Abstract
Gaucher disease is a lysosomal storage disorder caused by deficiency in lysosomal acid β-glucosidase (GlcCerase), the enzyme responsible for the catabolism of glucosylceramide. One of the most prevalent disease-causing mutations, N370S, results in an enzyme with lower catalytic activity and impaired exit from the endoplasmic reticulum. Here, we report that the iminosugar isofagomine (IFG), an active-site inhibitor, increases GlcCerase activity 3.0 ± 0.6-fold in N370S fibroblasts by several mechanisms. A major effect of IFG is to facilitate the folding and transport of newly synthesized GlcCerase in the endoplasmic reticulum, thereby increasing the lysosomal pool of the enzyme. In addition, N370S GlcCerase synthesized in the presence of IFG exhibits a shift in pH optimum from 6.4 to 5.2 and altered sensitivity to SDS. Although IFG fully inhibits GlcCerase in the lysosome in an in situ assay, washout of the drug leads to partial recovery of GlcCerase activity within 4 h and full recovery by 24 h. These findings provide support for the possible use of active-site inhibitors in the treatment of some forms of Gaucher disease.
Keywords: lysosome, pharmacological chaperone, lysosomal storage disease
Gaucher disease is a lysosomal storage disorder caused by mutations in acid β-glucosidase (GlcCerase; EC 3.2.1.45), the enzyme responsible for the catabolism of glucosylceramide to ceramide and glucose (1, 2). Deficiency of GlcCerase activity results in the progressive accumulation of glucosylceramide, primarily within macrophages, and it ultimately leads to clinical manifestations of anemia, hepatosplenomegaly, bone lesions, and, in more severe cases, central nervous system impairment (3). The disease is classified clinically into three types based on the age of onset and the degree of neurological involvement. Type 1 Gaucher disease is characterized by adult onset and the absence of neurological symptoms, whereas patients with type 2 or 3 disease experience juvenile or infantile onset and exhibit mild to severe neurological impairment.
Although >200 different mutations in GlcCerase have been reported, the N370S and L444P missense mutations represent the most prevalent ones in the Western hemisphere, with the former found in 70% of Ashkenazi Jews with the disease (3–6). The N370S mutation is associated with type 1 disease, and it results in an enzyme with lower catalytic activity (3–16% of wild type) and impaired exit from the endoplasmic reticulum (ER) (7–9).
Currently, enzyme-replacement therapy (ERT) and substrate-reduction therapy are the only approved treatment options for patients with Gaucher disease (3). ERT improves the visceral and hematologic manifestations of the disease, but it is costly and not effective in neuronopathic forms of Gaucher disease because of the inability of recombinant enzymes to cross the blood–brain barrier (10). Miglustat (N-butyldeoxynojirimycin), an iminosugar inhibitor of glucosylceramide synthase, also produces clinical improvement in selected patients with mild or moderate disease, but the responses are slower and less robust than those observed with ERT (11–14). Its administration is associated with significant side effects, although they tend to be transient (11). The application of gene therapy for Gaucher disease is still in its initial stages of development (3). Thus, a need exists for the generation of alternative therapeutic options.
The observation that many lysosomal enzyme variants, including the GlcCerase variant N370S, exhibit impaired biosynthesis but retain substantial catalytic activity has prompted the hypothesis that small-molecule active-site inhibitors might facilitate proper folding and trafficking of these lysosomal enzymes, thus increasing their activity in cells to a level sufficient to avoid or alleviate disease. The ability of several glucose and galactose analogs to increase the activity of the defective enzymes in Gaucher and Fabry disease cells, respectively, has been recently demonstrated by several groups (15–20). Although the increased enzyme activity is believed to result from improved folding and trafficking of the mutant enzymes, insight into the actual mechanisms whereby these small molecules exert their action is limited. In this study, we have examined the effect of the iminosugar isofagomine (IFG) on the synthesis, stability, and catalytic properties of the GlcCerase mutant N370S. We present evidence that IFG increases GlcCerase activity by several mechanisms.
Results
GlcCerase Activity in N370S Fibroblasts Is Enhanced by IFG Treatment.
In our initial experiments, 20 iminosugar derivatives were incubated with patient fibroblasts for 3–5 days followed by assays of cell lysates for GlcCerase activity. Five of the compounds gave an ≈2-fold increase in GlcCerase activity, and they were further profiled. All were potent inhibitors of GlcCerase. The compound IFG (Fig. 1A) was chosen as the lead compound based on its high solubility in water; its oral availability and wide tissue distribution, including the brain, in mice; and its lack of acute toxicity in mice at high doses (data not shown). The effect of 10–100 μM IFG on GlcCerase activity in N370S fibroblasts is summarized in Table 1. After 3 days of treatment with 30 or 100 μM IFG, GlcCerase activity was 2.2- to 2.4-fold higher compared with untreated cells. IFG treatment for 5 days led to an even greater enhancement (2.4- to 3.0-fold) over the untreated level. Optimal enhancement was observed at IFG concentrations of 30 μM. Interestingly, GlcCerase activity was also enhanced in normal fibroblasts but to a much lesser extent (1.3 ± 0.3-fold) after treatment with 30 μM IFG for 5 days (Table 1).
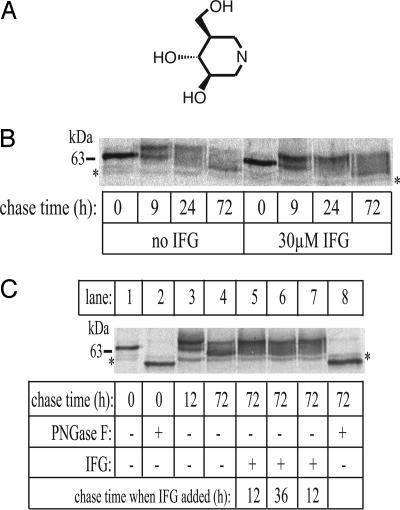
Fig. 1.
Effect of IFG on N370S GlcCerase biosynthesis and trafficking. (A) Molecular structure of IFG. (B) Cells were cultured for 5 days with or without 30 μM IFG before labeling with 750 μCi of [35S]methionine/[35S]cysteine for 1 h. After labeling medium was replaced with normal growth medium, cells were harvested at the indicated times, and the GlcCerase was immunoprecipitated and analyzed by SDS/PAGE and autoradiography, as described in Methods. (C) Cells cultured in the absence of IFG were labeled with 750 μCi of [35S]methionine/[35S]cysteine for 1 h. After the labeling medium was replaced with normal growth medium, the cells were chased for various times. In lanes 5–7, IFG was added to a final concentration of 30 μM at the chase times indicated. Newly synthesized GlcCerase molecules were immunoprecipitated and treated with (lanes 2 and 8) or without peptide:N-glycosidase F (PNGase F) (lanes 1, 3–7) to remove all N-glycans before SDS/PAGE and autoradiography. The asterisks in B and C denote nonspecific bands.
Table 1.
Effect of IFG on GlcCerase activity
| Cell type | [IFG], μM | Treatment time, days | Fold enhancement over untreated cells∗ |
|---|---|---|---|
| N370S | 10 | 5 | 2.4 ± 0.2 |
| 30 | 3 | 2.4 ± 0.2 | |
| 5 | 3.0 ± 0.6 | ||
| 100 | 3 | 2.2 ± 0.1 | |
| 5 | 2.6 ± 0.2 | ||
| Wild type | 30 | 5 | 1.3 ± 0.3 |
GlcCerase activity was measured in fibroblast lysates after treatment with IFG for 3 or 5 days. The GlcCerase activity of untreated N370S fibroblasts was 2.8 ± 0.5% of that measured in wild-type fibroblasts. Data shown are the mean ± SD of at least three experiments.
∗, P < 0.01 for all values.
IFG Inhibits GlcCerase at Neutral and Acidic pH.
We next sought to understand the mechanisms whereby IFG treatment results in increased GlcCerase activity. As an active-site inhibitor, IFG could exert its effects in the ER (by assisting folding) and/or the lysosome (by stabilizing the enzyme at acidic pH). To act in these compartments, IFG must be able to interact with GlcCerase at both neutral and acidic pH. As summarized in Table 2, IFG strongly inhibits wild-type and N370S GlcCerase at both neutral and acidic pH with IC50 values in the nanomolar range. The N370S enzyme was 3- to 4-fold less sensitive to the inhibitor compared with the wild-type enzyme. Both enzymes were ≈6-fold more sensitive to IFG at pH 7.2 than at pH 5.2.
Table 2.
IFG inhibits wild-type and N370S GlcCerase at neutral and acidic pH
| pH | IC50, nM |
|
|---|---|---|
| Wild type | N370S | |
| 5.2 | 30 | 128 |
| 6.4 | 9 | 26 |
| 7.2 | 5 | 18 |
GlcCerase activity was measured in wild-type and N370S fibroblast lysates in the presence of various concentrations of IFG. IC50 values, determined by plotting the percent inhibition of GlcCerase activity vs. log [IFG], represent the mean of two independent experiments. Standard errors were less than 5% for all IC50 values shown.
IFG Facilitates the Folding and Transport of Newly Synthesized GlcCerase Out of the ER.
Studies of the biosynthesis of the N370S GlcCerase have revealed that it exits the ER more slowly and less efficiently than the wild-type enzyme (9, 21). Consequently, IFG binding to newly synthesized N370S GlcCerase in the ER could facilitate folding and enhance its transport from this organelle. To explore this possibility, N370S fibroblasts were cultured for 5 days in the presence or absence of 30 μM IFG, labeled for 1 h with [35S]methionine/[35S]cysteine, and then chased in medium with or without IFG for up to 72 h. Aliquots of cells were harvested at various times, and the GlcCerase was immunoprecipitated and subjected to SDS/PAGE and autoradiography. An autoradiograph of a representative experiment is shown in Fig. 1B. At 0-h chase time, equal amounts of a 64-kDa species were present in untreated and treated cells. This species was completely converted to a 57-kDa form by endoglycosidase H, establishing that its N-linked glycans are all of the high-mannose type (data not shown). Subsequently, the 64-kDa form was processed to an endoglycosidase H-resistant, 69-kDa species, indicating that it had been transported to the Golgi, where the N-linked glycans were processed to complex-type oligosaccharides. By 24 h, the 64-kDa species was no longer detectable; and, between 24 and 72 h, the 69-kDa form was gradually converted to a 59-kDa form. This conversion has been reported to be caused by the trimming of N-linked glycans in the lysosome (22).
The presence of IFG had two striking effects. It enhanced the amount of N370S GlcCerase that is transported out of the ER (compare the 9-h lanes), and it slowed the conversion of the 69-kDa species to the 59-kDa form (compare the 72-h lanes). Quantitative assessment of several pulse–chase experiments showed that, on average, ≈60% of the newly synthesized GlcCerase in the untreated N370S fibroblasts was degraded in the first 24 h of the chase, presumably in the ER. When IFG was present, the degradation decreased, with 70–100% of the newly synthesized enzyme trafficking to the lysosome. The GlcCerase that reached the lysosome was stable for at least 72 h, regardless of the presence or absence of the drug.
To assess the effects of IFG on the lysosomal stability of mutant enzyme synthesized in the absence of the compound, N370S fibroblasts were pulse-labeled and chased for 12 or 36 h before the addition of IFG. The cells were then chased for an additional 60 or 36 h, respectively, and the amount of GlcCerase was compared with an equivalent sample in which the drug was omitted. A representative autoradiograph is shown in Fig. 1C. Lane 3 shows that by 12-h chase, the majority of the newly synthesized N370S GlcCerase remaining at this time had been transported out of the ER. Quantitative analysis of this experiment revealed that the addition of IFG to the culture medium at 12 or 36 h of the chase did not appreciably affect the level of GlcCerase, consistent with the previous experiments showing that the mutant enzyme that exits the ER is stable for at least 72 h. However, the addition of IFG decreased the conversion of the 69-kDa form of the enzyme to the 59-kDa form (compare lane 4 with lanes 5–7). Treatment of the 0- and 72-h chase samples with PNGase F, which cleaves all forms of N-linked glycans, produced a single polypeptide with an apparent molecular mass of 57 kDa, demonstrating that no substantial proteolytic cleavage had occurred during the 72-h chase period (compare lanes 2 and 8) and confirming that the processing only involves oligosaccharide trimming.
IFG Increases the Lysosomal Pool of GlcCerase.
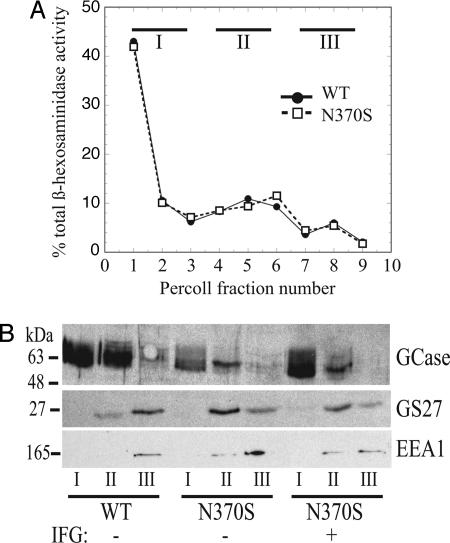
To prove that the GlcCerase made in the presence of IFG was in fact trafficking to lysosomes, we performed Percoll gradient fractionation on control and N370S fibroblast lysates after treatment for 5 days with 100 μM IFG. This procedure separates dense lysosomes from other organelles (23). As shown in Fig. 2A, the majority (67%) of lysosomal β-N-acetylhexosaminidase activity was recovered at the bottom of the gradient. The fractions were combined into three pools: pool I (fractions 1–3), containing the dense lysosomes; pool II (fractions 4–6), containing endosomes, Golgi complex, and plasma membrane; and pool III (fractions 7–9), containing soluble proteins.
Fig. 2.
IFG increases the lysosomal pool of N370S GlcCerase. (A) Wild-type and N370S fibroblast cultures were treated with or without 100 μM IFG for 5 days followed by fractionation of homogenized lysates on Percoll gradients. Fractions were collected, and β-N-acetylhexosaminidase activity was measured. The percent of total activity in each fraction is plotted. (B) Fractions were combined into three pools and subjected to SDS/PAGE and Western blot analysis. GCase, GlcCerase.
The distribution of GlcCerase, the early endosomal marker EEA1, and the Golgi SNAP protein receptor GS27 was determined by subjecting aliquots of the Percoll gradient pools to SDS/PAGE followed by Western blotting (Fig. 2B). As shown, GlcCerase had a distribution in all samples similar to that of the β-N-acetylhexosaminidase, with the majority being recovered in pool I. The lack of endosomal and Golgi markers in pool I confirms the fidelity of the Percoll fractionation. These data clearly demonstrate that the increased GlcCerase in IFG-treated N370S cells is in fact lysosomal. Furthermore, the increased GlcCerase signal in the Western blot of the IFG-treated N370S sample provides additional confirmation that this compound increases the amount of GlcCerase protein present in N370S fibroblasts.
The Relative Specific Activity of N370S GlcCerase Is Increased by IFG Treatment.
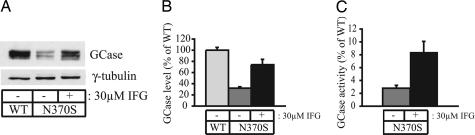
Because IFG facilitates N370S GlcCerase trafficking to the lysosome, presumably through improving its folding, we considered the possibility that the compound might also alter the catalytic properties of the enzyme. To explore this possibility, we treated N370S fibroblasts with 30 μM IFG for 5 days, and then we measured GlcCerase protein levels by Western blotting along with enzyme activity (Fig. 3), which allowed us to calculate a “relative” specific activity. Western blot analysis of GlcCerase revealed three distinct forms of the enzyme previously observed by others (Fig. 3A; refs. 24 and 25). Importantly, quantitation of the Western blots revealed a 2.3 ± 0.3-fold increase in GlcCerase protein levels in the IFG-treated N370S cells vs. a 3.0 ± 0.6-fold increase in enzyme activity (P < 0.01, n = 8). This increase corresponds to a 30% increase in the relative specific activity of GlcCerase. It is also noteworthy that the 5 days of treatment with 30 μM IFG resulted in the level of N370S protein attaining 79 ± 3% of the value measured in wild-type cells (Fig. 3B). These findings indicate that the enhanced GlcCerase activity in IFG-treated N370S fibroblasts can be attributed to both higher levels of GlcCerase and an increase in the relative specific activity of the enzyme. In contrast, the compound did not enhance the relative specific activity of wild-type GlcCerase because both enzyme levels and activity increased by 1.3-fold in fibroblasts treated for 5 days with 30 μM IFG.
Fig. 3.
Effect of IFG on the relative specific activity of N370S GlcCerase (GCase). (A) Representative Western blot. (B) Cells were grown for 5 days with or without 30 μM IFG. Lysates were prepared, and equivalent protein aliquots were subjected to SDS/PAGE and Western blot analysis (see Methods). GlcCerase levels were obtained by densitometry analysis, and the mean amount of GlcCerase ± SD is plotted relative to wild-type levels (n = 8). (C) GlcCerase activity in lysates from treated and untreated N370S fibroblasts was measured in triplicate. The mean activity ± SD is plotted relative to the GlcCerase activity in wild-type cells (n = 8). Activity measurements were normalized to total protein.
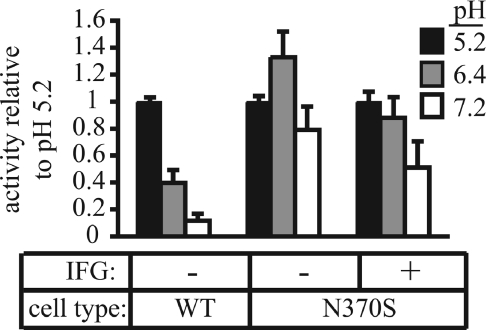
The GlcCerase activity measurements shown in Fig. 3 were performed at pH 5.2, the typical pH used in assays of this enzyme (22). When we assayed the wild-type and N370S GlcCerase at pH 5.2, 6.4, and 7.2, we found that the wild-type enzyme was most active at pH 5.2 (Fig. 4). By contrast, the N370S enzyme had its peak activity at pH 6.4. Remarkably, the pH-dependent activity of N370S GlcCerase from fibroblasts treated for 5 days with 30 μM IFG was shifted toward normal, with the peak activity being restored to pH 5.2. This increase in activity at pH 5.2 can account for the increase in the relative specific activity of the N370S enzyme noted in Fig. 3.
Fig. 4.
The pH optimum of GlcCerase activity from IFG-treated N370S cells is altered. Wild-type and N370S fibroblasts were cultured for 5 days with or without 30 μM IFG. Before harvesting, IFG was washed out of treated cells as described in Methods. Cell lysates were prepared at various pH values, and activity was measured in triplicate. The bar graphs represent the mean of four (wild-type) or seven (N370S) experiments with the activity at pH 5.2 set at 1.0.
IFG-Treated N370S GlcCerase Exhibits Altered Sensitivity to SDS.
We next sought to determine whether additional properties of the enzyme were changed. To explore whether N370S GlcCerase synthesized in the presence of IFG had an altered response to the ionic detergent SDS, cell lysates at pH 5.2 or 6.4 were incubated with 1.0 mM SDS before determination of enzyme activity (Table 3). This treatment caused a small decrease in the activity of the wild-type enzyme at both pH 5.2 and 6.4. The activity of N370S GlcCerase from untreated cells was decreased by 64% in the presence of SDS at pH 5.2, but it was stimulated nearly 3-fold at pH 6.4. By contrast, the N370S GlcCerase from IFG-treated cells was more inhibited by the SDS at pH 5.2 and less stimulated at pH 6.4, revealing another change in the behavior of the enzyme.
Table 3.
IFG-treated N370S GlcCerase has an altered response to SDS
| Cell type | pH | IFG | Relative GlcCerase activity |
|---|---|---|---|
| WT | 5.2 | − | 0.78 ± 0.04 |
| 6.4 | − | 0.90 ± 0.04 | |
| N370S | 5.2 | − | 0.338 ± 0.082∗ |
| + | 0.275 ± 0.093∗ | ||
| 6.4 | − | 2.96 ± 0.58† | |
| + | 2.11 ± 0.62† |
Cell lysates from wild-type (WT), N370S, and IFG-treated N370S fibroblasts were incubated with or without 1.0 mM SDS at room temperature for 30 min before measurement of GlcCerase activity. Results are presented as SDS/control. Separate cell lysates were prepared for experiments at either pH 5.2 or pH 6.4.
∗, P < 0.05 (n = 4);
†, P = 0.001 (n = 5).
GlcCerase Activity Is Inhibited in Lysosomes but Recovers Rapidly After Drug Washout.
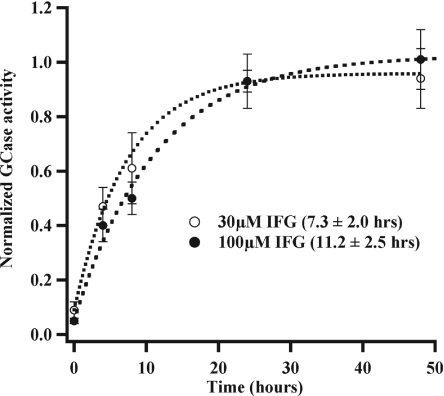
Because IFG inhibits GlcCerase in in vitro assays performed at the acidic pH of the lysosome, we examined whether the drug inhibits the activity of the enzyme in this compartment by performing in situ activity assays. Fibroblasts were incubated with 5-(pentafluorobenzoylamino)fluorescein di-β-d-glucopyranoside (PFB-FDGlu), a fluorogenic substrate that is internalized by pinocytosis and delivered to the lysosomes, where it can be hydrolyzed by GlcCerase (26). As shown in Fig. 5, either 30 or 100 μM IFG fully inhibits in situ GlcCerase activity. After washout of the drug, GlcCerase activity recovered to almost 50% of untreated levels by 4 h, and it recovered completely by 24 h. These data suggest that although IFG inhibits GlcCerase activity, rapid recovery occurs after removal of the IFG.
Fig. 5.
GlcCerase (GCase) inhibition by IFG recovers after drug washout. Cells were cultured for 5 days with 30 or 100 μM IFG before analysis. In situ GlcCerase activity was assessed in triplicate at various washout times. The mean normalized N370S GlcCerase activity ± SD at each washout time is shown. Time constants for exponential fits to the data are indicated in parentheses.
Discussion
It has been previously reported that the iminosugar N-nonyldeoxynojirimycin and related derivatives increase the activity of the N370S GlcCerase ≈2-fold when added for 5 days to cultures of fibroblasts expressing this mutant enzyme (15, 16). Because these compounds are active-site inhibitors of GlcCerase, the authors speculated that they might be acting as pharmacological chaperones to assist the folding of the mutant enzyme in the ER, thereby avoiding degradation by the ER-associated quality-control system and enhancing transport out of the ER to the Golgi and lysosome. This postulate is consistent with the prior demonstration by others that the trafficking of newly synthesized N370S GlcCerase from the ER to the Golgi is impaired (9, 21). However, the studies of Sawkar and colleagues (15, 16) did not directly address the mechanism(s) whereby the iminosugars enhance the cellular activity of N370S GlcCerase. Our findings provide evidence that the iminosugar IFG acts as a pharmacological chaperone to assist the folding of the N370S GlcCerase into a form that avoids degradation in the ER and is efficiently transported to the lysosome. Further, and unexpectedly, the N370S GlcCerase synthesized in the presence of IFG exhibits altered kinetic properties.
The pulse–chase experiments showed that IFG greatly increased the amount of newly synthesized GlcCerase that traffics out of the ER. This increase is likely the result of a direct pharmacological chaperone effect because we found that IFG interacts with GlcCerase at the neutral pH of the ER lumen. The enhanced transport of the N370S GlcCerase from the ER accounts for much of the effect of IFG on the total level of GlcCerase. A second but related mechanism whereby IFG increases the total activity of N370S in fibroblasts is by facilitating the synthesis of enzyme with altered catalytic properties that result in enhanced activity at lower pH values. Thus, the relative specific activity of the N370S GlcCerase from IFG-treated cells is increased 30% when assayed at pH 5.2. This increase can be accounted for by a shift of the pH optimum from 6.4 to 5.2. In addition, the N370S GlcCerase from IFG-treated cells exhibited an altered response to SDS. These differences in the properties of the mutant enzyme could arise by several mechanisms. First, IFG binding to the active site of the enzyme during the folding process in the ER could result in the mutant protein achieving a final conformation that differs from what occurs in the absence of the chaperone. This altered conformation could affect the properties of the enzyme. Because both the shift in pH optimum and the sensitivity to SDS were measured in the absence of IFG, the altered conformation would have to be stable. Alternatively, the binding of IFG to the mutant GlcCerase could favor some type of chemical modification, either in the ER or in the lysosome, which, in turn, influences the properties of the enzyme.
An additional effect of IFG is to slow the conversion of the 69-kDa form of GlcCerase to the 59-kDa species that occurs in the lysosome. This shift in molecular mass results from the trimming of sugars from complex-type glycans present on the mature GlcCerase rather than arising from proteolysis (Fig. 3; refs. 21 and 22). The basis for the protective effect of IFG is not clear because the drug does not inhibit neuraminidase, β-galactosidase, or β-N-acetylhexosaminidase in in vitro assays (data not shown). These glycosidases are the most likely to mediate the trimming of the glycans. IFG binding to GlcCerase in the lysosome may induce a conformational change in the protein that makes its complex-type glycans less accessible to lysosomal glycosidases, or it may alter the interaction of GlcCerase with another protein (such as its activator, saposin C), thereby slowing the processing of the glycans. In addition, it is not clear whether the glycan trimming makes the protein more susceptible to turnover in the lysosome. Because the survival of the GlcCerase that reaches the lysosome is quite long, even in the absence of IFG, it is difficult to measure the t½ of the enzyme accurately. Therefore, we cannot exclude the possibility that IFG also prolongs the survival of the enzyme in the lysosome.
It has been reported that GlcCerase activity can be as low as 11–15% of normal before storage of substrate occurs in a cell-culture model of Gaucher disease (27). In patients receiving Cerezyme infusions with clinical improvement in their blood counts and lessening of bone crisis, the increase of GlcCerase activity in the bone marrow may only be 1.7- to 9.6-fold (28). These findings indicate that the 2.4- to 3.0-fold increase in GlcCerase activity observed with IFG treatment may have clinical benefit.
One caveat is that IFG is an inhibitor of GlcCerase in the lysosome, at least under the conditions of our fibroblast assays. Therefore, to be an effective therapeutic agent, this inhibitory effect must be reversible. Using an in situ assay of GlcCerase activity, we found that the inhibition caused by both 30 μM and 100 μM IFG was relieved by almost 50% within 4 h of removal of the drug from the medium, and it was completely reversed by 24 h. Because the increased GlcCerase activity induced by IFG persists for much longer than 24 h, it should be possible to achieve a net gain in enzyme activity by intermittent dosing of the iminosugar.
It is also of note that it requires three orders of magnitude higher concentrations of IFG to increase the GlcCerase activity in cells compared with the amount needed to inhibit GlcCerase in the in vitro assays (10–100 μM vs. 5–100 nM). The most likely explanation for this difference is that IFG is transported into the ER and the lysosome very inefficiently. Nevertheless, it should be possible to achieve the low-micromolar concentrations in plasma that will be required to enhance GlcCerase activity.
Of the ≈200 disease-causing mutations in GlcCerase, many still retain some residual activity (8). Therefore, it will be important to determine whether IFG functions as a pharmacological chaperone for these other variants. It has been shown that an alkylated derivative of IFG enhances the activity of the G202R mutant that is retained in the ER (16, 29). By contrast, the L444P mutant was unresponsive to this iminosugar.
Pharmacological chaperones have been reported to improve the impaired trafficking of two other lysosomal enzymes. Several groups have shown that 1-deoxygalactonojirimycin corrects the trafficking defect in six mutants of α-galactosidase A, the enzyme that is deficient in Fabry disease (17, 18, 30). This pharmacological chaperone binds to the active site of the enzyme at the neutral pH of the ER to assist folding and enhance transport out of this organelle, but it dissociates in the low-pH environment of the lysosome. The 1-deoxygalactonojirimycin was also shown to restore enzyme activity in the tissues of a transgenic mouse expressing one of the mutant enzymes (30). This same compound, as well as another galactose derivative, N-octyl-4-epi-β-valienamine, restored the activity of several mutant lysosomal β-galactosidases that have been associated with GM1-gangliosidosis and Morquio B disease (31).
In summary, these findings provide greater insight into the mechanism of action of pharmacological chaperones, and they raise the possibility that such agents may be useful in the treatment of some forms of Gaucher disease.
Methods
Reagents.
PNGase F was obtained from New England Biolabs (Ipswich, MA). Conduritol β-epoxide (CBE) and the fluorogenic substrates 4-methylumbelliferyl β-d-glucoside and 4-methylumbelliferyl N-acetyl-β-d-glucosaminide were purchased from Sigma (St. Louis, MO). IFG was obtained from Amicus Therapeutics (New Brunswick, NY). Tran35S-label was purchased from MP Biomedicals (Irvine, CA). Antibodies to GS27 and EEA1 were purchased from BD Biosciences (San Jose, CA). The rabbit anti-human GlcCerase polyclonal antibodies were generated at Amicus Therapeutics. The 8E4 monoclonal anti-human GlcCerase antiserum was a gift from Raphael Schiffmann and Gary Murray at the Developmental and Metabolic Neurology Branch of the National Institute of Neurological Disorders and Stroke (Bethesda, MD).
Cell Lines and Culture.
Wild-type primary skin fibroblasts (CRL-1509) and fibroblasts from a patient homozygous for the N370S mutation (DMN89.15) were obtained from the American Type Culture Collection (Manassas, VA) and the National Institutes of Health (Bethesda, MD), respectively. Cells were maintained at 37°C in DMEM (Mediatech, Herndon, VA) supplemented with 10% FBS (Sigma) and 100 units of penicillin per 0.1 mg of streptomycin per ml. New cultures were initiated every 6 weeks to avoid any changes associated with cell senescence.
Enzyme-Activity Measurements.
Cells were plated in 12-well plates and treated with IFG for various times, and GlcCerase activity was measured in cell lysates as follows. Intact cell monolayers were washed several times with PBS followed by incubation with 1.5 ml of complete medium (twice for 15 min at 37°C) before lysate preparation. Cells were lysed by the addition of 150 μl of reaction buffer (0.1 M sodium citrate/0.2 M sodium phosphate/0.1% Triton X-100/0.25% sodium taurocholate, pH 5.2) to the wells followed by circular agitation with a plastic cell scraper. In some cases, the reaction buffer was adjusted to pH 6.4 or pH 7.2. Lysates were cleared by centrifugation, and protein content was determined by using the micro BCA protein assay kit (Pierce, Rockford, IL). Reactions containing equivalent amounts of total cell protein were initiated by the addition of substrate (3 mM 4-methylumbelliferyl β-d-glucoside), and they were quenched with 1 ml of 400 mM glycine buffer (pH 10.8). The fluorescent product generated was measured (excitation, 360 nm; emission, 450 nm) with a model 450 fluorometer (Turner, Sunnyvale, CA). In all cases, GlcCerase activity increased in a linear fashion proportional with lysate volume, confirming that residual drug was not inhibiting GlcCerase activity under these assay conditions. Parallel samples were treated with CBE, a specific covalent inhibitor of GlcCerase, before reaction initiation to assess the contribution of nonlysosomal β-glucosidase. This activity was typically observed to constitute 5% or less of the total activity measured. For the inhibition studies, various concentrations of IFG were added to cell lysates for 10 min before reaction initiation.
For experiments assessing the altered sensitivity to SDS, the detergent was added to aliquots of treated and untreated cell lysates to a final concentration of 1.0 mM. These lysates were incubated for 30 min at room temperature followed by determination of GlcCerase activity as described above.
In situ GlcCerase activity was measured by using the fluorogenic substrate PFB-FDGlu (Invitrogen, Carlsbad, CA). Cells plated in 48-well format (5 × 104 cells per well) were treated for 5 days with 30 or 100 μM IFG. On day 5, cells were assayed for in situ activity immediately (time 0), or they were washed three times with PBS (5 min per wash) and then incubated with DMEM with 10% FBS at 37°C until they were assayed 4–48 h later. Briefly, cells were labeled with PFB-FDGlu (50 μg/ml) in phenol red-free, serum-free DMEM with 25 mM Hepes for 2 h at 37°C, washed once with ice-cold PBS, and then lysed with 50% CelLytic (Sigma) plus 0.2 M glycine/NaOH. Lysates were transferred to 96-well black-walled, clear-bottom plates and measured for PFB-F fluorescence (excitation, 485 nm; emission, 535 nm). Activity in treated cells was averaged and normalized against activity from untreated cells at the same time point. The data were fitted with exponential functions to estimate time constants for IFG washout.
Determination of GlcCerase Protein Levels.
Cell lysates (10 μg of total protein) were subjected to SDS/10% PAGE, transferred to nitrocellulose membranes, and immunoblotted with an anti-human GlcCerase rabbit polyclonal antibody (1:1,000 dilution). GlcCerase levels on subsaturated films were quantitated by using densitometry with an Image Station 440 CF (Kodak, Rochester, NY).
Metabolic Labeling and Immunoprecipitation.
Confluent fibroblasts were cultured in 60-mm plates with or without IFG for 5 days before labeling with 750 μCi (1 Ci = 37 GBq) of [35S]methionine/[35S]cysteine for 1 h. After labeling, the medium was replaced with normal methionine-containing growth medium, and the cells were incubated for various chase times. After brief trypsinization, the cells were collected in PBS and pelleted. Lysates were prepared with lysis buffer (50 mM Tris, pH 8.0/50 mM NaCl/0.5% Triton X-100/0.5% sodium deoxycholate/protease inhibitor mixture), and GlcCerase was immunoprecipitated by overnight incubation with the GlcCerase-specific monoclonal antibody 8E4. After the addition of protein A–Sepharose for 1 h, beads were washed three times with wash buffer (50 mM Tris, pH 8.0/150 mM NaCl/0.5% Triton X-100/0.5% sodium deoxycholate/protease inhibitor mixture) and once with 100 mM Tris (pH 8.0). In some experiments, immunoprecipitates were divided into two equal aliquots and treated or not with 1 unit of PNGase F for 3 h at 37°C before SDS/PAGE and autoradiography.
Percoll Gradient Fractionation.
Confluent cells grown in 6-cm plates were incubated in the presence or absence of 100 μM IFG for 5 days. The cells were harvested and analyzed as described in ref. 23.
Acknowledgments
This work was supported by National Institutes of Health Grant CA08759 (to S.A.K.) and by Amicus Therapeutics.
Abbreviations
- CBE
conduritol β-epoxide
- ER
endoplasmic reticulum
- ERT
enzyme-replacement therapy
- GlcCerase
acid β-glucosidase
- IFG
isofagomine
- PFB-FDGlu
5-(pentafluorobenzoylamino)fluorescein di-β-d-glucopyranoside
- PNGase F
peptide:N-glycosidase F.
Footnotes
Conflict of interest statement: S.A.K. is a member of the Scientific Advisory Board of Amicus Therapeutics.
References
- 1.Brady RO, Kanfer JN, Bradley RM, Shapiro D. J Clin Invest. 1966;45:1112–1115. doi: 10.1172/JCI105417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grabowski GA. Adv Hum Genet. 1993;21:377–441. [PubMed] [Google Scholar]
- 3.Sawkar AR, D'Haeze W, Kelly JW. Cell Mol Life Sci. 2006;63:1179–1192. doi: 10.1007/s00018-005-5437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenson PD, Ball EV, Mort M, Phillips AD, Shiel JA, Thomas NS, Abeysinghe S, Krawczak M, Cooper DN. Hum Mutat. 2003;21:577–581. doi: 10.1002/humu.10212. [DOI] [PubMed] [Google Scholar]
- 5.Grabowski GA. Genet Test. 1997;1:5–12. doi: 10.1089/gte.1997.1.5. [DOI] [PubMed] [Google Scholar]
- 6.Horowitz M, Pasmanik-Chor M, Borochowitz Z, Falik-Zaccai T, Heldmann K, Carmi R, Parvari R, Beit-Or H, Goldman B, Peleg L, et al. Hum Mutat. 1998;12:240–244. doi: 10.1002/(SICI)1098-1004(1998)12:4<240::AID-HUMU4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 7.Grace ME, Graves PN, Smith FI, Grabowski GA. J Biol Chem. 1990;265:6827–6835. [PubMed] [Google Scholar]
- 8.Liou B, Kazimierczuk A, Zhang M, Scott CR, Hegde RS, Grabowski GA. J Biol Chem. 2006;281:4242–4253. doi: 10.1074/jbc.M511110200. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz M, Alfalah M, Aerts JM, Naim HY, Zimmer KP. Int J Biochem Cell Biol. 2005;37:2310–2320. doi: 10.1016/j.biocel.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Schiffmann R, Heyes MP, Aerts JM, Dambrosia JM, Patterson MC, DeGraba T, Parker CC, Zirzow GC, Oliver K, Tedeschi G, et al. Ann Neurol. 1997;42:613–621. doi: 10.1002/ana.410420412. [DOI] [PubMed] [Google Scholar]
- 11.Elstein D, Hollak C, Aerts JM, van Weely S, Maas M, Cox TM, Lachmann RH, Hrebicek M, Platt FM, Butters TD, et al. J Inherited Metab Dis. 2004;27:757–766. doi: 10.1023/B:BOLI.0000045756.54006.17. [DOI] [PubMed] [Google Scholar]
- 12.Weinreb NJ, Barranger JA, Charrow J, Grabowski GA, Mankin HJ, Mistry P. Am J Hematol. 2005;80:223–229. doi: 10.1002/ajh.20504. [DOI] [PubMed] [Google Scholar]
- 13.Aerts JM, Hollak CE, Boot RG, Groener JE, Maas M. J Inherited Metab Dis. 2006;29:449–456. doi: 10.1007/s10545-006-0272-5. [DOI] [PubMed] [Google Scholar]
- 14.Pastores GM, Barnett NL, Kolodny EH. Clin Ther. 2005;27:1215–1227. doi: 10.1016/j.clinthera.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Sawkar AR, Cheng WC, Beutler E, Wong CH, Balch WE, Kelly JW. Proc Natl Acad Sci USA. 2002;99:15428–15433. doi: 10.1073/pnas.192582899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawkar AR, Adamski-Werner SL, Cheng WC, Wong CH, Beutler E, Zimmer KP, Kelly JW. Chem Biol. 2005;12:1235–1244. doi: 10.1016/j.chembiol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Yam GH, Zuber C, Roth J. FASEB J. 2005;19:12–18. doi: 10.1096/fj.04-2375com. [DOI] [PubMed] [Google Scholar]
- 18.Yam GH, Bosshard N, Zuber C, Steinmann B, Roth J. Am J Physiol. 2006;290:C1076–C1082. doi: 10.1152/ajpcell.00426.2005. [DOI] [PubMed] [Google Scholar]
- 19.Alfonso P, Pampin S, Estrada J, Rodriguez-Rey JC, Giraldo P, Sancho J, Pocovi M. Blood Cells Mol Dis. 2005;35:268–276. doi: 10.1016/j.bcmd.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Lin H, Sugimoto Y, Ohsaki Y, Ninomiya H, Oka A, Taniguchi M, Ida H, Eto Y, Ogawa S, Matsuzaki Y, et al. Biochim Biophys Acta. 2004;1689:219–228. doi: 10.1016/j.bbadis.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Bergmann JE, Grabowski GA. Am J Hum Genet. 1989;44:741–750. [PMC free article] [PubMed] [Google Scholar]
- 22.Jonsson LM, Murray GJ, Sorrell SH, Strijland A, Aerts JF, Ginns EI, Barranger JA, Tager JM, Schram AW. Eur J Biochem. 1987;164:171–179. doi: 10.1111/j.1432-1033.1987.tb11008.x. [DOI] [PubMed] [Google Scholar]
- 23.Rohrer J, Schweizer A, Russell D, Kornfeld S. J Cell Biol. 1996;132:565–576. doi: 10.1083/jcb.132.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ron I, Horowitz M. Hum Mol Genet. 2005;14:2387–2398. doi: 10.1093/hmg/ddi240. [DOI] [PubMed] [Google Scholar]
- 25.Grabowski GA, White WR, Grace ME. Enzyme. 1989;41:131–142. doi: 10.1159/000469068. [DOI] [PubMed] [Google Scholar]
- 26.Lorincz M, Herzenberg LA, Diwu Z, Barranger JA, Kerr WG. Blood. 1997;89:3412–3420. [PubMed] [Google Scholar]
- 27.Schueler UH, Kolter T, Kaneski CR, Zirzow GC, Sandhoff K, Brady RO. J Inherited Metab Dis. 2004;27:649–658. doi: 10.1023/b:boli.0000042959.44318.7c. [DOI] [PubMed] [Google Scholar]
- 28.Beutler E, Kuhl W, Vaughan LM. Mol Med. 1995;1:320–324. [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmer KP, le Coutre P, Aerts HM, Harzer K, Fukuda M, O'Brien JS, Naim HY. J Pathol. 1999;188:407–414. doi: 10.1002/(SICI)1096-9896(199908)188:4<407::AID-PATH377>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 30.Fan JQ, Ishii S, Asano N, Suzuki Y. Nat Med. 1999;5:112–115. doi: 10.1038/4801. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda J, Suzuki O, Oshima A, Yamamoto Y, Noguchi A, Takimoto K, Itoh M, Matsuzaki Y, Yasuda Y, Ogawa S, et al. Proc Natl Acad Sci USA. 2003;100:15912–15917. doi: 10.1073/pnas.2536657100. [DOI] [PMC free article] [PubMed] [Google Scholar]