Abstract
Solar UV radiation is the most important environmental factor involved in the pathogenesis of skin cancers. The well known genotoxic properties of UVB radiation (290–320 nm) mostly involve bipyrimidine DNA photoproducts. In contrast, the contribution of more-abundant UVA radiation (320–400 nm) that are not directly absorbed by DNA remains poorly understood in skin. Using a highly accurate and quantitative assay based on HPLC coupled with tandem mass spectrometry, we determined the type and the yield of formation of DNA damage in whole human skin exposed to UVB or UVA. Cyclobutane pyrimidine dimers, a typical UVB-induced DNA damage, were found to be produced in significant yield also in whole human skin exposed to UVA through a mechanism different from that triggered by UVB. Moreover, the latter class of photoproducts is produced in a larger amount than 8-oxo-7,8-dihydro-2′-deoxyguanosine, the most common oxidatively generated lesion, in human skin. Strikingly, the rate of removal of UVA-generated cyclobutane pyrimidine dimers was lower than those produced by UVB irradiation of skin. Finally, we compared the formation yields of DNA damage in whole skin with those determined in primary cultures of keratinocytes isolated from the same donors. We thus showed that human skin efficiently protects against UVB-induced DNA lesions, whereas very weak protection is afforded against UVA. These observations emphasize the likely role played by the UVA-induced DNA damage in skin carcinogenesis and should have consequences for photoprotection strategies.
Keywords: carcinogenesis, DNA damage, mutagenesis, oxidative stress, DNA repair
Occurrence of skin cancers, which mostly arise from exposure to solar UV radiation, has constantly increased in the recent years due to changes in life habits. Harmful effects of UV radiation are mostly associated with both direct and indirect photoinduced damage to DNA (1) that can lead to the induction of mutations. The chemical nature and the formation efficiency of the DNA lesions greatly depend on the wavelength of the incident photons. UVB radiation (290–320 nm), the most energetic mutagenic and carcinogenic component of solar radiation, is directly absorbed by DNA, giving rise to dimeric photoproducts between adjacent pyrimidine bases. Two types of these bulky modifications are produced, namely cyclobutane pyrimidine dimers (CPDs) and pyrimidine (6-4) pyrimidone photoproducts (1). Evidence for the involvement of these lesions in photocarcinogenesis is provided by the high proportion of p53 mutations (TC to TT or CC to TT transitions) detected at bipyrimidine sites in skin tumors (2–4).
UVA radiation (320–400 nm), the other component of terrestrial UV radiation, is mutagenic in cultured cells (5, 6) and induces skin tumors in mice (7, 8). In addition, UVA has been shown to be involved in immunosuppression (9) and is suspected to play a major role in the induction of melanoma (10, 11), the most severe type of skin cancers. Consequently, the carcinogenic properties of UVA have become a matter of concern. The incorrect use of sunscreens that until recently afforded mostly UVB protection and allow longer exposure periods to sunlight (12), together with the use of artificial tanning equipments (13), lead to an increase in the overall amount of UVA received by the population of wealthiest countries.
A better assessment of the role of UVA has regained attention with recent mutagenesis studies in skin tumors (14). In contrast with UVB, the lesions responsible for the UVA-induced mutations are not clearly identified. UVA radiation is extremely poorly absorbed by DNA and its genotoxic effects have been explained mostly by the induction of oxidative stress. The formation of 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo) in much larger amount than strand breaks and oxidized pyrimidine bases (15, 16) strongly suggests a major role played by singlet oxygen (17). However, the UVA mutation spectrum in mammalian cells does not exhibit a predominance of G:C to T:A transversions (18, 19) that is considered as the mutagenic hallmark of 8-oxodGuo. This observation, together with the lack of increase in mutation rate in cells deficient in repair of 8-oxodGuo (20), suggests that other lesions are involved in UVA mutagenesis.
Interestingly, data have been gathered on the induction of cyclobutane pyrimidine dimers in DNA upon exposure of bacteria (21), mammalian cells (22–24), and skin (25, 26) to UVA radiation. As a striking trend, recent studies showed that the yield of CPD is higher than that of 8-oxodGuo in rodent cell lines (15, 27, 28) and in human skin cells (29) exposed to UVA. In contrast to UVB, UVA radiation preferentially induces the production of CPDs at TT sites without any detectable formation of pyrimidine (6-4) pyrimidone photoproducts (28–30). These observations clearly indicate that UVA radiation does not induce CPDs via a direct excitation pathway but more likely through a triplet energy transfer photosensitization mechanism.
Until now, the predominant formation of CPDs over oxidatively generated lesions has only been shown in cultured cells. The recent observation that 8-oxodGuo is produced more efficiently than CPDs in UVA-irradiated yeast (31) shows that the cell type and the cellular context strongly influence the UVA photochemistry of DNA. Therefore, the present study was designed to confirm in whole human skin the trends observed in UVA-irradiated cultured cutaneous cells. For this purpose, we accurately determined the yield of formation of a series of relevant DNA lesions within whole human skin exposed to UVA radiation. Results were compared with those obtained upon UVB irradiation. Individual DNA lesions were quantified by using the accurate HPLC analytical method associated either with tandem mass spectrometry (MS/MS) detection for bipyrimidine photoproducts or electrochemical detection for 8-oxodGuo. Using the same approach, information also was gained on the repair kinetics of CPDs in skin.
Results
Formation of Bipyrimidine Photoproducts Within Human Skin Exposed to UVB Radiation.
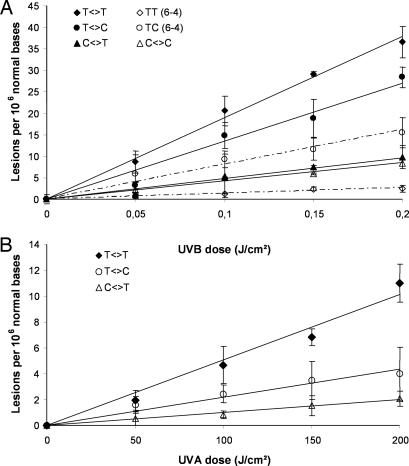
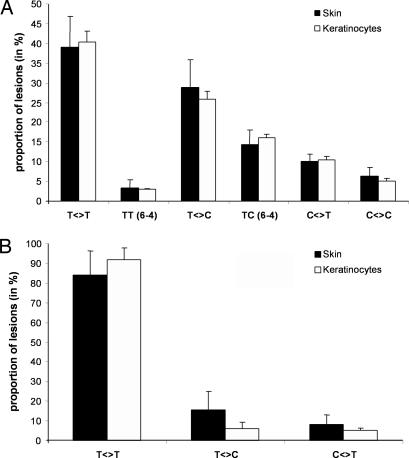
A first series of experiments was carried out to quantify the yield of formation of the bipyrimidine photoproducts within the skin of six donors immediately after exposure to UVB radiation. As shown in Fig. 1A, the formation of photoproducts was linear with respect to the applied UVB dose (0–0.2 J/cm2). This observation clearly indicates that no secondary photoreaction occurred under the experimental conditions used. The bipyrimidine photoproducts were generated in the DNA of human skin upon UVB irradiation in the following decreasing frequency: T<>T > T<>C > TC (6-4) > C<>T > C<>C > TT (6-4). The overall yield of formation of photoproducts was calculated from the linear regression of the dose-course plot for the sum of all bipyrimidine photolesions and for each donor (Table 1). Thus, the mean yield of formation was 518.8 lesions per 106 normal bases per J/cm2 within the whole skin exposed to UVB radiation. For the six donors, the difference between the highest (donor B) and the lowest (donor E) individual yield of photoproducts was <2-fold. It may be added that, as previously shown in cell culture (32–34), none of the Dewar valence isomers was detected, even at the highest applied UVB dose. The distribution of photoproducts in human skin was found to be similar to the one obtained within primary cultures of keratinocyte isolated from the same donors (Fig. 2A). Emphasis was placed on keratinocytes because other types of skin cells (melanocytes and fibroblasts) are present at much a lower frequency. The quantification of bipyrimidine photoproducts after extraction of DNA from a whole biopsy thus mostly reflects damage to keratinocytes of the different layers of the epidermis.
Fig. 1.
Formation of bipyrimidine photoproducts within human skin exposed to UVB (A) or UVA (B) radiation. The presented data corresponds to one representative donor. The results are expressed in lesions per 106 bases and are the average ± SD.
Table 1.
Sum of the yield of formation of all bipyrimidine photoproducts in irradiated human skin
| Donors | UVA |
UVB |
||
|---|---|---|---|---|
| Skin | Cultured keratinocytes | Skin | Cultured keratinocytes | |
| A | 0.065 ± 0.017 | 0.121 ± 0.024 | 431.0 ± 104.1 | 12,934 ± 278 |
| B | 0.113 ± 0.018 | 0.129 ± 0.004 | 656.8 ± 142.0 | 11,304 ± 124 |
| C | 0.077 ± 0.008 | 0.117 ± 0.013 | 546.7 ± 71.8 | 11,139 ± 620 |
| D | 0.076 ± 0.008 | 0.136 ± 0.006 | 511.4 ± 30.4 | 12,464 ± 318 |
| E | 0.080 ± 0.008 | 0.106 ± 0.010 | 425.1 ± 89.5 | 7,505 ± 215 |
| F | 0.076 ± 0.013 | 0.143 ± 0.007 | 542.2 ± 76.4 | 12,409 ± 253 |
| Mean | 0.081 ± 0.017 | 0.125 ± 0.014 | 518.8 ± 78.4 | 11,293 ± 1,811 |
| Ratio keratinocytes/skin | 1.5 | 21.8 | ||
For each donor, the yield (expressed in lesions per 106 normal bases per Joules per centimeter squared) was calculated from the linear regression for the sum of the level of all photoproducts with respect to the applied UV dose. The reported value is the slope ± SE. Mean represents the average ± SD.
Fig. 2.
Distribution of bipyrimidine photoproducts within human skin and primary keratinocytes upon exposure to either UVB (A) or UVA (B) radiation. The proportion (in a percentage) of each photoproduct was determined for each donor, and results were represented by the average ± SD.
Formation of CPD in Skin Exposed to UVA Radiation.
Then, the yield of formation of bipyrimidine photoproducts was determined in the whole human skin upon exposure to UVA radiation. The determined distribution of photoproducts was completely different from that obtained after UVB irradiation. Indeed, exposure to UVA led to the predominant formation of CPD at TT sites with much lower amounts of the corresponding TC or CT cyclobutane dimers. Neither C<>C, (6-4) photoproducts nor any of the Dewar valence isomers were detected in the DNA of irradiated skin. The formation of the three types of CPDs was found to be linear with respect to the applied UVA dose (0–200 J/cm2) (Fig. 1B). For each donor, the yield of formation was calculated from the linear regression of the plot for the sum of all UVA-induced CPD (Table 1). The difference between the highest and the lowest individual yield was also <2-fold, and the mean formation yield in human skin was 0.081 lesions per 106 normal bases per J/cm2. Moreover, the distribution of UVA-induced CPDs in the DNA of whole skin was strongly similar to the one obtained within primary culture of keratinocytes (Fig. 2B), where CPDs were produced in the following decreasing order of abundance T<>T ≫ T<>C > C<>T, as reported for other cell types (28, 29).
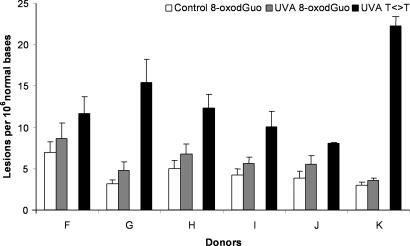
Larger Yield of T<>T than 8-oxodGuo in UVA-Irradiated Skin.
In another series of experiments, the induction of 8-oxodGuo, which is oxidatively generated damage, was quantified within the whole skin exposed to UVA radiation by using the HPLC-electrochemical assay. UVA radiation induced a slight increase in the level of 8-oxodGuo in the DNA, even for the highest applied UVA dose of 200 J/cm2. Although limited, the increase was found to be reproducible from one donor to the other (Fig. 3). The induction of T<>T also was determined by HPLC-MS/MS assay in the same samples. It was shown that T<>T was formed in a larger amount than 8-oxodGuo (Fig. 3). The yield of formation for 8-oxodGuo was 0.0071 lesions per 106 normal bases per J/cm2 in human skin exposed to UVA radiation, and the difference between the highest and the lowest individual yields was <4-fold (Table 2). The mean yield of T<>T formation was 0.066 lesions per 106 normal bases per J/cm2 in the DNA of UVA-irradiated whole skin (Table 2). The ratio between the level of the two lesions shows that T<>T are induced with a 9-fold higher frequency than 8-oxodGuo in the whole human skin exposed to UVA radiation.
Fig. 3.
Formation of 8-oxodGuo and thymine-thymine cyclobutane dimers within human skin exposed to UVA radiation (200 J/cm2). The results are expressed in lesions per 106 bases and are the average ± SD.
Table 2.
Yield of formation of 8-oxodGuo and thymine cyclobutane dimer (T<>T) within human skin exposed to UVA radiation
| Donors | 8-oxodGuo | T<>T | Ratio T<>T/8-oxodGuo |
|---|---|---|---|
| F | 0.0085 ± 0.0064 | 0.057 ± 0.007 | 6.7 |
| G | 0.0080 ± 0.0030 | 0.077 ± 0.009 | 9.6 |
| H | 0.0087 ± 0.0039 | 0.061 ± 0.004 | 7.0 |
| I | 0.0066 ± 0.0024 | 0.050 ± 0.005 | 7.6 |
| J | 0.0081 ± 0.0040 | 0.040 ± 0.003 | 5.0 |
| K | 0.0023 ± 0.0036 | 0.112 ± 0.004 | 48.8 |
| Mean | 0.0071 ± 0.0025 | 0.066 ± 0.025 | 9.4 |
The results (expressed in lesions per 106 normal bases per Joules per centimeter squared) represent the slope ± SE of the linear regression for 8-oxodGuo or T<>T with respect to the applied UV dose for each donor. The mean is average ± SD.
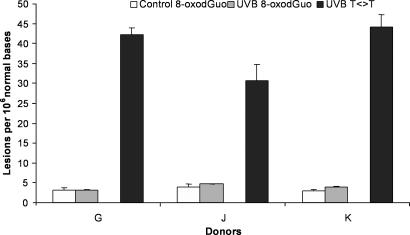
For three donors of the series, the formation of 8-oxodGuo also was quantified after UVB irradiation. Even at the highest dose applied, namely 0.2 J/cm2, only a very modest increase in the level of 8-oxodGuo was observed (Fig. 4). In contrast, T<>T was obtained in large amounts. Based on the sensitivity of the assays, it can be estimated that the yield of CPDs is at least two orders of magnitude higher than that of 8-oxodGuo in whole human skin exposed to UVB.
Fig. 4.
Formation of 8-oxodGuo and thymine-thymine cyclobutane dimers within human skin exposed to UVB radiation (0.2 J/cm2). The results are expressed in lesions per 106 normal bases and are the average ± SD.
Persistence of T<>T in UV-Irradiated Skin.
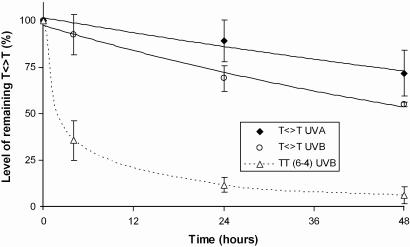
Because T<>T was found to be the major lesion produced after both UVA and UVB irradiations in the DNA of human skin, the persistence of this photoproduct was determined after an acute skin exposure to either UVA (100 J/cm2) or UVB (0.1 J/cm2) radiation. Interestingly, T<>T remained present in large amount within the DNA of human skin 48 h after the end of the irradiations (Fig. 5). It may be noted that for the respective applied doses, UVA produced ≈2-fold less T<>T than UVB radiation. However, the proportion of persistent T<>T 48 h after irradiation was significantly higher (P < 0.05) for UVA (72%) than for UVB radiation (55%). These low repair rates are in agreement with observations made by other groups on UV-irradiated human skin (35–37). The integrity of the repair capacities in the skin biopsies is shown by the efficient removal of TT (6-4) (Fig. 5).
Fig. 5.
Persistence of thymine-thymine cyclobutane dimers within human skin exposed to UVA (100 J/cm2) or UVB (0.1 J/cm2) radiation. Repair of TT (6-4) photoproducts within UVB irradiated skin also is shown. The results are expressed in percentage of residual lesions and are the average ± SD of data obtained with four different donors. The statistical significance in the level of remaining T<>T between UVA and UVB radiation was calculated by using the Student t test. Difference between UVA and UVB was found to be statistically significant at 24 h (P < 0.002) and 48 h (P < 0.05).
Attenuation of UV-Induced Damage by Skin.
Finally, we determined the extent of protection provided by the skin against the formation of bipyrimidine photoproducts induced upon exposure to UVA or UVB. For this purpose, primary keratinocytes were cultured from the skin of each of the donors (donors A–F), exposed to UVB and UVA radiations, and then the yield of formation of photoproducts was quantified. Although the relative proportions of the different photoproducts were similar in vivo and in culture, for both UVA and UVB radiations (Fig. 2), a major difference was observed concerning the absolute yields of formation (Table 1). Indeed, UVB radiation produced 22-fold more bipyrimidine photoproducts in keratinocyte monolayers than in the whole human skin. In contrast, the yield of CPD formation measured after UVA irradiation was only 1.5-fold lower in skin with respect to keratinocytes. Interestingly, the protection was the same for all photoproducts. The values of the attenuation factor for T<>T, the major damage induced by both UV components, is 22 and 1.7 for UVB and UVA, respectively.
Discussion
The formation of CPD in higher yield rather than oxidatively generated DNA lesions upon UVA irradiation represents recent new information that challenges the hypothesis that UVA-induced DNA damage are mostly mediated by oxidative stress. These data were obtained mostly in cultured cells (15, 27–29) and remained to be confirmed in whole skin. For this purpose, we determined the yield of a wide array of dimeric pyrimidine photoproducts by using an HPLC-MS/MS assay that allows the simultaneous and individual quantification of the 12 possible cyclobutane pyrimidine dimers, (6-4) photoproducts, and Dewar valence isomers (32). Quantitative data on the formation of 8-oxodGuo, the main UVA-oxidatively induced DNA damage, also were obtained.
The distribution of the bipyrimidine photoproducts induced by UVB radiation in the DNA of whole human skin was found to be very similar to that obtained in previous studies involving isolated DNA, human monocytes, or rodent cell lines (28, 32). The major lesion in human skin was T<>T, which was produced in a 10-fold higher yield than the TT pyrimidine (6-4) pyrimidone photoproduct. T<>C also was generated, in a 2-fold higher yield than TC (6-4) photoproducts. The overall amount of the two latter TC photoproducts was similar to that of the TT lesions, in agreement with previous HPLC-MS/MS measurements in human monocytes or human dermal fibroblasts exposed to the same UVB source (32, 34). Similar to results obtained on isolated DNA and cultured cells, CT and CC sites also were much less reactive than TT and TC sequences in the DNA of human skin. The present results contrast with those of previous studies in which the quantification of the photoproducts in the DNA of human skin exposed to UVB radiation was achieved by using the HPLC 32P-postlabeling assay. These works led to the conclusion that T<>C was the major lesion generated (38–40). However, it may be pointed out that the same approach failed to provide reproducible data concerning the formation of CPDs in skin exposed to simulated sunlight (36, 37, 41–46).
Our results clearly demonstrate that CPDs also are produced in significant yield in the whole human skin exposed to UVA radiation, in agreement with data obtained by immunohistochemistry (25, 47, 48). CPDs were predominantly produced at TT sites after UVA irradiation of human skin. Interestingly, the ratio between the yield of CPDs produced at TT compared with those at TC and CT sites was much higher than upon exposure to UVB radiation. In addition, no other photoproducts such as pyrimidine (6-4) pyrimidone photoproducts and Dewar valence isomers could be detected in human skin exposed to UVA radiation. These observations, which are in agreement with results obtained in CHO (28, 30) and human skin cells (29), show that the mechanism of formation of CPDs upon UVA and UVB irradiation is different. The distribution of dimeric photoproducts suggests that a photosensitization reaction involving a triplet energy transfer mechanisms rather than a direct excitation process takes place upon exposure of skin to UVA, similarly to what is observed in the effects of some phototoxic drugs (49–51). Moreover, in contrast to a widely accepted hypothesis, we also found that, like in cultured cells (15, 28, 29), T<>T was produced in a larger amount than 8-oxodGuo in human skin exposed to UVA. UVA-mediated photosensitization processes are expected to be partly involved in photocarcinogenesis and, in particular, in the induction of melanoma (10, 52). Emphasis has been placed in the past on photo-oxidative processes that may occur in the dermis, but, in the light of our present results, photosensitized formation of cyclobutane dimers should not be neglected.
The results reported above show that the UV photochemistry of DNA is roughly the same in cultured mammalian cells and in whole skin, at least in terms of relative frequency of the different photoproducts. However, the structure of the skin was found to greatly affect the relative sensitivity of DNA to UVA and UVB radiations. It is universally presumed that the upper epidermal layers in human skin, most particularly the stratum corneum, and the melanin would protect the basal layer against DNA damage formation by blocking the penetration of a significant portion of the UV spectrum. However, to our best knowledge, only one study has evaluated the role played by the multilayered structure of the skin against the induction of CPD by either UVB radiation or simulated sunlight in the DNA of engineered human skin (53). To determine the extent of protection afforded by skin against the radiation emitted by our UVA and UVB sources, we compared the yield of formation of DNA damage within the whole human skin with that determined in monolayer cultures of keratinocytes obtained from the same donor. This approach allowed us to establish that the organization of the human skin efficiently protects against UVB-induced formation of DNA bipyrimidine lesions by a factor of ≈22. In contrast, a very weak protection is afforded by the skin against UVA; the corresponding factor is 1.5. Interestingly, immunohistochemical studies have shown that the frequency of DNA photoproducts is constant through all of the epidermis thickness of skin exposed to UVB radiation (25, 48). It therefore is most likely that the efficient UVB protection is provided by the components of the stratum corneum (urocanic acid and aromatic amino acids) and by melanin.
CPD is the major UVA-induced DNA lesion, and skin does not afford efficient photoprotection against its formation. These two features are likely worsened in terms of deleterious biological effects by the fact that CPDs are persistent DNA lesions in UVA-irradiated skin. Indeed, we observed that TT CPD remained present in a large amount in the DNA of human skin 48 h after UVA or UVB irradiations have ceased. Similar observations previously were made after exposure of skin to simulated solar radiation (35–37, 42, 43, 54). Interestingly, we found that the level of unrepaired UVA-induced TT CPD was higher than after UVB irradiation. This result is reminiscent of a similar fact observed in primary culture of skin keratinocytes (33). The effect of UVA on the repair of CPD remains to be clearly understood. Obviously, TT cyclobutane dimers are the same whether they are produced by UVA or UVB. The reduced repair capacities of UVA-irradiated cells could be explained by a different in-cell cycle arrest after irradiation (55, 56) or by a degradation of DNA repair protein by the oxidative stress induced by UVA.
Conclusion
The data obtained in the present work further emphasize the likely role played by cyclobutane pyrimidine dimers in UVA genotoxicity. Indeed, this class of damage was found to be produced in larger amounts than oxidatively generated damage. In addition, CPDs were shown to persist over a rather long period after UVA irradiation. It thus may be anticipated that part of the UVA-induced mutations are due to the presence of CPDs. This proposal is in agreement with a series of recent mutagenesis data that clearly implicate CPDs, unlike 8-oxodGuo, as major promutagenic DNA photoproducts induced by UVA radiation (14, 19, 20, 30, 57). The TT cyclobutane dimer is known to be rather poorly mutagenic. However, a number of mutations at TT sites were observed in rodent cells exposed to UVA (30). It may be added that TC and CT CPDs also are produced upon UVA irradiation of skin, although in an ≈10-fold lower yield than the TT dimer. Cytosine-containing CPDs are known to be mutagenic and the TC to TT transition is a major mutagenic event in skin tumors (2–4). It is likely that these transitions are mostly the result of UVB-induced damage, but the present results show that a UVA contribution cannot be ruled out. The observation that UVA may induce the formation of CPDs also may have some mechanistic implications into its immunosuppressive properties. Indeed, DNA damage has been shown to be involved in UVA-induced immunosuppression (58). The likely biological significance of the UVA-induced CPDs makes necessary additional work to definitively establish the underlying mechanism of formation. Determination of an action spectra in skin similar to that obtained in rodent cell line (15) should provide at least a partial answer to this question. In addition, the contribution of UVA-induced CPDs to the overall DNA damage induced by sunlight also remains to be determined within a complete terrestrial solar spectrum.
Materials and Methods
Skin Sample Preparation and Cell Culture.
Human skins were obtained immediately after breast plastic surgery from healthy donors with their inform consent (Centre Hospitalier Universitaire de Grenoble and Clinique des Alpes, Grenoble, France). All donors were Caucasian (16–62 year old), and their skin phototype was between II and III according to the Fitzpatrick classification (59). The whole skin was rinsed four times in PBS, for 30 sec in 70% ethanol, and five times again in PBS. Then, 6-mm punch biopsies were made, placed in a 35-mm Petri dish with PBS, and stored in the dark until irradiation.
For half of the donors, keratinocytes were isolated from whole skin. Briefly, to obtain primary keratinocytes, the skin, after washing with ethanol 70%, was rinsed 10 times in PBS containing 100 units/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B, and finally was immersed overnight in 0.25% trypsin at 4°C. The action of trypsin was stopped by the addition of DMEM with 10% FCS. Epidermis was detached from dermis and homogenized. After centrifugation, harvested primary keratinocytes were seeded in Keratinocyte serum-free medium supplemented with 1.5 ng/ml EGF/25 μg/ml bovine pituitary extract/75 μg/ml primocin and then cultured at 37°C in a humidified atmosphere containing 5% CO2. For all experiments, cells were used at passages 2 or 3.
UV Sources and Irradiation Procedure.
The UVB source used was a VL 215 G irradiator (Bioblock Scientific, Illkirch, France) fitted with two 15-W tubes with a spectrum distribution mostly emitting at 312 nm as described in ref. 33. The irradiance was measured by a VLX 3W radiometer (Vilbert Lourmat, Marne la Vallée, France) equipped with a 312-nm probe. The sample effectively received an average irradiance of 0.3 mW/cm2 with the lamp placed 80 cm above the irradiated targets.
The UVA source used was a Waldman UVA 700L irradiator fitted with a high pressure lamp MSR 700 (700 W) (Waldman, Villingen-Schwenningen, Germany) with an emission spectrum providing mostly photons of wavelength >330 nm as described in ref. 29. The irradiance effectively received by the sample was 40 mW/cm2.
Petri dish-containing skin biopsies were exposed immediately and lid removed to UVB radiation or on ice to UVA radiation. Just after irradiation, skin biopsies were dripped dry, frozen in liquid nitrogen, and kept at −80°C until the DNA extraction step. Sham-irradiated skin biopsies were treated similarly and kept in the dark during irradiation. To study the persistence of DNA photoproducts in human skin, three biopsies were pooled per point. Typically after irradiation with UVB (0.1 J/cm2) or UVA (100 J/cm2) radiation, PBS was replaced by fresh medium (DMEM/F12), and skin biopsies then were incubated at 37°C for increasing period.
For cultured cells irradiation, keratinocytes were seeded at 5 × 105 cells per 100-mm Petri dish (UVB irradiation) or 2 × 105 cells per 60-mm Petri dish (UVA irradiation) and grown to subconfluence during 5 days. Practically, just before irradiation, culture medium was removed, and the cells were rinsed twice with PBS. Irradiations then were performed in PBS with the lid removed at room temperature for UVB or on ice for UVA. Immediately after treatment, keratinocytes were trypsinized and recovered by centrifugation. The cell pellet then was frozen and kept at −80°C until extraction. In all cases, irradiations of whole human skin or primary keratinocytes were performed in triplicate.
DNA Extraction and Digestion.
Two different protocols were used to extract DNA from whole skin and primary keratinocytes. For human skin, after a grinding step DNA was extracted by using the DNEasy Tissue Kit obtained from Qiagen (Courtaboeuf, France). Briefly, the first step was the cold grinding of the biopsy by using a manual pestle cooled down with liquid nitrogen. The resulting powder then was recovered with 180 μl of the first lysis buffer ATL. After adding proteinase K, samples were incubated overnight at 55°C for a complete lysis of the tissue. An RNase A treatment and a second lysis step involving buffer AL were performed before loading the lysate samples onto the DNEasy mini spin column. DNA then was eluted in 200 μl of deionized water after efficient washing. The sample was freeze-dried overnight, and the resulting DNA residue was dissolved in 50 μl of a 0.1 mM deferroxiamine mesylate solution. To study the persistence of DNA photoproducts and the formation of 8-oxodGuo in human skin, the protocol was modified slightly to allow the simultaneous treatment of three and four biopsies, respectively. For this purpose, the volume of each buffer was doubled and elution was performed in two successive steps by using 200 μl of a 0.1 mM deferroxiamine mesylate solution each time. Samples then were freeze-dried overnight and dissolved into 50 μl of a 0.1 mM deferroxiamine solution for the measurement of DNA photoproducts or concentrated with a speed-vac for 8-oxodGuo analysis.
For keratinocytes, DNA extraction was performed by using a chaotropic method as reported in ref. 60. In brief, the cell pellet was efficiently homogenized by pipeting in the presence of Triton X-100 to remove the plasma membrane. Nuclei were isolated by centrifugation and made soluble by the addition of SDS. After successive treatment by RNases and proteinase, DNA was precipitated by sodium iodide and 2-propanol, and the resulting pellet was dissolved in 50 μl of a 0.1 mM deferroxiamine mesylate solution.
In all cases, DNA then was digested by incubation with 0.025 units of phosphodiesterase II/2.5 units of DNase II/0.5 units of nuclease P1 at pH 6 for 2 h at 37°C. An additional digestion step involving phosphodiesterase I and 2 units of alkaline phosphatase at pH 8 was performed. After this second digestion step, 0.1 M HCl was added, the sample was centrifuged, and then transferred into HPLC vials. The solution contained normal nucleosides and 8-oxodGuo, whereas the bipyrimidine photoproducts consisted of modified dinucleoside monophosphates. Cytosine-containing cyclobutane dimers were obtained as their uracil derivatives after fast and quantitative spontaneous deamination.
Analysis of DNA Lesions.
Analysis of bipyrimidine photoproducts.
The samples were freeze-dried overnight, and the resulting residues were made soluble in 40 μl of a 20 mM triethylammonium acetate solution just before analysis by HPLC-MS/MS. The samples were injected onto a series 1100 microHPLC system (Agilent Technologies, Massy, France) coupled to a API 3000 triple quadrupolar mass spectrometer (PerkinElmer/SCIEX, Thornhill, ON, Canada). The separation was achieved on an Uptisphere ODB octadecylsilyl silica gel column (150 × 2 mm; 3-μm particle size) from Interchim (Monluçon, France) with a gradient of acetonitrile in a 2 mM triethylammonium acetate solution. Chromatographic conditions and mass spectrometry features were as described in ref. 32. Normal nucleosides were quantified by HPLC UV with the detector set at 270 nm. The transitions used for the detection of the different bipyrimidine photoproducts were as follows: 545 → 447 for the TT cyclobutane dimer (T<>T), 545 → 432 for the TT (6-4) photoproduct [TT (6-4)], 531 → 195 for the TC and the CT cyclobutane dimers (T<>C and C<>T, respectively), 530 → 195 for the TC (6-4) photoproduct [TC (6-4)], and 517 → 195 for the CC cyclobutane dimer (C<>C).
Analysis of 8-oxodGuo.
The samples were analyzed by HPLC associated with a coulometric electrochemical detection with slight modifications of the method described in ref. 27. Briefly, the separation was performed on an Uptisphere ODB octadecylsilyl silica gel column (250 × 4.6 mm, 5 μm particle size) with an isocratic eluent (25 mM potassium phosphate, 8% methanol). The coulometric detection was provided by a Coulochem II detector equipped with a 5011 cell (ESA, Chelmsford, MA) with the potential of the two electrodes set at 200 and 450 mV, respectively. Normal nucleosides were quantified by a UV detector at the output of the HPLC column that was set at 254 nm.
The amount of each individual lesion determined in both HPLC-MS/MS and HPLC-electrochemical analyses was inferred from an external calibration obtained by injecting known amounts of the authentic compound. Similarly, the amount of analyzed DNA was determined from the area of the peak of dGuo after appropriate calibration of the UV detector.
Abbreviations
- CPD
cyclobutane pyrimidine dimmer
- 8-oxodGuo
8-oxo-7,8-dihydro-2′-deoxyguanosine
- MS/MS
tandem mass spectrometry.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
See Commentary on page 13567.
References
- 1.Cadet J, Sage E, Douki T. Mutat Res. 2005;571:3–17. doi: 10.1016/j.mrfmmm.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, Halperin AJ, Ponten J. Proc Natl Acad Sci USA. 1991;88:10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziegler A, Leffell DJ, Kunala S, Sharma HW, Gailani M, Simon JA, Halperin AJ, Baden HP, Shapiro PE, Bale AE, Brash DE. Proc Natl Acad Sci USA. 1993;90:4216–4220. doi: 10.1073/pnas.90.9.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumaz N, Drougard C, Sarasin A, Daya-Grosjean L. Proc Natl Acad Sci USA. 1993;90:10529–10533. doi: 10.1073/pnas.90.22.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stary A, Robert C, Sarasin A. Mutat Res. 1997;383:1–8. doi: 10.1016/s0921-8777(96)00041-9. [DOI] [PubMed] [Google Scholar]
- 6.Drobetsky EA, Turcotte J, Chateauneuf A. Proc Natl Acad Sci USA. 1995;92:2350–2354. doi: 10.1073/pnas.92.6.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pastila R, Leszczynski D. Photodermatol Photoimmunol Photomed. 2005;21:183–190. doi: 10.1111/j.1600-0781.2005.00156.x. [DOI] [PubMed] [Google Scholar]
- 8.de Laat A, van der Leun JC, de Gruijl FR. Carcinogenesis. 1997;18:1013–1020. doi: 10.1093/carcin/18.5.1013. [DOI] [PubMed] [Google Scholar]
- 9.Halliday GM. Mutat Res. 2005;571:107–120. doi: 10.1016/j.mrfmmm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Wang SQ, Setlow R, Berwick M, Polsky D, Marghoob AA, Kopf AW, Bart RS. J Am Acad Dermatol. 2001;44:837–846. doi: 10.1067/mjd.2001.114594. [DOI] [PubMed] [Google Scholar]
- 11.Setlow RB, Grist E, Thompson K, Woodhead AD. Proc Natl Acad Sci USA. 1993;90:6666–6670. doi: 10.1073/pnas.90.14.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasparro FP. Environ Health Perspect. 2000;108(Suppl 1):71–78. doi: 10.1289/ehp.00108s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westerdahl J, Ingvar C, Masback A, Jonsson N, Olsson H. Br J Cancer. 2000;82:1593–1599. doi: 10.1054/bjoc.1999.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agar NS, Halliday GM, Barnetson RS, Ananthaswamy HN, Wheeler M, Jones AM. Proc Natl Acad Sci USA. 2004;101:4954–4959. doi: 10.1073/pnas.0401141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kielbassa C, Roza L, Epe B. Carcinogenesis. 1997;18:811–816. doi: 10.1093/carcin/18.4.811. [DOI] [PubMed] [Google Scholar]
- 16.Pouget JP, Douki T, Richard M-J, Cadet J. Chem Res Toxicol. 2000;13:541–549. doi: 10.1021/tx000020e. [DOI] [PubMed] [Google Scholar]
- 17.Ravanat JL, Di Mascio P, Martinez GR, Medeiros MH, Cadet J. J Biol Chem. 2000;275:40601–40604. doi: 10.1074/jbc.M006681200. [DOI] [PubMed] [Google Scholar]
- 18.Sage E, Lamolet B, Brulay E, Moustacchi E, Châteauneuf A, Drobetsky EA. Proc Natl Acad Sci USA. 1996;93:176–180. doi: 10.1073/pnas.93.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kappes UP, Luo D, Potter M, Schulmeister K, Runger TM. J Invest Dermatol. 2006;126:667–675. doi: 10.1038/sj.jid.5700093. [DOI] [PubMed] [Google Scholar]
- 20.Kappes UP, Runger TM. Radiat Res. 2005;164:440–445. doi: 10.1667/rr3434.1. [DOI] [PubMed] [Google Scholar]
- 21.Tyrrell RM. Photochem Photobiol. 1973;17:69–73. doi: 10.1111/j.1751-1097.1973.tb06334.x. [DOI] [PubMed] [Google Scholar]
- 22.Kvam E, Tyrrell RM. Carcinogenesis. 1997;18:2379–2384. doi: 10.1093/carcin/18.12.2379. [DOI] [PubMed] [Google Scholar]
- 23.Perdiz D, Grof P, Mezzina M, Nikaido O, Moustacchi E, Sage E. J Biol Chem. 2000;275:26732–26742. doi: 10.1074/jbc.M001450200. [DOI] [PubMed] [Google Scholar]
- 24.Freeman SE, Ryan SL. Mutat Res. 1990;235:181–186. doi: 10.1016/0921-8777(90)90072-d. [DOI] [PubMed] [Google Scholar]
- 25.Young AR, Chadwick CA, Harrison GI, Nikaido O, Ramsden J, Potten CS. J Invest Dermatol. 1998;111:982–988. doi: 10.1046/j.1523-1747.1998.00436.x. [DOI] [PubMed] [Google Scholar]
- 26.Freeman SE, Hacham H, Gange RW, Maytum DJ, Sutherland JC, Sutherland BM. Proc Natl Acad Sci USA. 1989;86:5605–5609. doi: 10.1073/pnas.86.14.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douki T, Perdiz D, Grof P, Kuluncsics Z, Moustacchi E, Cadet J, Sage E. Photochem Photobiol. 1999;70:184–190. [PubMed] [Google Scholar]
- 28.Douki T, Reynaud-Angelin A, Cadet J, Sage E. Biochemistry. 2003;42:9221–9226. doi: 10.1021/bi034593c. [DOI] [PubMed] [Google Scholar]
- 29.Courdavault S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Mutat Res. 2004;556:135–142. doi: 10.1016/j.mrfmmm.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Rochette PJ, Therrien JP, Drouin R, Perdiz D, Bastien N, Drobetsky EA, Sage E. Nucleic Acids Res. 2003;31:2786–2794. doi: 10.1093/nar/gkg402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozmin S, Slezak G, Reynaud-Angelin A, Elie C, de Rycke Y, Boiteux S, Sage E. Proc Natl Acad Sci USA. 2005;102:13538–13543. doi: 10.1073/pnas.0504497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douki T, Cadet J. Biochemistry. 2001;40:2495–2501. doi: 10.1021/bi0022543. [DOI] [PubMed] [Google Scholar]
- 33.Courdavault S, Baudouin C, Charveron M, Canguilhem B, Favier A, Cadet J, Douki T. DNA Repair. 2005;4:836–844. doi: 10.1016/j.dnarep.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Courdavault S, Baudouin C, Sauvaigo S, Mouret S, Candeias S, Charveron M, Favier A, Cadet J, Douki T. Photochem Photobiol. 2004;79:145–151. doi: 10.1562/0031-8655(2004)079<0145:ucpddn>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 35.Young AR, Chadwick CA, Harrison GI, Hawk JL, Nikaido O, Potten CS. J Invest Dermatol. 1996;106:1307–1313. doi: 10.1111/1523-1747.ep12349031. [DOI] [PubMed] [Google Scholar]
- 36.Xu G, Snellman E, Bykov VJ, Jansen CT, Hemminki K. J Invest Dermatol. 2000;114:628–631. doi: 10.1046/j.1523-1747.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 37.Hemminki K, Xu G, Kause L, Koulu LM, Zhao C, Jansen CT. Carcinogenesis. 2002;23:605–609. doi: 10.1093/carcin/23.4.605. [DOI] [PubMed] [Google Scholar]
- 38.Bykov VJ, Marcusson JA, Hemminki K. Cancer Res. 1998;58:2961–2964. [PubMed] [Google Scholar]
- 39.Bykov VJ, Marcusson JA, Hemminki K. J Invest Dermatol. 2000;114:40–43. doi: 10.1046/j.1523-1747.2000.00821.x. [DOI] [PubMed] [Google Scholar]
- 40.Bykov VJ, Hemminki K. Carcinogenesis. 1996;17:1949–1955. doi: 10.1093/carcin/17.9.1949. [DOI] [PubMed] [Google Scholar]
- 41.Zhao C, Snellman E, Jansen CT, Hemminki K. J Invest Dermatol. 2002;118:180–184. doi: 10.1046/j.0022-202x.2001.01654.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhao C, Snellman E, Jansen CT, Hemminki K. Int J Cancer. 2002;98:331–334. doi: 10.1002/ijc.10216. [DOI] [PubMed] [Google Scholar]
- 43.Xu G, Snellman E, Jansen CT, Hemminki K. J Invest Dermatol. 2000;115:95–99. doi: 10.1046/j.1523-1747.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 44.Xu G, Snellman E, Bykov VJ, Jansen CT, Hemminki K. Mutat Res. 2000;459:195–202. doi: 10.1016/s0921-8777(99)00069-5. [DOI] [PubMed] [Google Scholar]
- 45.Bykov VJ, Sheehan JM, Hemminki K, Young AR. J Invest Dermatol. 1999;112:326–331. doi: 10.1046/j.1523-1747.1999.00523.x. [DOI] [PubMed] [Google Scholar]
- 46.Bykov VJ, Jansen CT, Hemminki K. Cancer Epidemiol Biomarkers Prev. 1998;7:199–202. [PubMed] [Google Scholar]
- 47.Young AR, Potten CS, Nikaido O, Parsons PG, Boenders J, Ramsden JM, Chadwick CA. J Invest Dermatol. 1998;111:936–940. doi: 10.1046/j.1523-1747.1998.00435.x. [DOI] [PubMed] [Google Scholar]
- 48.Chadwick CA, Potten CS, Nikaido O, Matsunaga T, Proby C, Young AR. J Photochem Photobiol B. 1995;28:163–170. doi: 10.1016/1011-1344(94)07096-7. [DOI] [PubMed] [Google Scholar]
- 49.Lhiaubet V, Paillous N, Chouini-Lalanne N. Photochem Photobiol. 2001;74:670–678. doi: 10.1562/0031-8655(2001)074<0670:coddpb>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 50.Sauvaigo S, Douki T, Odin F, Caillat S, Ravanat J-L, Cadet J. Photochem Photobiol. 2001;13:230–237. doi: 10.1562/0031-8655(2001)073<0230:AOFMPO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 51.Traynor NJ, Gibbs NK. Photochem Photobiol. 1999;70:957–959. [PubMed] [Google Scholar]
- 52.Wood SR, Berwick M, Ley RD, Walter RB, Setlow RB, Timmins GS. Proc Natl Acad Sci USA. 2006;103:4111–4115. doi: 10.1073/pnas.0511248103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Therrien JP, Rouabhia M, Drobetsky EA, Drouin R. Cancer Res. 1999;59:285–289. [PubMed] [Google Scholar]
- 54.Katiyar SK, Matsui MS, Mukhtar H. Photochem Photobiol. 2000;72:788–793. doi: 10.1562/0031-8655(2000)072<0788:koulic>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 55.Banrud H, Stokke T, Moan J, Berg K. Carcinogenesis. 1995;16:1087–1094. doi: 10.1093/carcin/16.5.1087. [DOI] [PubMed] [Google Scholar]
- 56.de Laat A, van Tilburg M, van der Leun JC, van Vloten WA, de Gruijl FR. Photochem Photobiol. 1996;63:492–497. doi: 10.1111/j.1751-1097.1996.tb03075.x. [DOI] [PubMed] [Google Scholar]
- 57.Besaratinia A, Synold TW, Chen H-H, Chang C, Xi B, Riggs AD, Pfeifer GP. Proc Natl Acad Sci USA. 2005;102:10058–10063. doi: 10.1073/pnas.0502311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuchel JM, Barnetson RS, Halliday GM. Photochem Photobiol Sci. 2005;4:577–582. doi: 10.1039/b504068j. [DOI] [PubMed] [Google Scholar]
- 59.Fitzpatrick TB. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 60.Ravanat JL, Douki T, Duez P, Gremaud E, Herbert K, Hofer T, Lasserre L, Saint-Pierre C, Favier A, Cadet J. Carcinogenesis. 2002;23:1911–1918. doi: 10.1093/carcin/23.11.1911. [DOI] [PubMed] [Google Scholar]