Abstract
Tertiary insects and arachnids have been virtually unknown from the vast western Amazonian basin. We report here the discovery of amber from this region containing a diverse fossil arthropod fauna (13 hexapod families and 3 arachnid species) and abundant microfossil inclusions (pollen, spores, algae, and cyanophyceae). This unique fossil assemblage, recovered from middle Miocene deposits of northeastern Peru, greatly increases the known diversity of Cenozoic tropical–equatorial arthropods and microorganisms and provides insights into the biogeography and evolutionary history of modern Neotropical biota. It also strengthens evidence for the presence of more modern, high-diversity tropical rainforest ecosystems during the middle Miocene in western Amazonia.
Keywords: Pebas Formation, Peru, Hexapoda, Arachnida, microorganisms
Ambers and other fossilized natural tree resins are common, documented in hundreds of Upper Paleozoic to Recent localities from around the globe. In exceptional cases, they can entomb pollen (1) or delicate and soft-bodied organisms that are poorly sampled or absent in the fossil record (2–4). Most of these amber-bearing deposits are restricted to the Northern Hemisphere: Only three South American Cenozoic localities have been reported, from the Eocene of Patagonia, Miocene of eastern Brazil, and Pleistocene of French Guyana (4, 5). We report the previously undescribed occurrence of fossil-bearing amber from the vast western Amazonian basin, a region of extraordinary biological diversity today, but whose fossil record has been virtually unknown for most modern groups. Although the Miocene Pebas Formation of northeastern Peru had long been investigated in paleontological studies [mollusks, fishes, and pollen (6–10)], amber clasts with organic inclusions are known from only a single level that we discovered in 2004. This amber is especially noteworthy for containing a diverse fossil arthropod fauna [at least 13 different families of Hexapoda (in the Collembola, Coleoptera, Diptera, Hemiptera, Hymenoptera, Orthoptera, Psocoptera, and Trichoptera) and 3 arachnid species] and abundant microfossil inclusions [pollen, spores (30 morphotaxa, including >20 fungi), algae, and cyanophyceae].
Results and Discussion
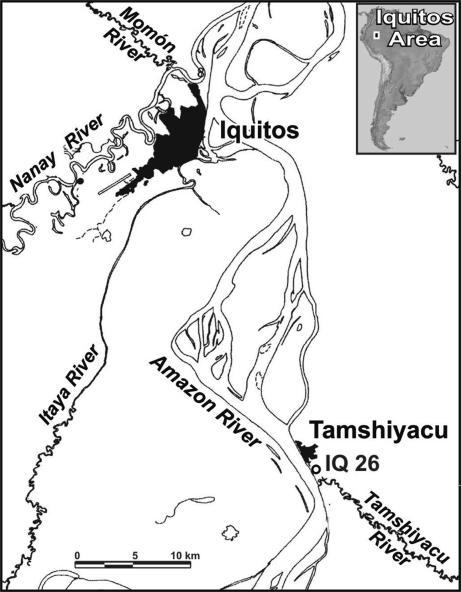
Three large and 25 smaller clasts (≈150 g, in 50-g + 50-g+ 30-g + smaller clasts) were recovered, two of which include trapped arthropods and pollen (see Figs. 2B, 3, and 4 and Tables 1 and 2). Others include spores or dispersed organs of cyanobacteria, fungi, and freshwater algae, as well as a few unicellular organisms (see Fig. 4 and Table 1). All of the amber clasts originate from a single level of the Pebas Formation [18–10 million years ago; “Solimões Fm” in Brazil (10, 11)] in the Tamshiyacu locality on the eastern bank of the Amazon River, ≈30 km upstream of Iquitos in northeastern Peru (Figs. 1 and 2). This outcrop has been intensively studied for biostratigraphy and lithogenesis (7, 10, 11); it consists of two coarsening-upward parasequences, together referred to the middle Miocene Crassoretitriletes Zone (≈15–12 Ma), based on the occurrence of C. vanraadshoovenii pollen (7, 10, 11). The amber-bearing level corresponds to a transgressive lag in the upper parasequence (TmB) (11), just above bottom lignites (Fig. 2A). “Rare detrital amber” was already mentioned within this parasequence (11), but it was not investigated further, and no fossil inclusions were reported. In fact, it appears to be relatively abundant and of large size within the locality. Even though the nature of the depositional environments of Pebas strata has been widely debated throughout the last decade (7, 10,11,12,13–14), with inferences ranging from fluvial, lacustrine, and brackish to tidal environments, most authors acknowledge the occurrence of episodic marine incursions, most likely of Caribbean origin (9–16).
Fig. 2.
Amber-bearing Tamshiyacu section (IQ 26, Iquitos area, northeastern Peru). (A) Middle Miocene amber-bearing level is indicated by black arrow. (B) Large amber clast from the level in A (length ≈ 70 mm) at natural size.
Fig. 3.
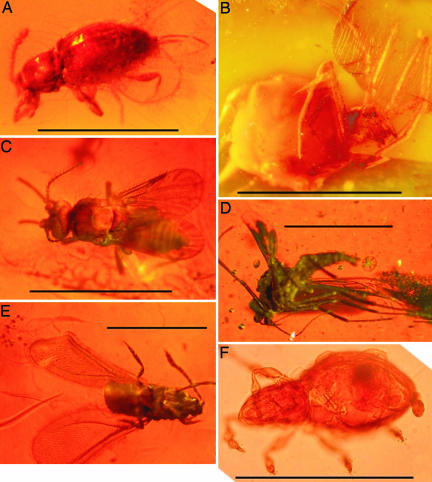
Photographs of Euarthropoda in amber from the Miocene of Iquitos (northeastern Peru). (A) Coleoptera: Cucujoidea: Sphindidae. (Scale bar, 0.6 mm.) (B) Psocoptera, family undetermined. (Scale bar, 4 mm.) (C) Diptera: Ceratopogonidae (female). (Scale bar, 0.7 mm.) (D) Diptera: Chironomidae (male). (Scale bar, 1 mm.) (E) Hemiptera: Aleyrodidae (male). (Scale bar, 0.8 mm.) (F) Arachnida: Acarina (mite). (Scale bar, 0.2 mm.)
Fig. 4.
Microfossils in Miocene amber from western Amazonia (Iquitos, northeastern Peru) in photographs taken under a light microscope. (A) Eubacteria. (B) Nostocaceae (Cyanobacteria). (C) Quilonia sp., pluricellate spores, specimens close to the modern Alternaria. (D) Polycellulaesporonites sp., also found in modern Alternaria species. (E) Pluricellulaesporites sp. (F) Scenedesmus sp. (G) Frasnacritetrus sp., four-branched spore (only two are visible here in the microscope focus). (H) Phragmothyrites sp., small subcircular ascostroma. (I) Hypoxylonites sp., fungal or algal spores. (J) Triporopollenites sp., pollen grain of a Proteaceae (Eudicotyledons: Magnoliophyta).
Table 1.
Microorganisms entrapped in amber from the middle Miocene of Tamshiyacu, nearby Iquitos (northeastern Peru)
| Binomen | Higher-level taxon |
|---|---|
| Mycophyta | |
| Psiamasporites fusiformis (Salard-Cheboldaeff & Locquin, 1980) | Amerosporae (“Fungi imperfecti” fungal or algal spore) |
| Inapertisporites clarkei (Kalgutkar & Jansonius, 2000) | Amerosporae (“Fungi imperfecti” fungal or algal spore) |
| Inapertisporites spp. | Amerosporae (“Fungi imperfecti” fungal or algal spore) |
| Monosporites cf. magnus (Kalmghutkar, 1993) | Amerosporae (“Fungi imperfecti” fungal or algal spore) |
| Monosporites sp. | Amerosporae (“Fungi imperfecti” fungal or algal spore) |
| Hypoxylonites spp. | Amerosporae (“Fungi imperfecti” fungal or algal spore) |
| Dicellaesporites sp. | Didymosporae (“Fungi imperfecti” = Deuteromycetes) |
| Dicellaesporites cf. obnixus (Norris, 1986) | Didymosporae (Deuteromycetes) |
| Dicellaesporites cf. perelongatus (Kalgutkar & Jansonius, 2000) | Didymosporae (Deuteromycetes) |
| Dicellaesporites africanus (Salard-Cheboldaeff, 1980) | Didymosporae (Ascomycetes) |
| Dicellaesporites inequabilis (Martinez-Hernandez & Tomasini-Ortiz, 1989) | Didymosporae (Deuteromycetes) |
| Dicellaesporites longus (Trivedi & Verma ex Kalgutkar & Jansonius, 2000) | Didymosporae (Deuteromycetes) |
| Dyadosporites cf. minor (Salard-Cheboldaeff & Locquin, 1980) | Didymosporae (Ascomycetes) |
| Kumarisporites sp. | Phragmosporae (Deuteromycetes) |
| Reduviasporonites sp. | Phragmosporae (Deuteromycetes) |
| Multicellites sp. | Phragmosporae (Deuteromycetes) |
| Pluricellulaesporites sp. | Phragmosporae (Deuteromycetes) |
| Quilonia spp. | Phragmosporae (Deuteromycetes, 1 sp. cf. Alternaria) |
| Multicellaesporites sp. | Phragmosporae (Deuteromycetes) |
| Diporicellaesporites fusoides (Salard-Cheboldaeff & Locquin, 1980) | Dematiaceae (Phragmosporae) |
| Diporicellaesporites (Elsik, 1968) sp. | Dematiaceae (Phragmosporae) |
| Dictyosporites (Felix, 1894) sp. | Dictyoporeae (Deuteromycetes) |
| Polycellulaesporonites (Chandra, Saxena, & Setty, 1984) sp. | Dictyoporeae (Deuteromycetes) |
| Frasnacritetrus spp. | Staurosporae (Deuteromycetes) |
| Trilobites sp. | Staurosporae (Deuteromycetes) |
| Phragmothyrites sp. | Microthyriales (Phragmothyrium, Ascomycetes) |
| Lichen | |
| Gen. et sp. indet. | Incertae sedis |
| Chlorophyta | |
| Scenedesmus sp. | Scenedesmaceae |
| Magnoliophyta | |
| Triporopollenites sp. | Proteaceae |
List is sorted by morphographic order for spores (22). Identification was by D.D.F.
Table 2.
Taxonomy of Hexapoda entrapped in amber from the middle Miocene of Tamshiyacu, nearby Iquitos (northeastern Peru)
| Amber sample/slide | Entrapped Hexapoda |
|---|---|
| IQ 26IA1 (6) | Diptera: Phoridae (female) |
| IQ 26IA (4) | Coleoptera: Cucujoidea: Sphindidae |
| IQ 26IA (1) | Diptera: Brachycera: Cyclorrhapha, family undetermined |
| IQ 26IA2B | Diptera: Chironomidae subfamily Orthocladiinae (male) |
| IQ 26IA1 (7 and 9) | two Diptera: Ceratopogonidae (female) |
| IQ 26IA (5) | Hemiptera: Aleyrodidae (male) |
| IQ 26IA2 (10) | Diptera larva, family undetermined |
| IQ 26IA2 | Psocoptera, family undetermined |
| IQ 26IA3 | Orthoptera wing fragments, family undetermined (?) + Hemiptera Aleyrodidae (female) |
| IQ 26IA3 | Hymenoptera: Ichneumonidae (?) |
| IQ 26IA1 | Hymenoptera: Chalcidoidea: Aphelinidae |
| Unlabeled | Diptera: Phlebotomidae (female) |
| Unlabeled | Diptera: Mycetophilidae + Hymenoptera: Scelionidae |
| Unlabeled | Hexapoda: Collembola |
| Unlabeled | Hexapoda: Trichoptera |
Identification was by A.N.
Fig. 1.
Map of the Iquitos area (northeastern Peru) showing the geographic location of the middle Miocene amber-bearing locality of Tamshiyacu (IQ 26), denoted by an open circle. Map was redrawn from ref. 10.
Amber allows the fossilized preservation of delicate plant structures [flowers or pollen (1, 17)], soft-bodied animals [e.g., nematodes, annelids, gastropods, arthropods, and small vertebrates (4, 17,18–19)], and microorganisms (20), which allows their comparison with recent organisms. Contrary to what occurs in Dominican amber, which preserves membrane structures, musculature, and nerve tissue of arthropods (4), the amber clasts from Iquitos only preserved cuticles. However, these organisms are sufficiently well preserved to be identified precisely and used as paleoenvironmental markers. In addition, this fossilized resin from Iquitos preserves partial cell contents of some microorganisms and pollen grains, as also observed in Baltic and Paris basin ambers (21). Because it represents a previously undescribed Neogene insect assemblage from western Amazonia, the entomofauna is entirely unique and reflects only an initial sample of what is likely to be a much more diverse assemblage, necessitating broader taxonomic and phylogenetic analyses. Nevertheless, preliminary identifications already reveal significant taxonomic and ecological diversity, including two humid-environment, ground-living Hexapoda (Collembola) and a Trichoptera (Hexapoda with aquatic larvae). Among the Diptera, the families Mycetophilidae, Chironomidae (Fig. 3D), Ceratopogonidae (two specimens; Fig. 3C), Phlebotomidae, and Phoridae are represented. The first four of these dipteran families frequently live in humid environments. An adult Coleoptera (Cucujoidea: Sphindidae) is also present (Fig. 3A). These beetles are myxomycophagous specialists, living in forests on, or inside, mold sporocarps. There are also male and female specimens of Hemiptera: Aleyrodidae (Fig. 3E), and two parasitoid Hymenoptera, one Chalcidoidea: Aphelinidae (which live in Hemiptera [Aleyrodoidea, Aphidoidea, Auchenorrhyncha, Psylloidea, and Coccoidea]), and one Scelionidae, plus four undetermined insects, including a Psocoptera (Fig. 3B). Representatives of the arachnids include at least three species of Acari (Fig. 3F). The numerous microorganisms found in the other amber clasts (Fig. 4) are fungi spores and conidia, cyanobacteria cells (Fig. 4B), eubacteria (Fig. 4A), and a few freshwater green algae. Among hundreds of individual spores and other microstructures, >30 different morphotaxa could be identified (Table 1). These include many previously undescribed species, which need to be studied comprehensively. The overall assemblage of known spore species documents an early and/or middle Miocene age (22), confirming contemporaneity for both this amber and the lithologic unit in which it is found (late early to early middle Miocene age Pebas Formation). A previously undescribed species of Frasnacritetrus (Staurosporea), a four-forked spore, is related to the modern genus Tetraploa [usually associated with Poaceae (grasses), Cyperaceae (sedges), or some tree species]. The rarity and fragility of these spores recovered from sediments suggest that they could represent contamination by recent Tetraploa (22). The presence of this specimen (Fig. 4G) within Miocene amber testifies to definitive occurrence of this genus in the early Neogene and could also indicate greater habitat complexity than might be expected in the dense rainforest-dominated environment suggested by the other amber organisms.
Arthropods and microorganisms play an exceptionally important role in modern terrestrial ecosystems (4), and amber-preserved specimens provide key paleoenvironmental information for the middle Miocene ecosystems of the western Amazonian Basin. Resin entombment of an organism is a rapid preburial process (4). Further, amber is insoluble in water (3) but with a density close to it (1.04–1.10; Iquitos clast density = 1.06), which allows its long-distance transportation in running water and preservation in various proximal and downstream depositional environments (often associated with wood debris), including delta-plain and/or tidal environments (4). Occurrence of amber containing terrestrial organisms is consistent with previous reconstructions of the depositional environment of the Tamshiyacu sedimentary series (11, 14), accumulating near the shore of a tidally influenced “marine-like megalake” (10). Some amber deposits (notably Baltic amber) are known to be reworked, depending on both their density and the salinity of the transporting water. The Iquitos amber clasts are unlikely to be reworked from much earlier deposits because of the following: (i) the Iquitos resin flows have kept their original shape (Fig. 2B); (ii) several trapped spore taxa have an early to middle Miocene stratigraphical range, indicating contemporaneity with the surrounding sedimentary matrix (Pebas Fm.); and (iii) the composition and aspect of this Amazonian amber precludes the occurrence of any subsequent tectonic deformation and/or sediment burial effect, such as that often observed in reworked ambers (23, 24).
Many recent families of conifers and angiosperms generate resins (25), but only a few of them have been documented to be amber producers (4) through anatomical studies of associated amber and wood specimens [Pinaceae (17, 26); Caesalpiniaceae (27)]. It is currently impossible to identify the specific amber-producing tree(s) for the Iquitos specimens, but the presence of fossil wood and excellent and laterally extensive outcrops suggest that intensive sampling should permit recovery of such connected specimens in the near future. In the interim, however, infrared spectrometry (26), solubility, and chemical properties indicate that the Iquitos amber originates from an angiosperm tree. Resin production is known to have both seasonal and diurnal fluctuations [resin exudations are more frequent during the warm season and in higher daily temperatures (4)]. The alternating bands observed in the Iquitos amber clasts can be inferred to represent successive resin flows, as proposed for similarly banded ambers (18). Periods of intensive amber production have been interpreted to be due to either frequent storm damage to source trees or intense forest fires [notably in relation to periods of drastic paleoenvironmental changes (28)] or to the abundance of mature trees in surrounding forests (23). The preliminary sample is too small to provide unequivocal conclusions regarding which of these causes might have been responsible for the resin exudation forming the Iquitos ambers.
The available insect sample (14 specimens) is sufficiently large to provide several additional paleoecological inferences. As in all other known amber insect associations, Hymenoptera and Diptera: “Nematocera” dominate. This dominance relates to a probable bias of attraction of these insect groups to the resin, particularly with respect to the position of the exudate in the tree or to the behavior and local habitat of the trapped organisms (2, 4, 29). However, the insects in the Iquitos amber seem to be associated with a humid terrestrial environment, probably in a forest, which is consistent with the identified fern spores and the large amount of unidentified lignitic plant remains from the same layers (11). Unfortunately, the lignitic fragments are partly transformed into vitrinite, which precludes any infrafamiliar identification of the associated trees. The high diversity (13 different families for 14 specimens) and ecological disparity (presence of parasitoids, phytophagous, myxomycophagous, blood feeding, and detritivorous taxa) observed in even just this preliminary sample suggest a rich and complex entomofauna for the middle Miocene of western Amazonia (Table 2), consistent with broader continental and global conditions associated with the high Miocene floral diversity (30) and the Miocene Climate Optimum (12). In addition, the Iquitos amber yields some Hexapoda with aquatic affinities (Trichoptera, whose larvae are strictly aquatic) and a small ground soil fauna (Collembola), as also occurs in Baltic amber (28).
Light microscopy also revealed a preserved microcenosis. The abundance of fungus spores and other microorganisms and the comparative rarity of pollen (one pollen grain only) reveal that these small resin flows most likely formed in the closed undergrowth of a moist forest, on the bark of the trunk or branches of the amber-producing tree(s). Some of the fungal spores belong to parasite or saprophyte groups found on wood, which could have colonized tree injuries (broken branches or insect attacks) and might have been trapped in the resin flow during cicatrization. The occurrence of epiphytous predators, such as spiders, may also promote carcass concentrations, as observed in the amber clast that contained several spider webs (4, 18).
The Cenozoic history and evolution of terrestrial arthropods and microorganisms is poorly documented in South America; for instance, fossil insects were previously known only from three lacustrine Oligocene Brazilian basins (31). Later in the Cenozoic, nothing has been recorded from the Amazon area before Holocene copal from Santander (Colombia), <1,000 years old (32). Comparisons with the entomofaunas of the Oligocene–Miocene Dominican and Oligocene Chiapas amber (Mexico) shall be of great palaeobiogeographical interest, because Dominican and Mexican ambers represent mid-Cenozoic faunas of the two main transitional areas between North and South America. Direct comparisons should be possible because the potential taphonomic biases related to the chemical nature of the ambers would be very similar in the three cases, because they have comparable angiosperm origins.
Previous studies suggested that the Miocene of the Amazon Basin sheltered high-diversity ecosystems, which were supposed to have originated from climatic change (30), island biogeography (33), or variable habitat (34). Yet, throughout Neogene times northern South America is likely to have formed a single floristic province, of which western Amazonia was a part [based on pollen studies (7)]. The same palynostratigraphic data (7) indicated that the regional vegetation already was a tropical rainforest, with dominant swamp, alluvial plain, and aquatic elements, with intermittent mangrove systems, throughout the Crassoretitriletes Zone [middle Miocene (7, 30)]. At the same time, open habitats occurred, as revealed by typical floristic elements, such as Poaceae (grasses).
The amber sample testifies both to high annual rainfall [with marked seasonality (10)] and to exceptionally high plant and animal diversity (30), providing more robust inferences about the timing of establishment of high biodiversity and more modern ecosystems and climate regimes in western Amazonia. For example, the diverse animals, plants, fungi, and cyanobacteria entrapped in this initial small amber sample substantiate that humid and densely forested environments in a tropical climate must have been present by the middle Miocene in western Amazonia. Future work on this assemblage should more widely elucidate the pattern of changes in biotic diversity, palaeoenvironment, and paleoecology of an area that experienced drastic environmental changes (7, 8) throughout the Miocene (12, 30).
Materials and Methods
The amber clasts were hand-picked directly within the amber-bearing level, for a width of ≈200 m along a vertical riverbank. This preliminary sampling method may have introduced a bias in the frequency of arthropods within the amber inclusions (without screening, we could not collect enough small fragments that might contain more arthropods). Systematic screening of the amber-bearing level is needed. The amber clasts and preparations are temporarily housed in the Muséum d'Histoire Naturelle (Paris, France) and belong to the collections of the Museo de Historia Natural (Lima, Peru).
Acknowledgments
We thank Michel Lemoine and Gaël De Ploëg for their help in specimen preparation, Laurent Marivaux for improving a previous version of the manuscript, and George Poinar and Bruce MacFadden for helpful commentary. This work was supported by the Environnements et Climats du Passé: Histoire et Evolution (ECLIPSE) Program of France, the Centre National de la Recherche Scientifique, the Institut de Recherche pour le Développement, the National Aeronautics and Space Administration, the Field Museum (Chicago, IL), and the American Museum.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.De Franceschi D, Dejax J, De Ploëg G. C R Acad Sci. 2000;330:227–233. [Google Scholar]
- 2.Poinar GO., Jr . Life in Amber. Stanford, CA: Stanford Univ Press; 1992. [Google Scholar]
- 3.Anderson KB, Crelling JC, editors. Amber, Resinite, and Fossil Resins. Vol 617. Washington, DC: Am Chemists Soc; 1995. pp. 11–17. American Chemists Society Symposia Series. [Google Scholar]
- 4.Martínez-Delclòs X, Briggs DEG, Peñalver E. Palaeogeogr Palaeoclimatol Palaeoecol. 2004;203:19–64. [Google Scholar]
- 5.Ensaf A, Nel A, Garrouste R. Mitt Schweiz Entomol Gesell. 2003;76:245–248. [Google Scholar]
- 6.Nuttall CP. Br Mus Nat Hist Bull Geol. 1990;45:165–371. [Google Scholar]
- 7.Hoorn C. Palaeogeogr Palaeoclimatol Palaeoecol. 1993;105:267–309. [Google Scholar]
- 8.Hoorn C, Guerrero J, Sarmiento GA, Lorente MA. Geology. 1995;23:237–240. [Google Scholar]
- 9.Monsch K. Palaeogeogr Palaeoclimatol Palaeoecol. 1998;143:31–50. [Google Scholar]
- 10.Wesselingh FP, Räsänen ME, Irion G, Vonhof HB, Kaandorp R, Renema W, Romero Pittman L, Gingras M. Cainozoic Res. 2002;1:35–81. [Google Scholar]
- 11.Gingras MK, Räsänen ME, Pemberton SG, Romero LP. J Sedimentary Res. 2002;72:871–883. [Google Scholar]
- 12.Kaandorp RJG, Vonhof HB, Wesselingh FP, Romero Pittman L, Kroon D, van Hinte JE. Palaeogeogr Palaeoclimatol Palaeoecol. 2005;221:1–6. [Google Scholar]
- 13.Räsänen ME, Linna A, Santos J, Negri F. Science. 1995;269:386–390. doi: 10.1126/science.269.5222.386. [DOI] [PubMed] [Google Scholar]
- 14.Hernández RM, Jordan TE, Dalenz Farjat A, Echavarría L, Idleman BD, Reynolds JH. J South Am Earth Sci. 2005;19:495–512. [Google Scholar]
- 15.Vonhof HB, Wesselingh FP, Kaandorp RJG, Davies GR, van Hinte JE, Guerrero J, Räsänen M, Romero-Pitmann L, Ranci A. GSA Bull. 2003;115:983–993. [Google Scholar]
- 16.Hovikoski J, Räsänen M, Gingras M, Roddaz M, Brusset S, Hermoza W, Romero Pittman L, Lertola K. Geology. 2005;33:177–180. [Google Scholar]
- 17.Conwentz H. Monographie der Baltischen Bernsteinbäumeqq. Germany: Leipzig; 1890. [Google Scholar]
- 18.Weitschat W, Wichard W. Atlas der Pflanzen und Tiere in Baltische Bernstein. Munich: Pfeil; 1998. [Google Scholar]
- 19.Arnold EN, Azar D, Ineich I, Nel A. J Zool. 2002;258:7–10. [Google Scholar]
- 20.Greenblatt CL, Baum J, Klein BY, Nachshon S, Koltunov V, Cano RJ. Microb Ecol. 2004;48:120–127. doi: 10.1007/s00248-003-2016-5. [DOI] [PubMed] [Google Scholar]
- 21.Dejax J, De Franceschi D, Lugardon B, De Ploëg G, Arnold V. C R Acad Sci. 2001;332:339–344. [Google Scholar]
- 22.Kalgutkar RM, Jansonius J. Synopsis of Fossil Fungal Spores, Mycelia and Fructifications. Dallas: Am Assoc of Stratigraphic Palynologists Foundation; 2000. [Google Scholar]
- 23.Henwood A. Mod Geol. 1993;19:35–59. [Google Scholar]
- 24.Grimaldi DA. In: Amber, Resinite, and Fossil Resins, Anderson KB, Crelling JC, editors. Vol 617. Washington, DC: Am Chemists Soc; 1995. pp. 203–217. American Chemists Society Symposia Series. [Google Scholar]
- 25.Metcalf ECR, Chalk L. Anatomy of the Dicotyledones: Leaves, Stems, and Wood in Relation to Taxonomy with Notes on Economic Uses. Oxford: Clarendon; 1950. [Google Scholar]
- 26.Pielinska A. Metalla Bochum. 1997;66:25–28. [Google Scholar]
- 27.De Franceschi D, De Ploëg G. Geodiversitas. 2003;25:633–647. [Google Scholar]
- 28.Grimaldi DA, Shedrinsky A, Wampler TP. In: Studies on Fossils in Amber, with Particular Reference to the Cretaceous of New Jersey. Grimaldi DA, editor. Leiden, Germany: Backhuys; 2000. pp. 1–76. [Google Scholar]
- 29.Poinar GO, Jr, Poinar R. The Amber Forest: A Reconstruction of a Vanished World. Princeton: Princeton Univ Press; 1999. [Google Scholar]
- 30.Van Der Hammen T, Hooghiemstra H. Quaternary Sci Rev. 2000;19:725–742. [Google Scholar]
- 31.Martins-Neto RG, editor. First Simposio Brasileiro de Paleoarthropodologia and First International Meeting on Paleoarthropodology. São Paulo: Ribeiro Preto; 2000. p. 23. [Google Scholar]
- 32.Mendes LF. Bol Soc Portug Entomol. 1997;168:245–250. [Google Scholar]
- 33.Nores M. J Biogeogr. 1999;26:475–485. [Google Scholar]
- 34.Hooghiemstra H, Van Der Hammen T. Earth-Sci Rev. 1998;44:147–183. [Google Scholar]