Abstract
The size and shape of organs depend on cellular processes such as cell proliferation, cell survival, and spatial arrangement of cells. In turn, all of these processes are a consequence of positional identity of individual cells in whole organs. Links of positional information with organ growth and pattern expression of genes is a little-addressed question. We show that differences in vestigial expression between neighboring cells of the wing blade autonomously and nonautonomously affect cell proliferation along the proximo-distal axis. On the other hand, uniform expression of vestigial inhibits cell proliferation and also perturbs the shape of wing blade altering the preferential orientation of cell divisions. Our observations provide evidence that local cell interactions, triggered by differences in vestigial expression between neighboring cells, confer positional values operating in the control of growth and shape of the wing.
Keywords: cell proliferation, organ shape, wing blade, organ growth
Organ size and shape are species-specific. Both parameters result from the coordination of cell proliferation, cell death, and arrangement of cells in specific patterns. During the last decades, our knowledge regarding the genetic basis of the cell cycle and cell survival has been greatly advanced, but the systemic relationships between gene expression patterns in cells and their proliferation only now are beginning to be established (1–5).
Cell dissociation and mixing experiments (6) have shown that the regional characteristic set of genes expressed in cells is related to their position in organs and confer differential recognition properties in pattern reconstruction (6). Regeneration experiments, on the other hand, demonstrated that intercalary growth of organs depended on discrepancies in positional identity between neighboring cells (7). Two different views have been proposed to provide cells their positional identity. An “externalistic” view suggests that the positional values of cells depend on the concentration of diffusible proteins (morphogens) received by proliferating cells from faraway sources. The role attributed to cells in the “morphogen” model is merely receptive (8–10). An alternative “internalistic” view posits that the positional values result from local cell interactions between neighboring cells in the anlage (6, 7). The differences of positional values between mutant and wild-type (WT) cells, in genetic mosaics, elicit intercalary cell proliferation to reach a normal size and pattern. The growth of organs finally ceases when differences in positional values among cells become minimal. The proliferative response around new positional references, which leads to reconstruct a normal pattern and organ size, is denominated “accommodation” (11).
The wing of Drosophila melanogaster is an experimental model to study the genetic mechanisms of organ patterning and growth. Wings have two primary orthogonal axes of growth, one antero-posterior (A/P) and the other dorso-ventral (D/V). As an outgrowth, the wing additionally shows a proximo-distal (P/D) axis, perpendicular to both A/P and D/V axes (12). The product of vestigial (vg) is a nuclear protein, which interacts with scalloped (sd) forming a transcriptional factor complex (13, 14), essential in the genetic specification of distal territories in the wing (wing blade) (15, 16). vg is symmetrically expressed from early larval development in both sides of D/V boundary, integrating inputs from the A/P (dpp signaling) and D/V (wg and N signaling) axes of growth (17–19). Recent reports (1, 20) have shown that the lack of function of the Vg–Sd complex prevents the development of the wing blade, affecting cell survival in distal wing territories. Unlike the lack of function of the Vg–Sd complex, the overexpression in vivo (1, 14, 18, 21–23) or in cell culture (1) can promote cell proliferation. These data have suggested that the Vg–Sd complex stimulate cell proliferation per se, in a constitutive manner (1).
In this report, we analyze the relationship between the distribution of Vg and the positional information of wing blade cells. Our findings indicate that the graded distribution of Vg can trigger local cell interactions between neighboring cells, globally modulating the growth and shape of the wing blade. These data favor the internalistic view to explain the control of growth and shape of organs.
Results
vestigial Is Heterogeneously Expressed Along the P/D and A/P Axes of Growth.
The complementary activation of two independent enhancers [vg D/V boundary enhancer vgBE (19) and vg quadrant enhancer vgQE (18)] defines the heterogeneous pattern of vg expression along the A/P and P/D axes of growth (17–19). Vg is expressed 60 h after egg laying (AEL), forming a symmetrical gradient at both sides of the D/V boundary (Fig. 1; see Fig. 7A, which is published as supporting information on the PNAS web site) (24). Vg expression is also detected in provein territories at higher levels than in interveins, from the middle of the third instar on (96 h AEL) (Figs. 1 and 7B). These heterogeneities in Vg distribution remain later during pupal development (Fig. 7C). This graded expression of vg suggests that it might be related to the positional information in the wing blade cells.
Fig. 1.
vg is heterogeneously expressed in the wing blade. (A) The expression of Vg (yellow) and Wg (blue) are shown in the wing blade (surface view to the left and longitudinal confocal sections to the right) in wing discs of late third instar larvae. (B) Intensity profiles of fluorescence along the P/D (Upper) and A/P (Lower) axes of growth. Yellow and blue lines correspond to vg and wg expression, respectively in B. D/V boundary and vein territories are indicated as D/V and (2–5) in A and B.
The Loss of Function of vestigial Autonomously and Nonautonomously Affects the Cell Proliferation of Wing Blade Cells in a Position-Dependent Manner.
Previous works have reported that morphogenetic mosaics defective in vg (18) or sd (25) expression grow poorly (3–5 cells) and disappear from the wing blade 24 h after clone initiation (data not shown). This finding indicates that vg expression is formally required for the survival of wing blade cells (1, 20). The cell survival and growth of mutant cells for vg were not favored in a Minute (M) genetic background either, dying quickly as it happens in a WT background (data not shown). Alternatively, we generated mutant clones for vg (vgnull has deleted all exons; ref. 20) that simultaneously express the inhibitor of cell death puckered (UAS-puc2A) (26) (Fig. 2A; see Fig. 8 A and B, which are published as supporting information on the PNAS web site). The size of these clones depends on their position along the P/D axis of growth, being recovered with higher frequency and larger size in the proximal territories of the vg expression domain (Fig. 2A). However, these clones express high levels of cell death markers (activated caspase-3) and tend to be extruded from the wing blade in larval stages (Fig. 8 A and B), thus failing to appear in the adult wing. To better modulate the lack of function of vg, we generated an RNAi construct of vg (vg-RNAi). The overexpression of vg-RNAi in either several G4 territories [distalless-G4 (dllG4md23), engrailed-G4 (enG4) (Fig. 2 B and C), and apterous-G4 (apG4md544)] or in clones diminishes the expression of vg in a dose (temperature)-dependent manner (Fig. 2D). The down-regulation of vg expression was correlated with autonomous size reductions of the wing blade (Fig. 2 B and C). These reductions were not associated with changes in cell death or cell size during imaginal development (data not shown). Interestingly, we also observe nonautonomous reductions (−13.5%) in surrounding WT territories along all axes of growth (Fig. 2 B and C) (see Discussion). Furthermore, clones of vg-RNAi frequently show (81%, n = 55) an atypical morphology, becoming narrower where the amount of vg is higher (Fig. 2D and 8 C and D). Thus, cell survival and proliferation of vg-expressing cells is closely correlated with the quantity of Vg and its pattern of expression in the wing blade.
Fig. 2.
The loss of function of vg autonomously and nonautonomously reduces growth in the wing blade in a dose and position manner. (A) Plot summarizing the size distribution of clones defective in Vg expression that simultaneously overexpress the cell death inhibitor puc. Clones are represented as red shadows in A. Notice that the size and frequency of clones diminishes in those territories expressing higher levels of vg (48–72 h AEL). (B) The overexpression of a vg-RNAi driven by dllG4md23 reduces the size of the wing blade along the axes of growth in a dose-dependent manner (increasing reductions with increase of temperature). (C) The overexpression of a vg-RNAi construct driven by enG4 (red wing) reduces the WT size of the wing (blue wing), autonomously (posterior compartment) and nonautonomously (anterior compartment, −13.5%). (D) The overexpression clones of a vg-RNAi construct (green) autonomously diminish the expression of Vg (red) and show an atypical triangular shape (48–72 h AEL) (these clones are narrower when approaching the wing margin). Wg expression (blue) reveals the D/V boundary of the wing and delimits wing blade territories.
The Homogenous Expression of vg Autonomously and Nonautonomously Reduces the Cell Proliferation in the Wing Blade in a Dose-Dependent Manner.
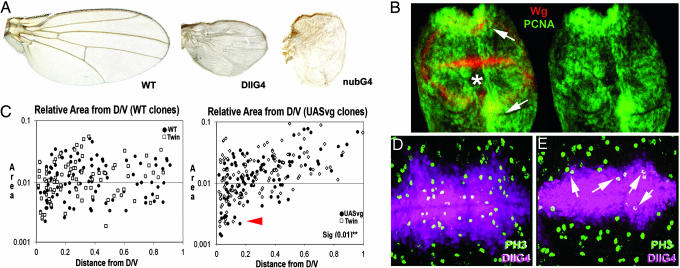
We investigated the capacity of vg to promote cell proliferation (1), driving the transgene UAS-vestigial in either different Gal4 territories of the wing or in genetic mosaics. Surprisingly, the regional overexpression of vg (under the control of apG4md544, nubbin-G4 (nubG4K), spalt-G4EPv (salG4EPv), and dllG4md23) reduces the size of the wing blade in an autonomous and nonautonomous manner (Fig. 3A; see Fig. 9A, which is published as supporting information on the PNAS web site) (17). In these experiments, FACS and adult measurements of cell size were not significantly different from wild type (data not shown). Cell death does not explain reductions of the wing size either (e.g., 44% in salG4EPv/UAS-vg) because the expression of activated Caspase-3 does not differ from wild type (data not shown), and the inhibition of cell death (overexpressing UAS-puc2A, UAS-P35, or in a mutant background DfH99) only slightly rescues wing growth (7.4%) (data not shown). Thus, reductions in wing size are mainly due to a lower cell proliferation such as it happens in the loss of function of vg.
Fig. 3.
Cell proliferation in the wing blade is maintained in response to heterogeneities of vg expression between neighboring cells. (A) The overexpression of vg under the control of several G4 at 25°C alters the growth and shape of the wing blade. The growth of the wings is strongly reduced in the experimental conditions shown in A. (B) The expression of the reporter proliferating cell nuclear antigen (PCNA) (green) is down-regulated in the wing blade (asterisk) but is up-regulated in the wing hinge (arrows) when vg is overexpressed under the control of ptcG4 (ptcG4 is expressed in a narrow band of anterior cells along the A/P compartment border). Wg expression (red) reveals the D/V boundary of the wing and delimits wing blade territories in B. (C) Size distribution of either WT clones (Left, ●) or vg-expressing clones (Right, ●) and their associated twins (□) in the wing along the P/D axis. The increment of area is represented in y axis of the graphs, whereas the distance of clones and twins to the D/V boundary is shown in the x axis in C. Unlike the WT clones and their twins, the size of vg-expressing clones and their twins is increased when far away from the D/V boundary and also are smaller than controls near the wing margin (red arrowhead). (D) Mitoses (green, PH3) in the WT dllG4md23 domain (purple). (E) Mitoses (green, PH3) in a mutant background dllGmd23/UASvg preferably appear in cells confronting different levels of vg (arrows). dllG4md23 domain is shown in purple.
We evaluated the effects of vg on cell proliferation in the wing blade comparing the area and the expression of cell proliferation reporters [BrdU, proliferating cell nuclear antigen (PCNA), and PH3] between vg-overexpressing cells and WT cells. The overexpression of vg in wing blade territories autonomously reduces the area (Fig. 3A) and the expression of cell proliferation reporters (Figs. 3 B, D, and E and 9 A, E, and F; see also Fig. 10 A and B, which is published as supporting information on the PNAS web site) (details regarding density of mitotic figures are published as Supporting Text, section iii, which is published as supporting information on the PNAS web site). Cell proliferation also diminishes in a dose-dependent manner because the lack of growth is strongly restored at 17°C (Fig. 9A). The most prominent reduction in size is visible along the P/D axis of growth (Fig. 3A).
In contrast to the wing blade, vg-expressing cells in proximal wing territories (wing hinge) causes tubular outgrowths that are composed by mutant and wild type surrounding cells (Fig. 9 B–D) (23). The overgrowths in the wing hinge are correlated with an autonomous and nonautonomous increment of mitosis (Fig. 10A), proliferating cell nuclear antigen (PCNA) expression (Fig. 3B) and BrdU incorporation (Fig. 10C). These findings indicate that the autonomous and nonautonomous effects of vg on cell proliferation depend on the positional location along the P/D axis.
The Differences in the Amount of Vg Between Neighboring Cells Locally Stimulates Cell Proliferation in the Wing Blade.
To quantitatively evaluate the autonomous and nonautonomous effects of cells overexpressing vg along the axes of growth, we carried out an analysis of twin clones (48–72 h AEL) by using the system FRT/Gal80 (MACRM system). The area of vg-expressing clones increases proportionally with the distance to the wing margin in a continuous manner (Figs. 3C and Fig. 9B), confirming data of Liu et al. (25). Interestingly, the same variation in size is detected in their associated twin clones (Figs. 3C and Fig. 9B). These data suggest that differences in vg expression between neighboring cells drive cell proliferation, autonomously and nonautonomously, in the wing blade along the P/D axis (see discussion and Fig. 11, which is published as supporting information on the PNAS web site). This conclusion is reinforced by data obtained expressing vg under the control of dllG4md23. dllG4md23 expression does not reach more proximal cells of vg expression domain, thus allowing the confrontation of juxtaposed cells with different levels of Vg (Fig. 3 D and E). In mutant territories, mitoses and proliferating cell nuclear antigen (PCNA) expression are preferably located in mutant cells in contact with or next to WT cells (Figs. 3 D and E and 9 E and F).
Contrary to what happens in the P/D axis, the growth of vg-expressing clones and associated twins is not affected by their position along the A/P axis (data not shown). In this axis, the heterogeneous pattern of vg possibly appears later in development or is related to vein differentiation. In fact, vg-expressing clones in adult wings tend to appear on vein territories (Fig. 9C).
The Effect of Vg on Cell Proliferation Is Associated to Changes in the Length of the Cell Cycle.
How are the different phenotypes of vg on growth correlated with the cell cycle? In accordance with FACS analyses obtained by Delanoue et al. (1) with enG4, the overexpression of vg in the apG4md544 or enG4 domains accelerates the G1/S transition (Fig. 12A, which is published as supporting information on the PNAS web site). However, these authors bypassed the fact that (i) G2/M transition also is delayed in these experiments (Fig. 12 A–C) and (ii) vg has differential effects on growth in different territories of the wing discs (see above). Thus, we overexpressed vg only in the wing blade under the control of dllG4md23 or salG4EPv. Paradoxically, dllG4md23 and salG4EPv FACS experiments revealed the same profile of cell cycle (Fig. 12 B and C) than apG4md544 and enG4 experiments. Two conclusions can be drawn from these results. (i) The excess or lack of growth is not explained by the effect of vg on a particular regulatory point of cell cycle (G1/S transition) because compensatory mechanisms retard the G2/M phasing. (ii) The control of growth is not cell autonomously determined but rather related to cell interactions between mutant and WT surrounding cells as in normal development (see Discussion).
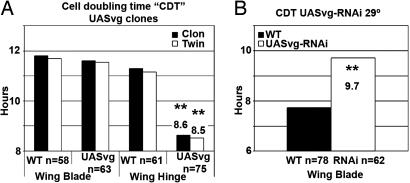
How is the length of the whole cell cycle modified in conditions of the lack or excess of function of vg? We calculated the cell doubling time of vg-expressing clones and twins (60 AEL) located in the wing hinge or the wing blade territories. Interestingly, outgrowths of wing hinge were correlated with a higher rate of cell proliferation and a shorter cell doubling time (Fig. 4A). On the contrary, the lack of function of vg in the wing blade increases the length of cell cycle (Fig. 4B). These findings suggest that the lack or excess of growth is a consequence of changes in the whole length of cell cycle, not in any particular cell cycle transition.
Fig. 4.
The heterogeneous expression of vg in the wing blade modulates the whole length of cell cycle. The cell doubling time (CDT) (hours in the y axis of the graphs) corresponding to either vg-expressing cells and surrounding WT cells (A) or cells defective in vg expression (B). (A) vg-expressing clones and their associated twins in the wing hinge show the CDT shortened compared with controls and also in respect to clones appearing in the wing blade. (B) Loss-of-function clones of vg in the wing blade (cells expressing the vg-RNAi construct) show increased CDT compared with controls.
The Heterogeneous Distribution of Vg Favors Orientated Cell Division Along the P/D Axis.
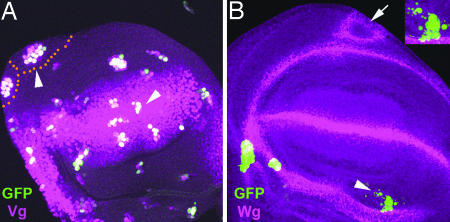
The shape of the wing has been correlated with the preferential orientation of cell divisions along the P/D axis of growth (27). To analyze the possible links between positional identity and the orientation of cell division, we performed a cell lineage study in a genetic background overexpressing vg under the control of several G4 lines (salG4EPv, apG4md544, and nubG4K). In these mutant backgrounds, cell markers reveal that clones grow abnormally with respect to the wild type (compare Fig. 5A and B), and the orientation of cell divisions is altered (Fig. 5C), leading to rounded wings. These findings explain why the homogenous expression of vg perturbs the shape of the wing (see Discussion).
Fig. 5.
The heterogeneous expression of vg favors the orientation of cell division in the wing blade along P/D axis. (A) Clonal growth (green) in a WT background is orientated preferentially along the P/D axis (arrow). (B) Clones (green) in a mutant background overexpressing vg (nubG4K/UASvg) fail to grow preferentially along the P/D axis (arrowheads). Wg expression defines wing blade territories (purple in A and B). (C) Graph summarizing the angle of cell division in respect to the D/V boundary (x axis) in either a WT wing blade (black bars) or in a mutant background (gray bars) overexpressing vg (nubG4K/UASvg). Notice that the orientation of cell division is perturbed in a mutant background overexpressing vg (nubG4K/UASvg) (gray bars) in respect to controls (black bars).
Wg Signaling Acts only as a Cell Survival Factor in the Late Stages of Wing Blade Development.
Wg signaling together with other signaling pathways leads to define the expression of vg in the wing blade (17), although its overexpression alone does not increase cell proliferation (2). However, we wondered whether the effects on growth associated with vg expression were independent of Wg signaling. Thus, we generated cell mutants for both Wg receptors [frizzled1 (fz1) and Dfrizzled2 (Dfz2)] that simultaneously overexpress vg (fz1Dfz2-UASvg mutant clones). Forty-eight hours after heat shock, fz1Dfz2-UASvg mutant clones autonomously showed the same profile of size distribution than only vg-expressing clones (Fig. 6A). Seventy-two hours after heat shock, however, fz1Dfz2-UASvg mutant cells disappear after cell death (Fig. 6B Inset) from the wing epithelium. Interestingly, the nonautonomous effects on growth and patterning of fz1Dfz2-UASvg clones were similar to those of vg-expressing clones (Fig. 6B). These observations suggest that Wg signaling only is required to the cell survival of vg-expressing cells, leaving to vg expression the observed effects on cell proliferation along the P/D axis (see Discussion).
Fig. 6.
The expression of Wg is required only to the cell survival of cells expressing vg. Shown are mutant clones for both receptors of Wg (fz1 and Dfz2) that simultaneously overexpress vg (green) (fz1Dfz2-UASvg). (A) The size of fz1Dfz2-UASvg clones in the wing is increased when they appear far away from the D/V boundary 48 h after heat-shock induction. (B) fz1Dfz2-UASvg clones disappear from the wing epithelium 72 h after heat shock. Nuclear GFP is fragmented as a reporter of cell death (arrowhead and B Inset). Nonautonomous effects on growth and patterning of fz1Dfz2-UASvg clones are approximately encircled with orange spots in A and also are indicated with a white arrow in B (notice the outgrowths in the wing discs). Vg and Wg expression are reported in purple in A and B, respectively.
Discussion
We have focused in this report on the role of patterning genes, such as vg, in the control of cell proliferation of the wing to reach a normal size. The graded expression pattern of vg in the wing blade (Figs. 1 and 11A) prompted us to investigate its relationship with cell proliferation and positional identity in the wing disk.
The homogenous expression of vg (by lack or excess of function) notably reduces the growth of the wing blade without changes in the cell size or rate of cell death during larval development (Figs. 2B and 3A). However, cell proliferation is locally maintained when different levels of vg between neighboring cells are confronted (Fig. 3E). These findings suggest that the homogenous rate of clone size throughout the wing blade (28, 29) requires local cell interactions between neighboring cells, and it is not cell autonomously determined by the amount of vg expression in cells. These observations clearly indicate that the autonomous expression of Vg–Sd complex does not stimulate cell proliferation per se.
Discrepancies in vg expression experimentally induced between neighboring cells can autonomously and nonautonomously (accommodation) affect the wing blade growth in a position-dependent manner (Figs. 2C and 3C). Thus, vg-expressing clones autonomously show extra cell proliferation and outgrowths when they appear in the wing hinge (refs. 23, 25, and 30; Figs. 3C and 11C); i.e., territories of the wing farther away from cells expressing high levels of vg. In contrast to examples of extra cell proliferation, reductions in size of the wing blade are observed in loss of function mosaics of vg (Fig. 2C and 11B). All of these effects on growth are continuously graded along the P/D axis in correlation with Vg distribution (Figs. 3C and 11A). These observations support the notion that vg expression is involved with a system of positional values, which is required to drive the growth in the wing blade. The effects on growth of Vg–Sd complex along the A/P axis are much less manifest, because the heterogeneities in its expression pattern possibly appear late during proliferation stages of wing discs.
Gain or loss of function mosaics of vg have also nonautonomous effects. Interestingly, the whole wing also can be reduced even though the rate of cell proliferation is locally maintained in confronted cells expressing different levels of vg (Figs. 2C and 3A). We interpret this result as a consequence of the following parameters being affected. (i) Significant discrepancies of positional values (or vg expression) between neighboring cells fail to exist. (ii) Confronted populations of cells in mosaics are unable to reach their maximal positional values. (iii) Confronted cells exchange their positional information in a continuous and graded manner. In the loss-of-function mosaics of vg, mutant cells never achieve the maximal positional value (maximal expression of vg), nonautonomously diminishing also the reference of positional values to neighboring cells and, hence, to the whole wing (see model in Fig. 11B). In these mosaics, the exchange of positional information apparently is perturbed, and mutant and WT territories are unable to intercalate the full scale of positional values corresponding to the WT situation, thus reducing the size of the whole wing. On the other hand, vg-overexpressing clones in distal territories of the wing blade show nonautonomously reduced cell proliferation because mutant and surrounding cells do not have significant discrepancies in positional values. Conversely, outgrowths of the wing hinge appear in response to vg-expressing cells because the cell proliferation in the mutant cell is locally maintained and surrounding cells intercalate higher values up to a continuous landscape of positional values (Fig. 11C). According to these interpretations, the proliferation is stimulated by cell interactions between neighboring cells (Fig. 11D), but the growth of organs is controlled globally by the full scale of positional values locally exchanged between neighboring cells (31).
Wg signaling is required to define the expression of vg (17) and, therefore, we analyzed the contribution of Wg signaling in phenotypes of vg-expressing clones. The conclusion of these experiments (Fig. 6) is that Wg signaling does not contribute to cell proliferation but acts as a cell survival factor in vg-expressing cells (wing blade cells) (2). In light of all these results, the notion and role of Wg as a morphogen perhaps should be revised as it was highlighted in ref. 32. On the other hand, they favor the internalistic view (Entelechia model; ref. 33) to explain the local control of growth in the wing blade. In this model, the heterogeneous expression of vg might be instrumental in specifying positional values, at least along the P/D axis (see model in Fig. 12D), leading to the intercalar growth (33). The molecules involved in the control of short-range signals to exchange positional information between neighboring cells remain unknown; however, they should be able to transform the positional value associated with the amount of Vg in the nucleus to neighboring cells, eliciting cell division and providing intermedial positional values (Fig. 11D).
The differential effects of Vg–Sd complex on growth are not correlated with changes in a single regulatory point of cell cycle (ref. 34; Fig. 12) but on changes in the whole length of the cell cycle (Fig. 4; ref. 35). These findings indicate that the length of cell cycle is not cell autonomously determined by the amount of Vg–Sd but, rather, is likely related to local signaling between neighboring cells. This hypothesis agrees with the fact that cells nonclonally related form clusters of cells in the same phase of the cell cycle throughout the wing blade (36).
The shape of the wing blade is strongly determined by the orientation of cell divisions (27). Somehow, the heterogeneous expression of vg contributes to polarize these orientations along the P/D axis (Figs. 5 and 11D) because the homogenous expression of vg can randomize the characteristic WT orientations. It is difficult to propose molecular mechanisms to control the orientation of cell divisions; however, adhesion molecules might be implicated, affecting cell affinity and cell recognition properties. Supporting this hypothesis, we know that vg modulates the level of dachsous (ds) in the membrane of wing blade cells (unpublished data), and ds expression participates in the preferential orientation of cell divisions (27). Taken as a whole, these results indicate that the orientation of cell division is intimately related to the mechanisms at work in positional identity specification (Fig. 11D).
Materials and Methods
Fly Strains.
All fly strains are described at http://flybase.bio.indiana.edu unless otherwise indicated. The salG4EPv [a gift from C. Cruz (Consejo Superior de Investigaciones Cientificas, Madrid, Spain) and R. Barrio (Centro de Investigacíon Cooperativa en Biociencias, Derio, Spain)] was constructed from salEPv enhancer (37), and its expression is restricted to sal territories in the wing. Crosses were carried out at 25°C unless otherwise indicated. All G4 lines are expressed from the middle second larval instar, except for salG4EPv and dllG4md23, which start in early third larval instar.
Histology.
BrdU labeling and inmunostainings of imaginal discs were performed according to standard protocols (details are published in Supporting Text, section v).
Genotypes and Mosaic Analysis.
y w hsp70-flp, Act FRT y+ FRT G4 UAS-GFP/UAS-vg73 (for imaginal and pupal phenotypes) (Fig. 9); y f36a FLP122, abx/Ubx FRT f+ FRT G4 UAS lacZ/UAS-vg73 (for adult phenotypes); y w hsp70-flp FRT42D Minute arm-lacZ/FRT42D vgnull (data not shown); y w hsp70-flp Tubulin α1-G4 UAS-GFPnls, UAS-vg73, Tubulin α1-Gal80 FRT2A/Ubi-GFP FRT2A in (Figs. 3 and 9); y w hsp70-flp Tubulin α1-G4 UAS-GFPnls, UAS-vg73, Tubulin α1-Gal80 FRT2A/fz1H51 Dfz2C1 FRT2A (Fig. 6); y w hsp70-flp Tubulin α1-G4 UAS-GFPnls, FRT42D Tubulin α1-Gal80/FRT42D vgnull, UAS-puc2A or + in (Figs. 2 and 8); f36a, f+44A f+52 M (2)l2 (our stock collection)/FRT42D vgnull (data not shown); y w hsp 122-flp, (nubG4K, salG4EPv, or apG4md544)/Act-FRT w+ FRTlacZ, UAS-vgZ in (Fig. 5). y w hsp70-flp Tubulin α1-G4 UAS-GFPnls, UAS-vg73, Tubulin α1-Gal80 FRT2A/fz1 Dfz2 FRT2A (Fig. 6). Specific details regarding conditions of mosaic analysis are published in Supporting Text, section i.
Generation of UASvg-RNAi.
The second exon of vg was amplified from a genomic sequence to generate the UASvg-RNAi transgene by standard procedures. Details regarding the construction are published in Supporting Text, section ii.
Measurements of Clones.
Individual clones and twins were traced and their perimeters, areas, and centroids were calculated by using the ImageJ 3.2 program (Figs. 3 and 9). We also measured the shortest distance from the centroid of each clone to the center of the D/V or A/P stripe of Wg or Ptc expression. The area and diameter along the A/P or D/V compartment boundary within the inner ring of Wg hinge expression were measured in each wing disk, averaging all parameters. To normalize variation of sizes and distances, the area and location of each clone and twin were considered as relative area (area of the clone/average of area encircled in the inner ring of Wg) or relative distance of the clone to D/V or A/P (distance of the clone to D/V or A/P border/diameter of the inner ring of Wg along the A/P or D/V boundaries). A logarithmic scale was chosen to plot relative clone and twin size. Two independent experiments were carried out taking data from 25 wing discs. Clones and twins were initiated (48–72 AEL) and fixed at the end of the third instar (115 h AEL). At least 20 planes per imaginal disk were taken for measurements.
Flow Cytometry.
Cell size (Fig. 12) and cell doubling time (Fig. 4) were performed as described in refs. 2 and 38, and details are published in Supporting Text, section iv. The orientation of cell division was measured as described in ref. 27. Cell size in adult wings was measured as described in ref. 31.
Supplementary Material
Acknowledgments
We thank A. Martínez-Arias and L. Johnston for helpful discussion and comments; J. F. de Celis, A. Baonza, and other members of A.G.-B.'s laboratory for their constructive criticisms; C. Cruz, R. Barrio, B. Edgar (Fred Hutchinson Cancer Research Center, Seattle, WA), J. Silber (University of Cambridge, Cambridge, U.K.), C. O'Kane (University of Paris, Paris, France), G. Struhl (Columbia University College of Physicians and Surgeons, New York, NY), G. Halder (University of Texas MD Anderson Cancer Center, Houston, TX), E. Martín-Blanco (Consejo Superior de Investigaciones Cientificas, Barcelona, Spain), I. Guerrero (Consejo Superior de Investigaciones Cientificas, Madrid, Spain), S. Carroll (University of Wisconsin, Madison, WI), M. Affolter (University of Basel, Basel, Switzerland), and Developmental Studies Hybridoma Bank for providing fly strains and antibodies; and R. Hernández for her skilful technical assistance. This work was supported by grants from the Dirección General de Investigación Científica y Técnica and an institutional grant from the F. Ramón Areces to the Centro de Biología Molecular Severo Ochoa. L.A.B-L. is a Fellow of Consejo Superior de Investigaciones Científicas in collaboration with PACISA-GIRALT (I3P-BPD2002-1).
Abbreviations
- AEL
after egg laying
- A/P
antero-posterior
- D/V
dorso-ventral
- P/D
proximo-distal.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Delanoue R, Legent K, Godefroy N, Flagiello D, Dutriaux A, Vaudin P, Becker JL, Silber J. Cell Death Differ. 2004;11:110–122. doi: 10.1038/sj.cdd.4401321. [DOI] [PubMed] [Google Scholar]
- 2.Johnston LA, Sanders AL. Nat Cell Biol. 2003;5:827–833. doi: 10.1038/ncb1041. [DOI] [PubMed] [Google Scholar]
- 3.Giraldez AJ, Cohen SM. Development (Cambridge, UK) 2003;130:6533–6543. doi: 10.1242/dev.00904. [DOI] [PubMed] [Google Scholar]
- 4.Duman-Scheel M, Johnston LA, Du W. Proc Natl Acad Sci USA. 2004;101:3857–3862. doi: 10.1073/pnas.0400526101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogulja D, Irvine KD. Cell. 2005;123:449–461. doi: 10.1016/j.cell.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 6.García-Bellido A. Dev Biol. 1966;14:278–306. doi: 10.1016/0012-1606(66)90017-0. [DOI] [PubMed] [Google Scholar]
- 7.Bohn H. J Embryol Exp Morphol. 1974;32:81–98. [PubMed] [Google Scholar]
- 8.Wolpert L. J Theor Biol. 1969;25:1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence P. J Exp Biol. 1966;44:607–620. doi: 10.1242/jeb.44.3.507. [DOI] [PubMed] [Google Scholar]
- 10.Stumpf H. Nature. 1966;212:133–138. doi: 10.1038/212430a0. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Bellido A, Cortes F, Milan M. Proc Natl Acad Sci USA. 1994;91:10222–10226. doi: 10.1073/pnas.91.21.10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blair SS. BioEssays. 1995;17:299–309. doi: 10.1002/bies.950170406. [DOI] [PubMed] [Google Scholar]
- 13.Halder G, Carroll SB. Development (Cambridge, UK) 2001;128:3295–3305. doi: 10.1242/dev.128.17.3295. [DOI] [PubMed] [Google Scholar]
- 14.Halder G, Polaczyk P, Kraus ME, Hudson A, Kim J, Laughon A, Carroll S. Genes Dev. 1998;12:3900–3909. doi: 10.1101/gad.12.24.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams JA, Bell JB, Carroll SB. Genes Dev. 1991;5:2481–2495. doi: 10.1101/gad.5.12b.2481. [DOI] [PubMed] [Google Scholar]
- 16.Williams JA, Paddock SW, Carroll SB. Development (Cambridge, UK) 1993;117:571–584. doi: 10.1242/dev.117.2.571. [DOI] [PubMed] [Google Scholar]
- 17.Klein T, Martínez-Arias A. Development (Cambridge, UK) 1999;126:913–925. doi: 10.1242/dev.126.5.913. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Sebring A, Esch JJ, Kraus ME, Vorwerk K, Magee J, Carroll SB. Nature. 1996;382:133–138. doi: 10.1038/382133a0. [DOI] [PubMed] [Google Scholar]
- 19.Klein T, Martínez-Arias A. Dev Biol. 1998;194:196–212. doi: 10.1006/dbio.1997.8829. [DOI] [PubMed] [Google Scholar]
- 20.Van de Bor V, Delanoue R, Cossard R, Silber J. Cell Death Differ. 1999;6:557–564. doi: 10.1038/sj.cdd.4400517. [DOI] [PubMed] [Google Scholar]
- 21.Simmonds AJ, Liu X, Soanes KH, Krause HM, Irvine KD, Bell JB. Genes Dev. 1998;12:3815–3820. doi: 10.1101/gad.12.24.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paumard-Rigal S, Zider A, Vaudin P, Silber J. Dev Genes Evol. 1998;208:440–446. doi: 10.1007/s004270050201. [DOI] [PubMed] [Google Scholar]
- 23.Baena-Lopez LA, Garcia-Bellido A. Development (Cambridge, UK) 2003;130:197–208. doi: 10.1242/dev.00187. [DOI] [PubMed] [Google Scholar]
- 24.Williams JA, Bell JB, Carroll SB. Genes Dev. 1991;5:2481–2495. doi: 10.1101/gad.5.12b.2481. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Grammont M, Irvine KD. Dev Biol. 2000;228:287–303. doi: 10.1006/dbio.2000.9939. [DOI] [PubMed] [Google Scholar]
- 26.Adachi-Yamada T, Fujimura-Kamada K, Nishida Y, Matsumoto K. Nature. 1999;400:166–169. doi: 10.1038/22112. [DOI] [PubMed] [Google Scholar]
- 27.Baena-López LA, Baonza A, García-Bellido A. Curr Biol. 2005;15:1640–1644. doi: 10.1016/j.cub.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 28.Resino J, Salama-Cohen P, García-Bellido A. Proc Natl Acad Sci USA. 2002;99:7502–7507. doi: 10.1073/pnas.072208199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.García-Bellido A, Merriam JR. Dev Biol. 1971;24:61–87. doi: 10.1016/0012-1606(71)90047-9. [DOI] [PubMed] [Google Scholar]
- 30.Kolzer S, Fuss B, Hoch M, Klein T. Development (Cambridge, UK) 2003;130:4135–4147. doi: 10.1242/dev.00608. [DOI] [PubMed] [Google Scholar]
- 31.Resino J, García-Bellido A. Mech Dev. 2004;121:351–364. doi: 10.1016/j.mod.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Martínez-Arias A. Nat Rev Mol Cell Biol. 2003;4:321–325. doi: 10.1038/nrm1078. [DOI] [PubMed] [Google Scholar]
- 33.García-Bellido A, García-Bellido A. Int J Dev Biol. 1998;42:353–362. [PubMed] [Google Scholar]
- 34.Delanoue R, Legent K, Godefroy N, Flagiello D, Dutriaux A, Vaudin P, Becker J, Silber J. Cell Death Differ. 2004;11:110–122. doi: 10.1038/sj.cdd.4401321. [DOI] [PubMed] [Google Scholar]
- 35.Reis T, Edgar BA. Cell. 2004;117:253–264. doi: 10.1016/s0092-8674(04)00247-8. [DOI] [PubMed] [Google Scholar]
- 36.Milan M, Campuzano S, Garcia-Bellido A. Proc Natl Acad Sci USA. 1996;93:11687–11692. doi: 10.1073/pnas.93.21.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrio R, De Celis JF. Proc Natl Acad Sci USA. 2004;101:6021–6026. doi: 10.1073/pnas.0401590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neufeld TP, de la Cruz AF, Johnston LA, Edgar BA. Cell. 1998;93:1183–1193. doi: 10.1016/s0092-8674(00)81462-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.