phase after watching model KT but failed her premodel motivation check on two consecutive days, becoming a side branch. In FS2, AM, who was initially successful, had such limited dexterity to extract rewards that he became frustrated and unwilling to participate and so became a side branch to the FS2 chain. Then, model CY was aggressive toward observer VV, and although VV observed one demonstration, she was subsequently reluctant to approach the apparatus. Because VV's reluctance to participate was due to social incompatibility with CY, she was paired with model ER and went on to succeed.
It might be objected that this methodology would exclude possible corruption of the behavior that may occur in the wild. However, our experiment was designed as a simulation of intergenerational transmission, where in the wild a youngster will typically have seen its elders perform extractive foraging techniques many times before making a successful attempt itself. Hence, we made observation of at least one demonstration a condition of inclusion.
Excluding the side branches, transmission data concern the performance of six participants in FS1 and five in FS2, all of whom performed like the respective models in their group, resulting in a significant difference in the performance of each method (lift:slide ratio in FS1 of 6:0 versus 0:5 in FS2, Fisher's exact test, P = 0.002). Four “blind coders,” unfamiliar with the identities of the chimpanzees, were able to determine with 100% accuracy whether they belonged to the lift or slide chain, after watching a short section of video from each chimpanzee's test phase. If side branches are included in the analysis to test the overall social learning effect (RN and MS in FS1, CY and AM in FS2; see Fig. 2), the contrast is even more robust (Fisher's exact test, P = 0.001).
The original models from each group (ER and GG) were both trained to use the tool to retrieve rewards. However, this was an added level of complexity that was not necessary, because participants (with the exception of AM) could usually reach the rewards with their fingers. The tool was used by all participants in FS1 with the exception of RI and MA. In FS2, the tool was used by only ER and CY. We do not believe that this contrast between FS1 and FS2 necessarily indicates social diffusion of tool use in FS1 because tool use seemed to be more helpful in extracting food by the lift method. For this reason, and because tool use was not the focus of the study, we do not discuss these results further.
No-model control group.
The six chimpanzees from FS3 who participated as controls were highly motivated to retrieve the reward, giving food grunts as they observed the apparatus being baited. Three of the six controls successfully opened the door, with two discovering the lift method and one discovering the slide method. The unsuccessful controls spent much of the 60-min trial period sniffing, biting, and hitting the apparatus, as well as playing with the tool and cable.
The purpose of the control group was to assess how chimpanzees would perform without the opportunity to observe a model and to determine whether one method and not the other could be discovered by chance. The behavior of the controls indicates that the task was quite difficult, because three failed to open the door, but that both the lift and slide methods could be discovered individually.
Child Study.
Transmission of modeled behavior.
Children in the diffusion chains exclusively performed the same method of door opening that had been introduced by the first child in each chain. Thus, the original techniques were transmitted with 100% fidelity to the eighth and final generation. Lift was significantly more common in the group seeded with lift than in the group seeded with slide (Fisher's exact test, N1 = 8, N2 = 8, P < 0.0001).
No-model control group.
Before being given a prompt, eight control children (53%) were successful, four discovering the slide method and four the lift method, results mirroring those obtained for the control chimpanzees. Significantly fewer children in the control group succeeded in using lift or slide compared with the diffusion chain groups seeded with the respective method [lift: χ2(2) = 11.24, P < 0.01; slide: χ2(2) = 11.24, P < 0.01]. After a prompt directing their attention to the door, four more control children were successful, with two discovering the slide method and two the lift method. Three children attempted repeatedly to open the door by sliding it but failed to do so, each then going on to discover the lift method. Three children remained unsuccessful even after the prompt.
Discussion
We obtained evidence of high-fidelity replication of alternative foraging techniques along two diffusion chains of chimpanzees. In FS1, the lift method was transmitted along a chain of six chimpanzees with two side branches, and in FS2, the slide method was transmitted along a chain of five chimpanzees with two side branches. In each linear chain, all participants performed the same technique as the original model with high fidelity: only one small corruption occurred (in BB's actions; see Fig. 2), which was not transmitted onwards. The comparative study with children also generated two distinct diffusion chains, with all children performing the same method as the first in the chain.
That children are cultural creatures is not in doubt. We conducted the child comparison as a benchmark, demonstrating how the young of a highly cultural species would respond to the Doorian fruit task. The fidelity of transmission by both species is remarkable given the ease of potential corruption demonstrated by successful individuals from the no-model control groups; half the chimpanzees and children in the control groups were able to discover either lift or slide techniques by individual exploration. The task thus presented a roughly equivalent challenge to both species. Nevertheless, for both chimpanzees and children, observation of a conspecific using one exclusive technique was sufficient to ensure transmission of this technique along multiple simulated “cultural generations.”
These diffusion chain findings support our earlier “open group diffusion” study (31), in which two chimpanzee groups were exposed to a single expert model performing a different method of tool use. Each method spread within the groups, but it was not possible to determine with certainty from whom the chimpanzees were learning. The present study therefore complements our previous work in that we can be certain how many cultural generations occurred (six and five generations, respectively, in the two groups) and who learned from whom. If repeated transmission events of the kind we have documented occurred in the wild, they would occur only after each individual had matured and raised their own offspring. Female chimpanzees have their first offspring at ≈13–14 years old (44, 45). Given a proportion of failed first births (44), the six cultural generations represented by our study would thus span ≈90 years in the wild, about twice the span of the longest ongoing field studies. Of course, the long developmental period is the very factor that our study inevitably lacks, and it would be interesting to extend our approach to longer-term studies in the future.
It is not the function of diffusion studies to dissect in depth the underlying mechanisms of transmission, although these must be sophisticated enough to ensure the replication of behavior across the generations. However, some limited inferences are suggested by the contrasts deriving from the three-group design. The no-model control condition indicates that for about half the chimpanzees (and children), opening an object like the Doorian fruit can be said to be within their untutored competence. Therefore, it seems reasonable to assume that half the participants from the diffusion chains would also have been able to open the Doorian fruit in a control condition. Their exclusive use of only one of the two available techniques may represent a form of “canalization” (46), whereby a chimpanzee's potentially limitless exploration of a problem is focused around only a subset of behaviors that they see performed by others. Similarly, it is likely that half of the participants in the chains would have failed the control condition, and hence their behavior suggests a more complex social learning mechanism, such as emulation or imitation (28), but further experiments will be required to establish this.
A number of aspects of our methodology may be critical to reveal the high fidelity of social transmission that we found in this study. We took care to ensure that, within the confines of the diffusion chain paradigm, the learning environment was as naturalistic as possible. In each group, starting with the trained model, chimpanzees were added to the chains based roughly on the order in which they had solved a tool-use task in a previous group diffusion study (31). With the exception of two links in the chain (KT to BO in FS1 and CY to VV in FS2), this predetermined order proved to be successful in facilitating social learning between model–observer pairs. All models maintained control of the Doorian fruit and performed at least one demonstration. Models were highly tolerant of close inspection by the observers (percentage of model's demonstrations observed: FS1 = 65%, FS2 = 54%). In addition, 10 chimpanzees participated in co-action, which occurs when the model allows the observer to participate intimately in their behavior (47). Two types of co-action were observed: co-action of door opening (lift or slide) and co-action of reward retrieval once the door was open. Co-action of tool use between mothers and offspring has been reported in captive tufted capuchin monkeys using sticks to dip for syrup (48) and in wild chimpanzees during termite fishing (4). Co-action is distinct from scrounging, in which the observer exploits the actions of the model by stealing the food they have worked for, which in some cases may impede social learning (49). The prevalence of co-action in the present study between both related and unrelated pairs indicates that models and observers were highly tolerant of each other and the testing situation, which likely mimics learning between familiar individuals in the wild.
Side branches occurred in both chains because either the model was unsuccessful/unmotivated (RN in FS1, AM in FS2) or aggression occurred between the model and observer (KT to BO in FS1, CY to VV in FS2). The latter highlights the importance of tolerance and reinforces the hypothesis that opportunities for social learning in the wild may be restricted by the level of tolerance between individuals (50) and that not all individuals within a population may be good models for social learning (51).
Materials and Methods
Apparatus.
For the chimpanzees, the Doorian fruit apparatus was a rectangular polycarbonate box (L × W × H: 20 × 17 × 13 cm) with an opaque door (5.5 cm2) on the front (Fig. 1A), which could be opened to retrieve a food reward inside. The door could be opened by using either of two methods: lifting (Fig. 1 B and D) or sliding (Fig. 1C). The resting state of the door was closed, because the sliding mechanism of the frame was spring-loaded and the lifted door fell closed by gravity. In addition, small magnets in the frame ensured that approximately equal force was required to initiate each method.
In the chimpanzee study, the Doorian fruit was mounted on a 22-cm-high metal platform bolted to the floor of the research room, one of five interconnected rooms within the chimpanzees' indoor area. It could be baited by the experimenter (VH) from outside the research room by dropping food rewards down a connecting pipe (3.5-cm internal diameter). A metal tool (30 × 4 cm), attached to a 65-cm cable and located on the floor of the research room, was available to the chimpanzees to aid in the retrieval of rewards from the Doorian fruit if they could not extract them with their fingers.
For the child study, children were tested by EF in a quiet room in their play school, with the Doorian fruit bolted on to a small table at their waist height. The apparatus was scaled down slightly, given children's smaller hand size (L × W × H: 17 × 16 × 15 cm; door: 4 × 5 cm). Because chimpanzee participants did not consistently use the tool, and because tool use was not the subject of the experimental hypothesis, children were not supplied with a tool in the later child study. Children used their fingers to reach the reward, a toy marble, located just behind the door.
Participants.
Chimpanzee participants were 22 individuals at the Yerkes National Primate Research Center's Field Station. The Field Station is home to two chimpanzee groups, FS1 and FS2, with 15 and 14 members, respectively. Each group has an outdoor enclosure (FS1: 697 m2; FS2: 520 m2) with climbing structures, as well as a heated indoor building composed of five interconnected rooms with nesting sites and swings. The two groups can hear but not see each other, because their enclosures are ≈200 m apart, separated by a small hill. A description of the background of each group can be found in Table 2, which is published as supporting information on the PNAS web site. The chimpanzees assigned to the diffusion chains were nine members of FS1 (one male and eight females, mean age of 22 y, range of 11–42 y) and seven members of FS2 (two males and five females, mean age of 24 y, range of 11–33 y). Six chimpanzees from FS3 group, housed elsewhere at Yerkes, participated as controls (three males and three females, mean age of 28 y, range of 16–38 y).
The child study was conducted with children between the ages of 3 y and 3 y and 10 mo, who were recruited from two play schools in Scotland, United Kingdom. They were assigned to the same conditions as the chimpanzees: lift group, mean age of 3 y and 5 months, n = 8; slide group, mean age of 3 y and 7 mo, n = 8; and no-model control group, mean age of 3 y and 4 mo, n = 15.
Chimpanzee Study.
Procedure.
In both FS1 and FS2, a high-ranking female was trained to use either the lift (FS1) or slide (FS2) method to open the Doorian fruit and to use the tool to retrieve a reward from inside (see Movies 1 and 2). Proficiency was judged by the female's ability to open the door using the trained method at least 10 times on two consecutive days. The order in which individuals had mastered a different foraging problem in our previous study (31) was used to guide assignment to each position in the chain, but the order was changed if there were dominance confounds, such that a new observer might displace the model before observing their technique. These criteria and other social incompatibilities meant that only around half of the chimpanzees who had taken part in the earlier “open diffusion” study could be used to construct the diffusion chains.
Unlike many experimental studies of chimpanzee social learning, which mostly use human models kept at a distance, we conducted our experiment with chimpanzee models who were observed by conspecifics in the same room. The demonstration apparatus was in exactly the same position and was exactly the same apparatus with which observers would later interact. This arrangement simulates the natural environment, in which the observer can closely inspect the model's actions and move freely around the tool use site. Close social interaction is believed a critical part of an ecologically valid learning environment (50, 52, 53).
Observation phase.
Both model and observer were called into the research room from their outside enclosure. The experimenter then repeatedly baited the Doorian fruit with rewards until the observer had witnessed 10 demonstrations. Observation of a demonstration was determined by the presence of the observer within 1 m of the Doorian fruit, with their body oriented toward it at the moment that the model opened the door. This criterion was used because observers frequently moved about the room, and it was not always possible to determine their exact gaze direction. We judged that an observer who was close to the apparatus and orientated toward it was likely to gain relevant information about the model's behavior.
In practice, observers sometimes displaced the model from the apparatus before they had witnessed 10 complete demonstrations. Once the model had been displaced, or the observer had witnessed 10 demonstrations, whichever came first, the model was attracted out of the research room and into an adjacent room by offering a banana, leaving the observer alone to potentially interact with the apparatus.
Test phase.
Once the model left the research room, the Doorian fruit was rebaited and the observer was given a 10-min period to retrieve a reward. After retrieval of the first reward, the observer was given a further 20 min to retrieve an additional nine rewards. If successful, the observer became the model for the next chimpanzee in the chain, irrespective of the method they used to open the door. For logistical reasons this next step was conducted on the following day, such that each link in the chain occurred on a different day.
Premodel motivation check.
Before a new model was allowed to perform in front of the next observer in the chain, they were given an individual check to ensure that they were motivated to interact with the apparatus before the observer was introduced. This phase was included to eliminate the possibility that a model would not perform in the presence of the observer, who would then be able to interact with the apparatus before witnessing a demonstration.
Exclusion from chains.
Individuals were not included as models to continue chains if during the observation phase, they did not approach the Doorian fruit and hence did not observe the demonstrations. All excluded individuals became dead-end side branches to the linear chains and were replaced by the next individual on the list of potential participants.
No-model control group.
Six chimpanzees from the FS3 group participated as controls to determine how chimpanzees would respond to the apparatus in the absence of a model, and to investigate whether one method, and not the other, could be discovered by individual exploration. The Doorian fruit was placed outside their enclosure for 24 h before testing, to reduce any neophobic responses. Each chimpanzee was tested individually within one of their indoor rooms. The apparatus was baited in full view of the participant who was then given a 60-min period to interact with the apparatus. If a control chimpanzee was successful, the apparatus was rebaited for a total of 10 reward retrievals.
Child Study.
Procedure.
As with the chimpanzees, at the start of each chain, a child was trained to exclusively use one opening method: slide or lift. The second child in the chain was then brought into the room and asked to wait while the first child performed two demonstrations; then it would be his/her turn. No explicit instructions were given about watching or teaching. After the model's demonstrations, the observer had two solo attempts and if successful became the model for the next child, and so on down the chain. Unlike the chimpanzee study, each step in the child diffusion chain was conducted on the same day.
No-model control group.
Children were introduced to the Doorian by the experimenter (EF), who said, “lots of boys and girls have had a turn playing with this box, now it's your go.” If the child was unsuccessful after 2 min of interaction with the apparatus, the experimenter offered an explicit hint: “look at the front of the box, at the door, what do you think you do?” Children were allowed to interact with the Doorian until they were successful, which was defined as opening the door fully (either by lifting or sliding), until they refused to participate after a further prompt (“look at the front of the box, at the door, what do you think you do?”), or after 4 min and 30 sec without success. Data were later analyzed for responses both before and after a hint was given.
Analysis.
Videotapes were scored to determine the number of “lift door” and “slide door” actions used by each participant in their role as both observer and model. For the chimpanzee study, tapes were scored by two experimenters, and any discrepancies in scores were reconciled by reviewing the videotapes. In addition, four coders unfamiliar with the identities of each participant or the hypotheses of the study were asked to estimate whether each chimpanzee came from a lift or slide chain. For both chimpanzees and children, the tendency to use one method over the other was compared by using Fisher's exact test. All statistics are two-tailed.
Supplementary Material
Acknowledgments
We thank Kristin Bonnie, Devyn Carter, and the animal care staff of Yerkes Field Station for their support and assistance with the chimpanzees; Andy Burnley for his expert construction of the Doorian Fruit apparatus; Yerkes Field Station Engineering for their logistical support; the children and teachers of Westfield Nursery School and Cupar Preschool Playgroup for their participation in the child portion of the study; and T. Matsuzawa and J. Silk for insightful discussion of our results. Yerkes is fully accredited by the American Association for Accreditation for Laboratory Animal Care. The chimpanzee portion of this study was conducted at the Yerkes National Primate Research Center's Field Station and was supported by a project grant from the Biotechnology and Biological Sciences Research Council (to A.W.), National Institutes of Health Grant RR-00165, the Living Links Center of Emory University, and the University of St. Andrews. The child study was supported by a grant from the Economic and Social Research Council (to A.W.). A.W. was supported by a Leverhulme Fellowship.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.McGrew WC. The Cultured Chimpanzee: Reflections on Cultural Primatology. New York: Cambridge Univ Press; 2004. [Google Scholar]
- 2.Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama V, Tutin CEG, Wrangham RW, Boesch C. Nature. 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- 3.Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama V, Tutin CEG, Wrangham RW, Boesch C. Behaviour. 2001;138:1484–1516. [Google Scholar]
- 4.McGrew WC. In: Primate Bio-social Development: Biological, Social, and Ecological Determinants. Chevalier-Skolnikoff S, Poirier FE, editors. New York: Garland; 1977. pp. 261–288. [Google Scholar]
- 5.Boesch C. Anim Behav. 1991;41:530–532. [Google Scholar]
- 6.Lonsdorf E. Anim Behav. 2005;70:673–683. [Google Scholar]
- 7.Lonsdorf EV, Eberly LE, Pusey AE. Nature. 2004;428:715–716. doi: 10.1038/428715a. [DOI] [PubMed] [Google Scholar]
- 8.Matsuzawa T. In: Chimpanzee Cultures. Wrangham R, McGrew WC, de Waal FBM, Heltne MA, editors. Cambridge, MA: Harvard Univ Press; 1994. pp. 351–370. [Google Scholar]
- 9.Matsuzawa T, Yamakoshi G. In: Reaching into Thought. Russon A, Bard K, Parker ST, editors. Cambridge, UK: Cambridge Univ Press; 1996. pp. 211–232. [Google Scholar]
- 10.Inoue-Nakamura N, Matsuzawa T. J Comp Psychol. 1997;111:159–173. doi: 10.1037/0735-7036.111.2.159. [DOI] [PubMed] [Google Scholar]
- 11.Paquette D. Hum Evol. 1992;7:17–30. [Google Scholar]
- 12.Hirata S, Morimura N. J Comp Psychol. 2000;114:291–296. doi: 10.1037/0735-7036.114.3.291. [DOI] [PubMed] [Google Scholar]
- 13.Celli ML, Tomonaga M, Udono T, Teramoto M, Nagano K. Psychologia. 2001;44:70–81. [Google Scholar]
- 14.Sumita K, Kitahara-Frisch J, Norikoshi K. Primates. 1985;26:168–181. [Google Scholar]
- 15.Tonooka R, Tomonaga M, Matsuzawa T. Jpn Psychol Res. 1997;39:259–265. [Google Scholar]
- 16.van Schaik CP, Ancrenaz M, Borgen G, Galdikas B, Knott CD, Singletin I, Suzuki A, Utami SS, Merrill M. Science. 2003;299:102–105. doi: 10.1126/science.1078004. [DOI] [PubMed] [Google Scholar]
- 17.Panger M, Perry S, Rose LM, Gros-Luis J, Vogel E, MacKinnon KC, Baker M. Am J Phys Anthropol. 2002;119:52–66. doi: 10.1002/ajpa.10103. [DOI] [PubMed] [Google Scholar]
- 18.Perry S, Baker M, Fedigan L, Gros-Louis J, Jack K, MacKinnon KC, Manson JH, Panger M, Pyle K, Rose L. Curr Anthropol. 2003;44:241–268. [Google Scholar]
- 19.Rendell L, Whitehead H. Behav Brain Sci. 2001;24:309–382. doi: 10.1017/s0140525x0100396x. [DOI] [PubMed] [Google Scholar]
- 20.Boesch C, Marchesi P, Marchesi N, Fruth B, Jonlian F. J Hum Evol. 1994;26:325–338. [Google Scholar]
- 21.McGrew WC, Ham RM, White LTJ, Tutin CEG, Fernandez M. Int J Primatol. 1997;18:353–374. [Google Scholar]
- 22.Tomasello M. In: Language and Intelligence in Monkeys and Apes. Parker ST, Gibson KR, editors. Cambridge, UK: Cambridge Univ Press; 1990. pp. 274–311. [Google Scholar]
- 23.Galef BGJ. In: The Biology of Traditions: Models and Evidence. Fragaszy D, Perry S, editors. Cambridge, UK: Cambridge Univ Press; 2003. pp. 159–186. [Google Scholar]
- 24.Laland KN, Hoppitt W. Evol Anthropol. 2003;12:150–159. [Google Scholar]
- 25.Matsuzawa T. Primate Origins of Human Cognition and Behavior. New York: US Publishing; 2001. [Google Scholar]
- 26.Biro D, Inoue-Nakamura N, Tonooka R, Yamakoshi G, Sousa C, Matsuzawa T. Anim Cognit. 2003;6:213–223. doi: 10.1007/s10071-003-0183-x. [DOI] [PubMed] [Google Scholar]
- 27.Tomasello M, Call J. Primate Cognition. New York: Oxford Univ Press; 1997. [Google Scholar]
- 28.Whiten A, Horner V, Litchfield CA, Marshall-Pescini S. Learn Behav. 2004;32:36–52. doi: 10.3758/bf03196005. [DOI] [PubMed] [Google Scholar]
- 29.Hannah AC, McGrew WC. Primates. 1987;28:31–46. [Google Scholar]
- 30.Huffman MA, Hirata S. Primates. 2004;45:113–118. doi: 10.1007/s10329-003-0065-5. [DOI] [PubMed] [Google Scholar]
- 31.Whiten A, Horner V, de Waal FBM. Nature. 2005;437:737–740. doi: 10.1038/nature04047. [DOI] [PubMed] [Google Scholar]
- 32.Bartlett FC. Remembering. Oxford: Macmillan; 1932. [Google Scholar]
- 33.Mesoudi A, Whiten A. J Cognit Cult. 2004;4:1–24. [Google Scholar]
- 34.Curio E, Ernst U, Vieth W. Z Tierpsychol. 1978;48:184–202. doi: 10.1126/science.202.4370.899. [DOI] [PubMed] [Google Scholar]
- 35.Curio E, Ernst U, Vieth W. Science. 1978;202:899–901. doi: 10.1126/science.202.4370.899. [DOI] [PubMed] [Google Scholar]
- 36.Laland KN, Plotkin HC. Anim Learn Behav. 1990;18:246–251. [Google Scholar]
- 37.Laland KN, Plotkin HC. Anim Learn Behav. 1993;21:35–41. [Google Scholar]
- 38.Galef BG, Allen C. Anim Behav. 1995;50:705–717. [Google Scholar]
- 39.Laland KN, Williams K. Behav Ecol. 1998;9:493–499. [Google Scholar]
- 40.Menzel EW, Jr, Davenport RK, Rogers CM. Folia Primatol. 1972;17:161–170. doi: 10.1159/000155425. [DOI] [PubMed] [Google Scholar]
- 41.Whiten A, Custance DM, Gomez J, Teixidor P, Bard KA. J Comp Psychol. 1996;110:3–14. doi: 10.1037/0735-7036.110.1.3. [DOI] [PubMed] [Google Scholar]
- 42.Horner V, Whiten A. Anim Cognit. 2005;8:164–181. doi: 10.1007/s10071-004-0239-6. [DOI] [PubMed] [Google Scholar]
- 43.Tomasello M, Kruger AC, Ratner HH. Behav Brain Sci. 1993;16:495–552. [Google Scholar]
- 44.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Cambridge, MA: Harvard Univ Press; 1986. [Google Scholar]
- 45.Stumpf R. In: Primates in Perspective. Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK, editors. Oxford: Oxford Univ Press; 2006. pp. 321–344. [Google Scholar]
- 46.Boesch C. In: Reaching into Thought: The Minds of the Great Apes. Russon A, Bard KA, Parker ST, editors. Cambridge, UK: Cambridge Univ Press; 1996. pp. 404–429. [Google Scholar]
- 47.Visalberghi E, Fragaszy D. In: Language and Intelligence in Monkeys and Apes: Comparative Developmental Perspectives. Parker ST, Gibson KR, editors. Cambridge, UK: Cambridge Univ Press; 1990. pp. 247–273. [Google Scholar]
- 48.Westergaard GC, Fragaszy DM. J Comp Psychol. 1987;101:159–168. [Google Scholar]
- 49.Giraldeau L, Lefebvre L. Anim Behav. 1987;35:387–394. [Google Scholar]
- 50.Coussi-Korbel S, Fragaszy DM. Anim Behav. 1995;50:1441–1453. [Google Scholar]
- 51.Boesch C, Tomasello M. Curr Anthropol. 1998;39:591–614. [Google Scholar]
- 52.de Waal FBM. Behav Brain Sci. 1998;21:689. [Google Scholar]
- 53.van Schaik CP, Deaner RO, Merrill MY. J Hum Evol. 1999;36:719–741. doi: 10.1006/jhev.1999.0304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.