Abstract
Spermatogonial transplantation has demonstrated a unique opportunity for studying spermatogenesis and provided an assay for spermatogonial stem cells. However, it has remained unknown whether germ cells that matured in a xenogeneic environment are functionally normal. In this investigation, we demonstrate the successful production of xenogeneic offspring by using spermatogonial transplantation. Rat spermatogonial stem cells were collected from immature testis and transplanted into the seminiferous tubules of busulfan-treated nude mouse testis. Using rat spermatids or spermatozoa that developed in xenogeneic surrogate mice, rat offspring were born from fresh and cryopreserved donor cells after microinsemination with rat oocytes. These offspring were fertile and had a normal imprinting pattern. The xenogeneic offspring production by interspecies germ cell transplantation and in vitro microinsemination will become a powerful tool in animal transgenesis and species conservation.
Keywords: fertilization, spermatogenesis, stem cell, transplantation
Spermatogenesis occurs by complex interactions between germ cells and Sertoli cells (1, 2). At the foundation of this process are spermatogonial stem cells that proliferate indefinitely. These cells have self-renewal activity and continuously produce progenitor cells, thereby supporting male reproduction throughout life. An important characteristic of spermatogonial stem cells is that they can reinitiate and reconstitute spermatogenesis after transplantation into a heterologous surrogate environment; microinjection of donor spermatogonial stem cells into infertile recipient testes allows donor stem cells to recolonize the empty niche to initiate donor-derived spermatogenesis in the recipient animals (3). The recipient animals can then produce normal fertile offspring from transplanted donor cells (4). This technique of germ cell transplantation provided valuable opportunities to use these cells for preservation of genetic information, genetic engineering, or infertility treatment (5–7).
Surprisingly, this process of donor stem cell colonization and subsequent spermatogenesis can occur in xenogeneic recipient testes. It was first demonstrated by Brinster and colleagues (8) in 1996 that rat spermatogonial stem cells can colonize and complete spermatogenesis in mouse testes. Although rat spermatogenesis was directly supported by mouse Sertoli cells (9), the speed and length of the spermatogenic cycle of the rat remained the same in the mouse testis, which indicated that the rate of the cell cycle is determined by germ cells alone (10). Furthermore, when mature sperm were retrieved from the epididymides of the recipient mice, they showed morphological characteristics of rat sperm (8). The xenogeneic transplantation technique was later extended to other animal species, and spermatogonial stem cells from hamsters, rabbits, pigs, bulls, primates, and humans produced spermatogenesis to different degrees in mouse seminiferous tubules (11–15).
Although these pioneering studies demonstrated the feasibility of stem cell transplantation and revealed a remarkable flexibility of interactions between germ cells and Sertoli cells, it has not been possible to produce offspring by using germ cells that matured in xenogeneic mouse testes, and it remains unknown whether they are functionally normal. It is possible that such germ cells have a normal morphological appearance but cannot fertilize eggs because of a lack of necessary components that must be acquired during spermatogenesis. In particular, the major steps in chromatin remodeling occur during the final stages of spermatogenesis, and a recent study showed that the decline in fertility due to abnormal chromatin structure becomes apparent in epidydimal sperm (16). Such defects are not apparent by morphology and require functional assessment. In fact, many morphological abnormalities are reported after spermatogonial transplantation, and these abnormalities become more severe with xenogeneic donors (9). For example, hamster spermatogonial stem cells showed defective spermiogenesis and often had clear abnormalities in head and acrosomal development (11). In this investigation, we examined the fertility of rat germ cells by using microinsemination. Rat spermatogonial stem cells were transplanted into nude mouse testes, and rat germ cells were assessed for their fertility potential by using in vitro microinsemination.
Results
Rat Offspring from Fresh and Cryopreserved Rat Spermatogonial Stem Cells That Were Transplanted in Immunodeficient Mice.
Donor cells were prepared from a transgenic rat strain that expresses EGFP gene ubiquitously, including in the spermatogenic cells. Both fresh and cryopreserved testis cells were used for transplantation, and all donor rats were heterozygous for the transgene. In case of cryopreservation, cells were stored in liquid nitrogen for 29 to 30 days. After thawing, ≈70–80% (73.9 ± 1.4%, mean ± SEM, n = 5) of the cryopreserved cells were viable, as assessed by trypan blue exclusion. These donor cells were transplanted into chemically castrated immunodeficient nude mice. Because recipient mouse testes do not have endogenous fluorescence, donor cells could be specifically identified by UV light excitation. A total of three experiments were performed using fresh and cryopreserved donor cells, and fresh or cryopreserved cells were microinjected into 8 or 22 recipient testes, respectively.
The testes of the recipients were analyzed 4–8 months after transplantation. This period corresponds to three to five cycles of rat spermatogenic cells (10). Because the pace of rat spermatogenesis is intrinsic to the germ cell genotype after spermatogonial transplantation (10), the rat spermatogonial stem cells had sufficient time to mature fully. Although we observed endogenous mouse spermatogenesis in some seminiferous tubules, both fresh and cryopreserved rat donor cells produced EGFP-expressing spermatogenic colonies, as evidenced by fluorescence under UV light (Fig. 1A and B), and all (30/30) recipient testes showed colonization to some degree. When we examined the recipient testes histologically, we found all stages of rat spermatogenic cells, including spermatozoa. Meiosis of donor-derived EGFP-positive cells was confirmed by immunostaining for synaptonemal complex protein 3 (SCP3), a component of the synaptonemal complex (Fig. 1C).
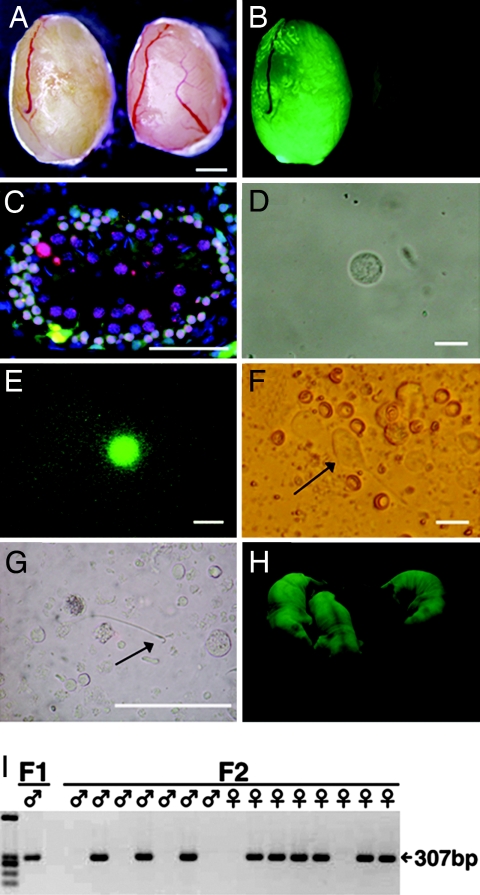
Fig. 1.
Rat spermatogenesis in mouse testis. (A and B) Macroscopic appearance of a nude mouse recipient testis that received EGFP-expressing rat testis cell transplantation (left). The recipient mouse was killed 5 months after transplantation. (B) Note the extensive colonization of donor cells under UV light. Control testis without transplantation did not show fluorescence (right). (C) Histological appearance of a recipient testis that was immunostained with anti-SCP3 antibody (red). The section was counterstained with Hoechst 33258 dye (blue) for nuclei. (D–G) A round spermatid (D and E), elongated spermatid (F, arrow), and spermatozoan (G, arrow) released from a segment of seminiferous tubule that was used to inject eggs. (E) The round spermatid fluoresced under UV light. (H) Rat offspring from a mouse recipient that received cryopreserved rat testis cell transplantation. EGFP expression was observed in the offspring. (I) PCR analysis using DNA extracted from individual F1 and F2 offspring. All EGFP-positive offspring contained the EGFP transgene. (Scale bars: A and B, 1 mm; C, 50 μm; D–F, 15 μm; G, 150 μm.)
To determine whether these rat germ cells were fertile, we next performed in vitro microinsemination, a technique commonly used to produce offspring from infertile animals and humans (17, 18). EGFP-positive seminiferous tubules were collected under UV light and mechanically dissociated to release donor-derived germ cells 124–205 days after transplantation. Whereas the round spermatids of the rats could be identified by their weak EGFP expression (Fig. 1 D and E), more differentiated cells do not express EGFP, and these cells were distinguished from endogenous mouse germ cells by their thinner head and longer tail (Fig. 1 F and G). In eight separate experiments, these cells were microinjected into 157 and 428 oocytes, respectively, for fresh and freeze-thawed donor cells (Tables 1 and 2). Because the number of eggs that could be injected per experiment was limited, in some experiments a suspension of germ cell from the recipient's testes was cryopreserved before microinsemination.
Table 1.
Microinsemination with germ cells that developed after transplantation of fresh rat testis cells
| Type of cells injected | No. of oocytes injected | Survival after 24 h (%) | Cleaved (%) | No. of embryos transferred (%) | No. of embryos implanted (%) | No. of pups (%) | EGFP fluorescence |
|---|---|---|---|---|---|---|---|
| Round spermatid | 141 | 97 (69) | 37 (26) | 92 (65) | 10 (7) | 2 (1) | 2 |
| Elongated spermatid | 16 | 8 (50) | 0 (0) | 8 (50) | 5 (31) | 0 (0) | 0 |
| Total | 157 | 105 (67) | 37 (24) | 100 (64) | 15 (10) | 2 (1) | 2 |
Shown are the results of two separate experiments. Embryos were cultured for 24 h before embryo transfer.
Table 2.
Microinsemination with germ cells that developed after transplantation of frozen rat testis cells
| Type of cells injected | No. of oocytes injected | Survival after 24 h (%) | Cleaved (%) | No. of embryos transferred (%) | No. of embryos implanted (%) | No. of pups (%) | EGFP fluorescence |
|---|---|---|---|---|---|---|---|
| Round spermatid | 336 | 198 (59) | 46 (14) | 164 (49) | 53 (16) | 8 (2) | 4 |
| Spermatozoan | 92 | 76 (83) | 14 (15) | 75 (82) | 22 (24) | 5 (5) | 3 |
| Total | 428 | 274 (64) | 60 (14) | 239 (56) | 75 (18) | 13 (3) | 7 |
Shown are the results of six separate experiments. Embryos were cultured for 24 h before embryo transfer.
In total, 97 (17%) of the eggs had progressed to the two-cell stage after 24-h culture. The survival rate of eggs that received freeze-thawed rat germ cells was consistently higher than that of eggs injected with fresh cells. Sperm sonication did not have a significant effect on in vitro development (data not shown). Of the 339 (58%) cultured eggs transferred to the uteri of psedopregnant females, 27% (90 of 339) implanted, and 4.4% (15 of 339) offspring were born. Normal offspring were obtained in experiments with different stages of haploid germ cells, but the success rate with spermatozoa was significantly higher than with elongated spermatids or round spermatids (P < 0.05 by t test), suggesting that maturity of the germ cells is correlated with successful offspring production. The cryopreservation of differentiated germ cells appeared to have a beneficial effect on microinsemination; offspring were obtained only when the germ cell suspension was cryopreserved after being collected from the seminiferous tubules (15 offspring from 420 injected oocytes), whereas no offspring were born with fresh-cell injection into 165 oocytes. This effect was statistically significant (P < 0.05 by t test).
When these offspring were placed under UV light, 60% (9 of 15) showed EGFP fluorescence, indicating that not all of the germ cells used in microinsemination contained EGFP transgene due to weak EGFP expression in haploid stages of germ cells. The offspring grew up to be fertile adults and showed no apparent abnormalities. The transgenes were transmitted stably to the next generation, which was confirmed by EGFP fluorescence and PCR analysis (Fig. 1 H and I).
Normal Imprinting Pattern and Fertility of the Xenogeneic Offspring.
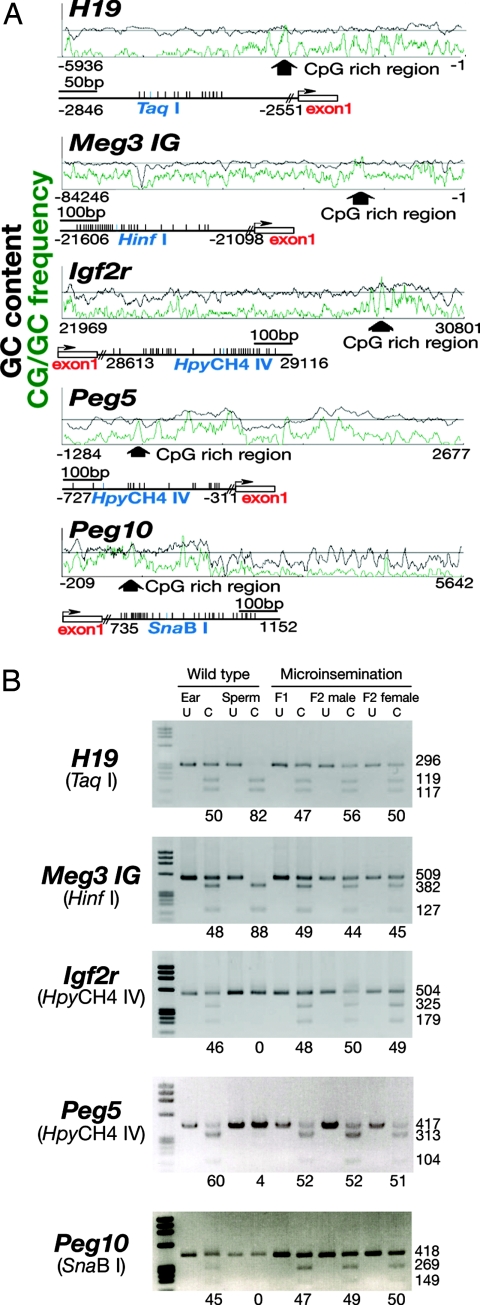
Because the manipulation of germline cells often induces abnormal imprint patterns (19, 20), we used combined bisulfite restriction analysis (COBRA) to examine the methylation patterns of some of the imprinted genes. DNA samples were collected from F1 and F2 offspring, and the degree of methylation in differentially methylated regions (DMRs) of two paternally imprinted regions (H19 and Meg3 IG) and three maternally imprinted regions (Igf2r, Peg5, and Peg10) was examined (Fig. 2A). COBRA showed that the imprinting pattern of offspring from the xenogeneic germ cells was normal in both F1 and F2 offspring. In contrast, control DNA from wild-type sperm showed androgenetic imprinting: methylation in paternal DMRs and demethylation in maternal DMRs (Fig. 2B). These results confirm our previous observation that spermatogonial stem cells are epigenetically resilient (21) and also suggest that spermatogonial stem cells can retain a normal imprinting pattern when the cells mature in a closely related xenogeneic microenvironment.
Fig. 2.
COBRA of genomic DNA from F1 and F2 pups derived from in vitro microinsemination. (A) Schematic diagram of the analyzed regions. (B) DNA methylation status of the analyzed locus. DNA was amplified by specific primers and the PCR products were digested with the indicated restriction enzymes. The percentage of methylation, which was estimated by the intensity of individual bands, is indicated below the gels. U, uncleaved; C, cleaved.
Discussion
Interspecies stem cell transplantation is one of the important technologies in stem cell research. For example, hematopoietic stem cells, the best-characterized stem cells in the body, can be induced to undergo complete differentiation after transplantation into xenogeneic mouse recipients, and this method now is a standard functional assay to detect human hematopoietic stem cells (22–25). Spermatogenesis is the only other self-renewing system for which a similar stem cell transplantation is available. Stem cells microinjected into the seminiferous tubules recolonize an empty niche of infertile recipient testes and achieve long-term reconstitution in a manner similar to hematopoietic stem cell transplantation (3). The spermatogonial transplantation technique has opened up several new possibilities in the study of spermatogenesis and can be used for treatment of infertility or transgenic animal production.
Although xenogeneic spermatogenesis was initially reported more than a decade ago (8), it has remained unknown whether the xenogeneic germ cells are fertile. In fact, several lines of evidence suggest the low quality of germ cells after germ cell transplantation. Russell et al. (26) originally reported several morphological abnormalities in spermatogenesis after syngeneic spermatogonial transplantation, and missing layers of germ cells or abnormalities in elongating phase of spermatogenesis were found in the germ cell colonies. Similar abnormalities were subsequently reported in xenogeneic transplantation. In addition to morphological abnormalities, it was recently found that the motility of sperm from transplanted animals was significantly lower than that of fertile control male mouse after syngeneic transplantation, and the fertilization rate and the blastocyst development rate was significantly reduced when germ cells from recipient mice were used (27). It is not surprising that the environmental damage created by germ cell ablation treatment and evolutional distance between donor and recipient animals negatively influenced the physiological interaction between xenogeneic germ cells and Sertoli cells. These results suggested xenogeneic germ cells do not have competence to fertilize eggs for offspring production.
Our success in xenogeneic offspring production depended critically on the recent development of an in vitro microinsemination technique with rat germ cells (28). Although the mouse microinsemination technique was developed more than a decade ago and extended to other animal species, application of this technology to rats was severely hampered, at least in part, by the relatively large sperm head and easily activated oocytes. However, we have recently demonstrated that not only spermatozoa but also round spermatids can be used to fertilize rat eggs (29). Moreover, we recently succeeded in the production of transgenic rats by using intracytoplasmic sperm injection (ICSI)-mediated DNA transfer (30). Thus, many procedures that were originally developed in mice are now applicable to rats, and the technique is apparently useful in expediting the offspring production from small fragments of germ cell colonies after spermatogonial transplantation.
Offspring from xenogeneic germ cells were previously reported using grafts of immature testis: Transplantation of immature testicular pieces into homotopic and heterotopic positions results in xenogeneic spermatogenesis (31–33). Spermatogenesis was observed in the grafts from various animal species (32, 33). Using this approach, we previously produced normal rabbit offspring by using rabbit sperm that developed in the mouse testis (31). Although this approach is useful for preserving the genetic information of the animals, its critical drawback is that germ cells in the graft are kept within the seminiferous tubule environment, which limits the genetic manipulation of germ cells. In contrast, in vitro genetic manipulation and spermatogonial transplantation can result in efficient transgenic or knockout animal production (6, 34), and the combination of these two technologies allows a wider range of applications with spermatogonial stem cells.
Interestingly, xenogeneic offspring were produced efficiently with freeze-thawed germ cells. In contrast, none of the transferred embryos could develop to term in experiments using fresh germ cells. Fertility of freeze-thawed round spermatids was initially demonstrated in mice (35), and, by using the same cryopreservation solution (glycerol and FBS), we previously obtained normal offspring from cryopreserved round spermatids (29). In that study, the success rates of offspring production were comparable between fresh and cryopreserved round spermatids. In this regard, the lower success rate of fresh cells in the present study suggests that xenogeneic transplantation affects the quality of spermatogenesis to some extent, although it does not completely abolish their fertility. Perhaps, not all of the germ cells are functionally competent in the xenogeneic microenvironment, and cryopreservation procedure may have selected relatively “normal” germ cells; abnormal germ cells may have more fragile cellular structures that are not conspicuous by morphological appearance. Another possibility is that components in the cryopreservation solution, or freeze-thaw procedure, may somehow confer fertility to xenogeneic germ cells. Further extensive studies are needed to clarify these issues.
Our success in xenogeneic offspring production indicates a promising possibility that the technique will be applicable to other animal species. The evolutionary separation between mouse and rat occurred 11 million years ago (8), which is comparable with the genetic distance between human and monkey. The spermatogonia culture technique is now being extended into several animal species, including rats (36, 37). Although normal offspring production from syngeneic transplantation has been reported for several species (38–41), the use of a smaller “surrogate father” reduces the cost and time, instead of keeping larger animals for transplantation and natural mating. Spermatogonial stem cells are readily expanded in vitro, genetically modified, and cryopreserved at will, thereby providing an ideal resource for germline modification (34, 36, 42). In vitro microinsemination allows the production of offspring in a controlled manner and also makes it possible to shorten generation time. It will be interesting to study whether the imprint pattern of the sperm is maintained exclusively by the donor organism and how xenogeneic sperm and epididymis interact with each other. Current technology may become an important option for animal transgenesis and conservation of germline cells.
Materials and Methods
Animals and Transplantation Procedure.
Donor cells were prepared from a transgenic rat line TgN (act-EGFP)Osb4 (a gift from M. Okabe, Osaka University, Osaka, Japan). This transgenic rat line expresses EGFP gene under the control of the β-actin promoter. The spermatogonia and spermatocytes of these rats express EGFP fluorescence, which gradually decreases after meiosis. Donor cells were collected from 10- to 15-day-old pups. Single cell suspension from the donor testes was prepared by two-step enzymatic digestion procedure using collagenase and trypsin (43). In some experiments, donor cells were frozen in liquid nitrogen as described in ref. 44. Donor cells were suspended at a concentration of 108 cells per milliliter in DMEM, supplemented as described (43).
For transplantation, KSN nude mice (Japan SLC, Shizuoka, Japan) were treated with busulfan (44 mg/kg) at 4 weeks old and were used as recipients at least 1 month after busulfan injection. To avoid bone marrow failure by busulfan treatment, all recipient mice received bone marrow transplantation from untreated healthy donors within a week after busulfan treatment. For the testicular injections, ≈10 μl of single-cell suspension was introduced into the seminiferous tubules. Microinjection involved efferent duct injection and filled 75–85% of the tubules in each recipient testis (43). The Institutional Animal Care and Use Committee of Kyoto University approved all of the animal experimentation protocols.
Analysis of Testes.
To visualize donor cell colonization, recipient testes was placed under UV light. A cluster of germ cells was defined as a colony when it occupied >50% of the basal surface of the tubule and was at least 0.1-mm long. For immunohistochemistry, testes were fixed in 4% paraformaldehyde and frozen in Tissue-Tek compound (Sakura Finetechnical, Tokyo, Japan) for cryosectioning. Sections were stained with Hoechst dye 33258 (Sigma, St. Louis, MO). Meiosis was detected by rabbit anti- SCP3 (45). Tetramethyl-rhodamine-conjugated goat anti-rabbit immunoglobulins were used as a secondary antibody (BioSource, Camarillo, CA).
Microinsemination.
Microinsemination was carried out with Piezo-driven micromanipulator, as described in ref. 28. Fragments of seminiferous tubules with EGFP fluorescence were dissected by fine forceps, and rat germ cells were recovered mechanically. Morphologically normal zygotes at the two-cell stage and nondegenerating one-cell stage, harvested at 24 h after microinsemination, were transferred into the oviductal ampullae of pseudopregnant Wistar rats. Offspring were recovered by caesarean section at 21 days after transfer. Data were analyzed by the Student t test.
PCR.
The EGFP transgene (≈307 bp) was amplified using specific primers (5′-TGAACCGCATCGAGCTGAAGGG-3′ and 5′-TCCAGCAGGACCATGTGATCGC-3′).
COBRA.
The methylation statuses of the imprinted genes were assessed by COBRA using specific primers (5′-GGAATTTATATAAGGTAATATTGTG-3′ and 5′-CTCATAAAACCCATAATTATAAAATC-3′ for H19; 5′-GGATTGTGGTTTTTTTATGGATTAGTG-3′ and 5′-CCTCTTTCCTTCCCAACTAACC-3′ for Meg3 IG; 5′-TAGTGGGGTATTTTTATTTGTTTGG-3′ and 5′-AACTATCCTAAAAATACAAAACTACAA-3′ for Igf2r; 5′-GGGAGGGGAGTATAAAATAGAG-3′ and 5′-TACTCCCAAACCTACAAATTC-3′ for Peg5; and 5′-TTTTATGTTTTTAGTGTATTAATGGG-3′ and 5′-CAAAACTCCATTTTATCTACCACC-3′ for Peg10) (21). The PCR products were digested with restriction enzymes that recognize sequences containing the CpG motif. The intensity of the digested DNA was quantified using Mac BASv25 software (Fuji Photo Film, Tokyo, Japan).
Acknowledgments
We thank Ms. A. Wada for her technical assistance and Dr. Y. Kaziro for encouragement. Financial support for this work was provided by the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan, and by grants from Core Research for Evolutional Science and Technology and the Human Science Foundation (Japanese). This work was also supported in part by Grants-in-Aid from the Scientific Research on Priority Areas (elucidation of glia–neuron network-mediated information processing systems) and by grants from the Genome Network Project from MEXT.
Abbreviation
- COBRA
combined bisulfite restriction analysis
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.de Rooij DG, Russell LD. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- 2.Meistrich ML, van Beek MEAB. In: Cell and Molecular Biology of the Testis. Desjardins C, Ewing LL, editors. New York: Oxford Univ. Press; 1993. pp. 266–295. [Google Scholar]
- 3.Brinster RL, Zimmermann JW. Proc Natl Acad Sci USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinster RL, Avarbock MR. Proc Natl Acad Sci USA. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avarbock MR, Brinster CJ, Brinster RL. Nat Med. 1996;6:693–696. doi: 10.1038/nm0696-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanatsu-Shinohara M, Ikawa M, Takehashi M, Ogonuki N, Miki H, Inoue K, Kazuki Y, Lee J, Toyokuni S, Oshimura M, et al. Proc Natl Acad Sci USA. 2006;103:8018–8023. doi: 10.1073/pnas.0601139103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Nat Med. 2000;6:29–34. doi: 10.1038/71496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clouthier DE, Avarbock MR, Maika SD, Hammer RE, Brinster RL. Nature. 1996;381:418–421. doi: 10.1038/381418a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell LD, Brinster RL. J Androl. 1996;17:615–627. [PubMed] [Google Scholar]
- 10.França LR, Ogawa T, Avarbock MR, Brinster RL, Russell LD. Biol Reprod. 1998;59:1371–1377. doi: 10.1095/biolreprod59.6.1371. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Biol Reprod. 1999;60:515–521. doi: 10.1095/biolreprod60.2.515. [DOI] [PubMed] [Google Scholar]
- 12.Dobrinski I, Avarbock MR, Brinster RL. Biol Reprod. 1999;60:515–521. doi: 10.1095/biolreprod60.2.515. [DOI] [PubMed] [Google Scholar]
- 13.Dobrinski I, Avarbock MR, Brinster RL. Mol Reprod Dev. 2000;57:270–279. doi: 10.1002/1098-2795(200011)57:3<270::AID-MRD9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 14.Nagano M, McCarrey JR, Brinster RL. Biol Reprod. 2001;64:1409–1416. doi: 10.1095/biolreprod64.5.1409. [DOI] [PubMed] [Google Scholar]
- 15.Nagano M, Patrizio P, Brinster RL. Fertil Steril. 2002;78:1225–1233. doi: 10.1016/s0015-0282(02)04345-5. [DOI] [PubMed] [Google Scholar]
- 16.Suganuma R, Yanagimachi R, Meistrich ML. Hum Reprod. 2005;20:3101–3108. doi: 10.1093/humrep/dei169. [DOI] [PubMed] [Google Scholar]
- 17.Kimura Y, Yanagimachi R. Biol Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 18.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Lancet. 1992;340:17–18. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- 19.Dean W, Bowden L, Aitchison A, Klose J, Moore T, Menesses JJ, Reik W, Feil R. Development (Cambridge, UK) 1998;125:2273–2282. doi: 10.1242/dev.125.12.2273. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki H, Ferguson-Smith AC, Shum ASW, Barton SC, Surani MA. Development (Cambridge, UK) 1995;121:4195–4202. doi: 10.1242/dev.121.12.4195. [DOI] [PubMed] [Google Scholar]
- 21.Kanatsu-Shinohara M, Ogonuki N, Iwano T, Lee J, Kazuki Y, Inoue K, Miki H, Takehashi M, Toyokuni S, Shinkai Y, et al. Development (Cambridge, UK) 2005;132:4155–4163. doi: 10.1242/dev.02004. [DOI] [PubMed] [Google Scholar]
- 22.Lapidot T, Pflumio F, Murdoch B, Williams DE. Science. 1992;255:1137–1141. doi: 10.1126/science.1372131. [DOI] [PubMed] [Google Scholar]
- 23.McCune JM, Namikawa R, Kaneshima H, Schultz LD, Liberman M, Weissman IL. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 24.Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, McCune JM. J Exp Med. 1990;172:1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larochelle A, Vormoor J, Hanenberg H, Wang JC, Bhatia M, Lapidot T, Moritz T, Murdoch B, Xiao XL, Kato I, et al. Nat Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 26.Russell LD, França LR, Brinster RL. J Androl. 1996;17:603–614. [PubMed] [Google Scholar]
- 27.Goossens E, Frederickx VF, Block G. De., Van Steirteghem AC, Tournaye H. Hum Reprod. 2003;18:1874–1880. doi: 10.1093/humrep/deg360. [DOI] [PubMed] [Google Scholar]
- 28.Hirabayashi M, Kato M, Aoto T, Sekimoto A, Ueda M, Miyoshi I, Kasai N, Hochi S. Transgenic Res. 2002;11:221–228. doi: 10.1023/a:1015210604906. [DOI] [PubMed] [Google Scholar]
- 29.Hirabayashi M, Kato M, Aoto T, Ueda M, Hochi S. Mol Reprod Dev. 2002;62:295–299. doi: 10.1002/mrd.10127. [DOI] [PubMed] [Google Scholar]
- 30.Hirabayashi M, Kato M, Ishikawa A, Kaneko R, Yagi T, Hochi S. Mol Reprod Dev. 2005;70:422–428. doi: 10.1002/mrd.20223. [DOI] [PubMed] [Google Scholar]
- 31.Shinohara T, Inoue K, Ogonuki N, Kanatsu-Shinohara M, Miki H, Nakata K, Kurome M, Nagashima H, Toyokuni S, Kogishi K, et al. Hum Reprod. 2002;17:3039–3045. doi: 10.1093/humrep/17.12.3039. [DOI] [PubMed] [Google Scholar]
- 32.Honaramooz A, Snedaker A, Boiani M, Schöler H, Dobrinski I, Schlatt S. Nature. 2002;418:778–781. doi: 10.1038/nature00918. [DOI] [PubMed] [Google Scholar]
- 33.Schlatt S, Foppiani L, Rolf C, Weinbauer GF, Nieschlag E. Hum Reprod. 2002;17:55–62. doi: 10.1093/humrep/17.1.55. [DOI] [PubMed] [Google Scholar]
- 34.Kanatsu-Shinohara M, Toyokuni S, Shinohara T. Biol Reprod. 2005;72:236–240. doi: 10.1095/biolreprod.104.035659. [DOI] [PubMed] [Google Scholar]
- 35.Ogura A, Matsuda J, Asano T, Suzuki O, Yanagimachi R. J Assist Reprod Genet. 1996;13:431–434. doi: 10.1007/BF02066177. [DOI] [PubMed] [Google Scholar]
- 36.Hamra FK, Chapman KM, Nguyen DM, Williams-Stephens AA, Hammer RE, Garbers DE. Proc Natl Acad Sci USA. 2005;102:17430–17435. doi: 10.1073/pnas.0508780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryu B-Y, Kubota H, Avarbock MR, Brinster RL. Proc Natl Acad Sci USA. 2005;102:14302–14307. doi: 10.1073/pnas.0506970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izadyar F, den Ouden K, Stout TAE, Stout J, Coret J, Lankveld DPK, Spoormakers TJP, Colenbrander B, Oldenbroek JK, van der Ploeg KD, et al. Reproduction. 2003;126:765–774. [PubMed] [Google Scholar]
- 39.Schlatt S, Foppiani L, Rolf C, Weinbauer GF, Nieschlag E. Hum Reprod. 2002;17:55–62. doi: 10.1093/humrep/17.1.55. [DOI] [PubMed] [Google Scholar]
- 40.Schlatt S, Rosiepen G, Weinbauer G, Rolf C, Brook PF, Nieschlag E. Hum Reprod. 1999;14:144–150. doi: 10.1093/humrep/14.1.144. [DOI] [PubMed] [Google Scholar]
- 41.Honaramooz A, Behboodi E, Megee SO, Overton SA, Galantino-Homer H, Echelard Y, Dobrinski I. Biol Reprod. 2003;69:1260–1265. doi: 10.1095/biolreprod.103.018788. [DOI] [PubMed] [Google Scholar]
- 42.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 43.Ogawa T, Aréchaga JM, Avarbock MR, Brinster RL. Int J Dev Biol. 1997;41:111–122. [PubMed] [Google Scholar]
- 44.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Ogura A, Toyokuni S, Shinohara T. Hum Reprod. 2003;18:2660–2667. doi: 10.1093/humrep/deg483. [DOI] [PubMed] [Google Scholar]
- 45.Chuma S, Nakatsuji N. Dev Biol. 2001;229:468–479. doi: 10.1006/dbio.2000.9989. [DOI] [PubMed] [Google Scholar]