Abstract
Cognitive control includes the ability to formulate goals and plans of action and to follow these while facing distraction. Previous neuroimaging studies have shown that the presence of conflicting response alternatives in Stroop-like tasks increases activity in dorsal anterior cingulate cortex (ACC), suggesting that the ACC is involved in cognitive control. However, the exact nature of ACC function is still under debate. The prevailing conflict detection hypothesis maintains that the ACC is involved in performance monitoring. According to this view, ACC activity reflects the detection of response conflict and acts as a signal that engages regulative processes subserved by lateral prefrontal brain regions. Here, we provide evidence from functional MRI that challenges this view and favors an alternative view, according to which the ACC has a role in regulation itself. Using an arrow–word Stroop task, subjects responded to incongruent, congruent, and neutral stimuli. A critical prediction made by the conflict detection hypothesis is that ACC activity should be increased only when conflicting response alternatives are present. Our data show that ACC responses are larger for neutral than for congruent stimuli, in the absence of response conflict. This result demonstrates the engagement of the ACC in regulation itself. A computational model of Stroop-like performance instantiating a version of the regulative hypothesis is shown to account for our findings.
Keywords: action regulation, cognitive control, neuroimaging, performance monitoring
Goals dominate human thinking and behavior. We feel able to exercise control over our thoughts and actions, and yet, we experience limitations to that control. “I can resist everything except temptation,” a character in an Oscar Wilde play once said. In general, we are good but not perfect at dealing with distraction. This fundamental truth is exploited in investigations of cognitive control. Cognitive control refers to the regulative processes that ensure that our thinking, memory retrieval, planning, and actions are in accordance with our goals. In this way, cognitive control helps us to resist temptations to satisfy other goals. Another important aspect of cognitive control concerns performance monitoring, or assessing whether the planning and action are consistent with intent.
A classic task that uses distraction in studying cognitive control is the color-word Stroop task (1, 2), which is among the most frequently used tasks in the cognitive and brain sciences. In this task, subjects are asked to name the ink color of written color words (e.g., say red to the word blue written in red ink). Results have consistently shown that people are much slower in naming the ink color of incongruent color words than in naming the ink color of a row of neutral xs, and people are often, but not always, faster when color and word are congruent (e.g., say red to the word red in red ink) than in the neutral condition. The finding that only a few errors are made on incongruent trials shows that people resist the temptation to read aloud the word, although with a temporal cost. The temptation is asymmetrical: In reading the word aloud (e.g., say blue to the word blue written in red ink), there are no interference and facilitation effects. Numerous analogs of the color-word Stroop task have been used by researchers. For example, in the manual arrow–word version of the task (3, 4), people are presented with congruent or incongruent combinations of arrows and words (e.g., a left- or right-pointing arrow and the word left or right), and they indicate the direction denoted by the arrow (arrow task) or word (word task) by pressing a left or right button.
Functional neuroimaging studies implicate an extensive network of cortical brain areas as a neural substrate of cognitive control, including the anterior cingulate cortex (ACC) and other medial prefrontal regions, lateral prefrontal cortex, and parietal areas (5–8). The exact contribution of these different brain regions to cognitive control is still under debate. Disagreement exists especially about the functional role of the ACC. In particular, theories differ in whether the ACC subserves monitoring or regulative functions during the performance of Stroop-like tasks. No task yet studied has been found to engage the entire ACC. Rather, previous research has suggested functional heterogeneity of the ACC (9, 10), with subdivisions involved in visceromotor and skeletomotor control, vocalization, pain, and cognitive control. The discussion about putative monitoring or regulative functions centers around a specific subarea of the ACC, often referred to as the dorsal part of the ACC. After seminal neuroimaging observations (11–13), involvement of this specific area in Stroop-like tasks has been confirmed by several subsequent studies and demonstrated in metaanalyses of the neuroimaging literature on Stroop-task performance (14–17). The present article specifically relates to the dorsal portion of the ACC to which monitoring and regulative functions in Stroop-like tasks have previously been ascribed.
According to the prevailing monitoring view (18, 19), ACC activity reflects the detection of response conflict. When conflicting response alternatives are activated in memory, the ACC conflict monitor alerts regulative processes subserved by lateral prefrontal brain regions. These regulative processes resolve the response conflict by biasing the response selection process toward the task-relevant response and away from the task-irrelevant response (18). To illustrate this for naming the ink color of the word blue written in red ink, the incongruent color word activates an incompatible response alternative, namely blue, leading to response conflict between red (the required response to the color) and blue (the nonrequired response to the word). The response conflict is detected by the ACC conflict monitor, which alerts the regulative system to selectively enhance the activation of the color naming response (say red) over the word reading response (say blue).
In support of the conflict monitoring view of ACC function, neuroimaging data, derived during Stroop-task performance, show that the ACC is more active on incongruent than on congruent and neutral color naming trials (11–17, 19). Moreover, it has been shown that the magnitude of the difference in ACC activity between incongruent and congruent stimuli depends on whether the previous trial was congruent or incongruent and on the proportion of congruent and incongruent stimuli in a block of trials (20, 21). Activity within the ACC is greater during trials with high levels of conflict (i.e., after a congruent trial and in a block of trials with mostly congruent stimuli) than during trials with low levels of conflict (i.e., after an incongruent trial and in a block of trials with mostly incongruent stimuli), in line with the conflict monitoring hypothesis.
Although these findings are in agreement with ACC conflict monitoring (18, 19), alternative explanations are possible. According to an alternative hypothesis of ACC function (5, 22–24), the area has a role in regulative processes, in collaboration with other medial prefrontal, lateral prefrontal, and parietal areas. For example, the ACC may be involved in the regulation of response selection processes, such that they meet the task demands (22–24). More top-down regulation is required on incongruent than on congruent trials, explaining the difference in ACC activity between trial types. Likewise, more regulation is required with high than with low levels of conflict, which explains the dependence of ACC activity on the previous trial type and the block type.
The aim of the present study was to further test the relative merits of the conflict monitoring and regulative hypotheses with respect to the role of the dorsal ACC in performing Stroop-like tasks. We report neuroimaging evidence from an arrow–word version of the Stroop task that favors the regulative hypothesis of ACC function and challenges the conflict monitoring view. Moreover, a computational model instantiating a version of the regulative hypothesis, the WEAVER++ model of Stroop-task performance (23, 24), is shown to account for our empirical findings concerning response latencies and ACC activity.
According to the monitoring hypothesis, ACC activity signals the presence of response conflict. A critical prediction made by the conflict hypothesis is therefore that ACC activity should be increased only when conflicting response alternatives are present. For example, in the arrow–word Stroop task, ACC activity should be higher for manually responding to the word left combined with a right-pointing arrow (an incongruent trial) than in responding to the word left without an arrow (a neutral trial). Most importantly, ACC activity should not differ between congruent trials (e.g., the word left combined with a left-pointing arrow) and neutral trials (e.g., the word left without an arrow), because competing response alternatives are absent on both trial types (18). In contrast, the regulative hypothesis not only predicts more ACC activity on incongruent than on neutral trials, but also less ACC activity on congruent than on neutral trials. More ACC activity is predicted for incongruent than for neutral trials, because more top-down regulation is required for incongruent than for congruent stimuli. Less ACC activity is predicted for congruent than for neutral trials, because the correct response is already activated by the distractor on congruent trials and therefore less regulation is required.
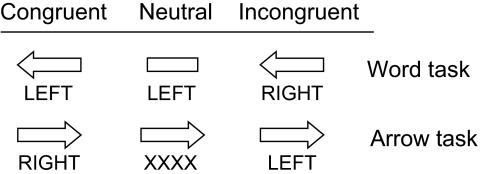
To test these predictions, we scanned subjects while they performed the arrow–word Stroop task. On each trial, subjects were presented with an arrow–word combination, and they indicated by means of a button press the direction denoted by the word (the word task) or arrow (the arrow task). Trials were blocked by task. On each trial, a congruent, incongruent, or neutral stimulus was presented. Example stimuli are shown in Fig. 1. Congruent, incongruent, and neutral trials were presented rapidly, in a randomly intermixed order to prevent subjects from anticipating and changing strategies for the different event types. On incongruent trials (e.g., a right-pointing arrow combined with the word left), the arrow and word indicated opposite responses (i.e., both left and right button presses), yielding response conflict. On congruent trials (e.g., a right-pointing arrow combined with the word right), word and arrow indicated the same response (here, a right button press), yielding no response conflict. On neutral trials in the word task, a word was presented in combination with a straight line (e.g., the word left combined with a straight line), so only one response was designated by the stimulus (here, a left button press). On neutral trials in the arrow task, an arrow was presented in combination with a row of xs (e.g., a right-pointing arrow combined with xxxx), so also on these trials only one response was designated by the stimulus (here, a right button press). The conflict monitoring hypothesis predicts a difference in ACC activity between incongruent and congruent stimuli, because of the presence of competing response alternatives, but not between congruent and neutral trials, because there are no competing response alternatives. In contrast, the regulative hypothesis predicts differences in ACC activity between all three trial types, because different amounts of regulation are required for the different stimulus types.
Fig. 1.
Examples of the stimuli used in the arrow and word tasks.
Results
Behavioral Performances.
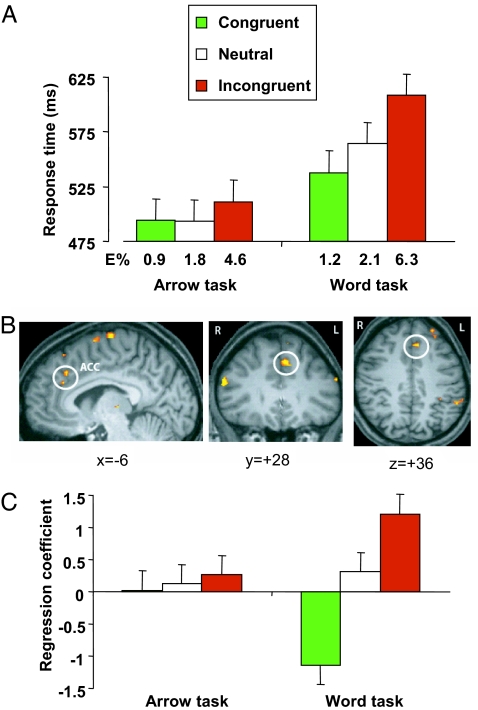
The latencies for the correct responses and the error rates were submitted to repeated-measurement ANOVAs with Stroop condition and task as independent variables. Specific effects were tested by applying t contrasts. Reaction time data showed that, consistent with earlier findings (3, 4, 14), responses to words were much slower on incongruent than on neutral trials and fastest on congruent trials (Fig. 2A). Responses to arrows were only slightly slower on incongruent than on neutral and congruent trials, while no difference between neutral and congruent trials was obtained.
Fig. 2.
The arrow–word findings. (A) Mean response times for the congruent, neutral, and incongruent conditions in the arrow and word tasks. Error rates (E%) are shown below the graph bar for each condition. Error bars indicate the SEM. (B) Among the brain regions showing increased activity on incongruent compared with congruent trials in the word task was the dorsal ACC [BA 32; Talairach and Tournoux (25) coordinates x = −4, y = 30, z = 36 and x = −8, y = 36, z = 28], the traditional locus of the Stroop effect. (C) Mean regression coefficients for congruent, neutral, and incongruent conditions, obtained for ACC voxels showing a Stroop effect in the word task. The regression coefficients estimate fMRI signal amplitude.
The response times exhibited effects of task, F(1,11) = 35.02, P < 0.001, and condition, F(2,22) = 22.89, P < 0.001. Task and condition interacted, F(2,22) = 14.25, P < 0.001. Responding to the words was slower on incongruent than on neutral trials, t(11) = 5.0, P < 0.001, slower on incongruent than on congruent trials, t(11) = 6.2, P < 0.001, and faster on congruent than on neutral trials, t(11) = 3.4, P < 0.003. Responding to the arrows was slower on incongruent than on neutral trials, t(11) = 2.0, P < 0.04, and slower on incongruent than on congruent trials, t(11) = 2.5, P < 0.02, whereas responding on neutral and congruent trials did not differ, t(11) = 0.2, P > 0.86. The error rates revealed an effect of Stroop condition, F(2,22) = 15.65, P < 0.001, but not of task, F(1,11) = 1.12, P > 0.31. Task and condition did not interact, F(2,22) < 1, P > 0.66. Overall, more errors were made on incongruent than on neutral trials, t(11) = 4.49, P < 0.001, and on incongruent than on congruent trials, t(11) = 4.32, P < 0.001, whereas the errors on congruent and neutral trials did not differ, t(11) = 1.31, P > 0.22.
Functional MRI (fMRI) Results.
Functional imaging data demonstrated that activity in the ACC was larger on incongruent than on congruent trials when subjects responded to the words (Fig. 2B), also consistent with earlier findings (11–17, 19). Other brain regions showing greater activity on incongruent than on congruent trials were the left supplementary motor area [BA 6; Talairach coordinates (25) x = −9, y = 12, z = 68 and x = −7, y = 0, z = 66], left precentral gyrus (BA 6; x = −20, y = −5, z = 47), left superior frontal gyrus (BA 9; x = −22, y = 48, z = 36), left inferior frontal gyrus (BA 44; x = −46, y = 14, z = 12), right middle frontal gyrus (BA 46; x = 54, y = 29, z = 20), bilateral inferior parietal lobe (BA 40; x = −50, y = −39, z = 36 and x = 44, y = −40, z = 47), and thalamus (x = −1, y = 22, z = 18).
To investigate whether the increase in ACC activity reflects response conflict, brain responses for all trial types in the ACC region of interest were obtained separately for all subjects. If ACC activity reflects conflict detection, then brain responses should not differ for congruent and neutral trials. On the other hand, if the ACC is involved in the regulation of response selection, then we predict that ACC activity should be modulated not only by incongruent distractors, but also by the presence of congruent distractors. Less activity should be obtained on congruent than on neutral trials, because the correct response is activated by the distractor on the former but not on the latter type of trial. Fig. 2C shows the mean regression coefficients for the incongruent, neutral, and congruent conditions in the arrow and word tasks. Note that the coefficients only indicate relative contribution. Negative values do not indicate deactivation, but only denote less activation. We observed more activity on incongruent than on neutral trials, t(9) = 3.5, P < 0.003, and more activity on incongruent than on congruent trials, t(9) = 24.2, P < 0.0001, in responding to the words (Fig. 2C). Importantly, the ACC was more active on neutral trials than on congruent trials, t(9) = 3.2, P < 0.001, even though conflicting response alternatives were absent on both types of trial. These findings support the predictions derived from the regulative hypothesis. In responding to the arrows, there were no differences in ACC activity among conditions, F(2,18) < 1, P > 0.86.
Discussion
The observed ACC activity in the absence of conflicting response alternatives provides evidence against the view that the area signals the presence of response conflict in Stroop-like tasks. Computer simulations by Botvinick et al. (18) showed that the conflict detection hypothesis predicts faster behavioral responses on congruent than on neutral trials, whereas ACC activity is the same on both trial types. If anything, ACC activity should be slightly larger on congruent than on neutral trials (18). Our findings are in clear disagreement with these predictions.
It is conceivable that the role of the ACC in Stroop-like tasks is not unitary. Perhaps one ACC subregion exhibits conflict specific increases in activity, as posited by the conflict detection hypothesis, whereas another ACC subregion exhibits activity independent of response conflict, as posited by the regulative hypothesis. However, this conjecture is not supported by our data. We functionally defined the ACC region of interest for each subject individually by selecting all voxels showing an effect of incongruent versus congruent stimuli. The difference between congruent and neutral stimuli was obtained for these voxels. Thus, the ACC region that was more active on incongruent than on congruent trials also exhibited a difference in activity between neutral and congruent trials, supporting the regulative hypothesis.
Moreover, it is possible that the neutral condition in the word task was not really free of conflict. Perhaps the absence of an arrow yielded a conflict in stimulus evaluation. According to Botvinick et al. (18), stimulus evaluation processes feed activation to relevant response channels in an ongoing fashion. Thus, conflicts occurring at early points in processing are likely to be reflected at the level of response selection. However, a conflict in stimulus evaluation as the cause of ACC activity leaves unexplained why reaction times and ACC activity did not differ among conditions in the arrow task.
Our rejection of ACC conflict detection rests on the assumption that the Stroop interference (incongruent vs. neutral) and facilitation (neutral vs. congruent) effects have a common source, namely that the effects arise during response selection. This is also what is assumed in Botvinick et al.’s model (18). It has been argued, however, that interference and facilitation in the Stroop task do not have a common base. Instead, facilitation is claimed to be caused by inadvertent responding to the distractor on some trials (26). In the color–word Stroop task, inadvertent responding to the distractor word in color naming yields apparent facilitation, because reading is faster than color naming. In our study, responding to the arrow was faster than responding to the word, which may explain the faster responding to the word on congruent than on neutral trials. However, ACC activity on congruent trials in responding to the word was much lower than the activity on all trial types in responding to the arrow (Fig. 2C). Thus, the difference between neutral and congruent trials in the word task cannot be caused by inadvertent responding to the distractor arrow on congruent trials.
According to the regulative hypothesis, Stroop effects arise during response selection, which requires therefore top-down regulation, as reflected by the ACC activity. What type of regulative process is mediated by the ACC? One possibility is that the ACC is involved in response inhibition (27, 28). During response selection, an activated response that does not meet the task demands is inhibited. Assuming that the distractor activates the associated response, response inhibition would occur only on incongruent trials. This would explain why ACC activity was higher on incongruent than on congruent trials. However, the response inhibition hypothesis cannot explain the increased ACC activity on neutral compared with congruent trials, because incorrect responses were not activated by the distractor on either type of trial.
Based on earlier reports (5, 22–24, 29, 30) and the present findings, we propose that, in performing Stroop-like tasks, the ACC is involved in selectively enhancing the activation of the correct response until a selection threshold is exceeded. This hypothesis explains why ACC activity was higher on incongruent than on neutral trials, because more activation enhancement is required on the former than on the latter type of trial. Moreover, the hypothesis explains why ACC activity was lower on congruent than on neutral trials, because the correct response is activated by the distractor on congruent but not on neutral trials.
Our claims about ACC function were put to a theoretical test by performing computer simulations of a model of the Stroop task that instantiates the selective enhancement view, namely WEAVER++ (23, 24). Elsewhere (23), it has been shown that this model accounts quantitatively for the key chronometric findings on Stroop task performance from 16 classic studies described in the comprehensive review of the literature by MacLeod (2). Moreover, the model has been applied successfully to neuroimaging findings derived during Stroop task performance (24).
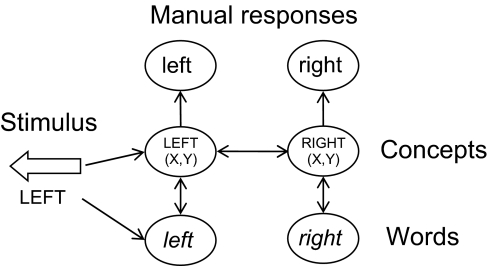
In WEAVER++, the arrow and word of arrow–word stimuli are linked to manual responses by an associative network (Fig. 3). Arrows are connected to concept nodes in the network and written words are connected to word nodes. Word nodes are connected to concept nodes, which are connected to manual response nodes. The nodes for the contrasting concepts LEFT(X, Y) and RIGHT(X, Y) are also connected. Stimuli activate responses via the associative network by means of spreading activation. Activation spreads through the network following a linear activation rule with a decay factor. Each node sends a proportion of its activation to the nodes it is connected to. For example, the written word left activates the word node left, which is followed by activation of the concept node LEFT(X, Y) and activation of the node for a left manual response. A left-pointing arrow directly activates the concept node LEFT(X, Y), which is followed by activation of the left manual response. Because the concept nodes are connected, the node for a right manual response will also receive some activation in perceiving the word left or a left-pointing arrow. If incongruent arrow–word combinations are presented to the network, the left and right manual response nodes both will be activated to a high level. Arrows will activate their response nodes more than words, because of the shorter network distance between arrows and manual response nodes than between words and response nodes. Thus, if the highest activated response node were selected, this would lead to the correct response in the arrow task but not in the word task. To achieve correct, task-dependent response selection in both tasks, the associative network is combined with condition–action rules that determine what is done with the activated nodes depending on the task. For example, if the task is to respond to the word, and the word left is presented together with a right-pointing arrow, a condition–action rule selects the concept node LEFT(X,Y) for the word and enhances the level of activation of the node until the left response node is selected. The response node is selected if its activation has reached a critical difference compared with the activation of the other response node.
Fig. 3.
Illustration of the associative network that connects arrow–word stimuli and manual responses in the WEAVER++ model (23, 24). Written words (e.g., left) activate corresponding word nodes in the network (i.e., left), which activate concept nodes [i.e., LEFT(X, Y)] and manual response nodes (i.e., left). Arrows (e.g., a left-pointing arrow) activate corresponding concept nodes [i.e., LEFT(X, Y)] and manual response nodes (i.e., left). The lines between nodes indicate excitatory connections.
We ran computer simulations of performance on the arrow–word Stroop task by using this model. As in earlier simulations (23, 24), processing in the model proceeded in discrete time steps. For each time step Δt during which activation enhancement was required, hypothetical ACC activity in the model was increased by a fixed amount. The total ACC activity is referred to as w, which indexes the amount of top-down regulation that is required by the response selection process in the model. It was assumed that a gamma function provides a reasonable model of the blood oxygenation level-dependent (BOLD) response (31). For the simulations it was assumed that:
¶ where du and critdiff are free parameters that affect the amount of top-down regulation required in the model. The du (duration) parameter is the duration of distractor input to the network and critdiff (critical difference) is the response threshold in the model. The parameter values were kept constant across distractor conditions and tasks. The response latencies were derived exactly as in earlier simulations of the model (23, 24).
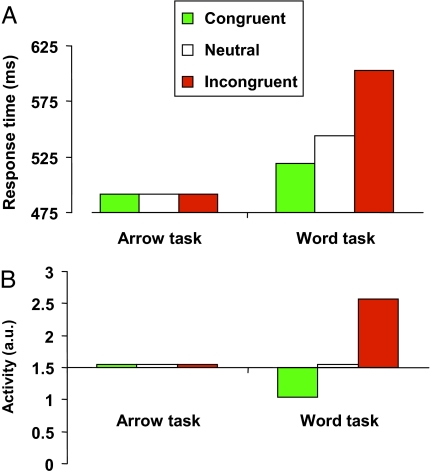
The computer simulations revealed that the responses to the words in the model were slower in the incongruent than in the neutral condition and they were faster in the congruent than in the neutral condition, whereas the responses to the arrows did not differ among conditions (Fig. 4A), as empirically observed (Fig. 2A). In the simulations, the ACC activity in the word task was larger in the incongruent than in the neutral condition and smaller in the congruent than in the neutral condition, whereas activity did not differ among conditions in the arrow task (Fig. 4B). This result also corresponds to what we empirically observed (Fig. 2C). Overall, there is a good agreement between the model and our empirical findings concerning response latencies and ACC activity, corroborating the regulative hypothesis of ACC function.
Fig. 4.
The arrow–word findings in WEAVER++ (23, 24) simulations. (A) Simulated response times for the congruent, neutral, and incongruent conditions in the arrow and word tasks. (B) Simulated ACC activity for the congruent, neutral, and incongruent conditions in the arrow and word tasks.
To conclude, our empirical findings show that ACC responses in performing an arrow–word Stroop task are larger for neutral than for congruent stimuli, in the absence of response conflict. This result demonstrates that ACC activity can be independent of response conflict, challenging the conflict monitoring view of ACC function. A computational model instantiating the alternative, regulative hypothesis of ACC function is shown to account for our findings.
Methods
Behavioral Protocol.
Twelve healthy Dutch human adults (eight female) performed the arrow and word tasks while undergoing fMRI. All subjects were right-handed. Their mean age was 23 years (range 21–28 years). The congruent and incongruent stimuli consisted of all possible combinations of left- and right-pointing arrows and the corresponding Dutch words links (left) and rechts (right). The neutral stimuli consisted of a word combined with a bar (in responding to the word), and an arrow combined with a row of five or six xs (in responding to the arrow). Every stimulus combination occurred twice, once with the word or xs above the arrow or bar, and once vice versa (vertical position). There were 20 different stimuli in total (2 incongruent arrow–word combinations, 2 congruent arrow–word combinations, 2 bar–word combinations, 2 arrow–5x combinations, 2 arrow-6x combinations, with each stimulus combination appearing in two vertical positions).
On each trial, subjects indicated by a left or right button press the direction denoted by the word or the arrow, depending on the task. Subjects were instructed to respond as quickly as possible while trying to avoid errors. Button presses were made by using the index and middle fingers of the left hand. On each trial, the stimulus was presented for 600 ms. The average interstimulus interval was 4,000 ms, jittered between 3,000 and 5,000 ms in steps of 500 ms, counterbalanced over conditions. There were 336 trials per subject. Subjects performed each task twice in an ABAB sequence, with the order of tasks counterbalanced across subjects.
fMRI Procedures.
Functional images of the whole brain were acquired on a 3-tesla MRI system (Trio, Siemens, Erlangen, Germany). Using a gradient-echo echo-planar scanning sequence, 32 axial slices were obtained for each subject (voxel size 3 × 3 × 3 mm, repetition time = 2,268 ms, field of view = 224 mm, echo time = 40 ms, flip angle = 75°). All functional images were acquired in one run that lasted for 27 min. After the acquisition of functional images, a high-resolution anatomical scan (T1-weighted magnetization prepared rapid gradient echo, 176 slices) was acquired.
fMRI data were analyzed with BrainVoyager 2000 and BrainVoyager QX (Brain Innovation, Maastricht,The Netherlands). Functional images were corrected for motion and slice time acquisition. Data were temporally smoothed with a high-pass filter removing frequencies below three cycles per time course. Functional images were coregistered with the anatomical scan and transformed into Talairach coordinate space using the nine-parameter landmark method of Talairach and Tournoux (25). Images were spatially smoothed with a FWHM Gaussian kernel of 6 mm.
Statistical analyses were performed in the context of the general linear model, including the six event types of interest (i.e., arrow task: congruent, neutral, incongruent; word task: congruent, neutral, incongruent). Event-related hemodynamic responses for each of the different event types were modeled as delta functions convolved with a synthetic hemodynamic response function. Random-effects group analyses were performed. The statistical threshold was set at P < 0.001 at the voxel level, uncorrected for multiple comparisons. For the region-of-interest analyses of the ACC, we first functionally defined the ACC region for each subject individually by selecting all voxels showing an effect of incongruent versus congruent stimuli in responding to the word (P < 0.001). The data of two subjects revealed no difference in ACC activity between incongruent and congruent trials, so they were excluded from the region of interest analyses. We obtained regression coefficients as indices of effect size for all voxels included in the region of interest, separately for each subject and event type. These regionally averaged regression coefficients were analyzed in repeated-measurement ANOVAs. Specific effects were tested by applying t contrasts to the regression weights obtained for the different event types.
Acknowledgments
We thank Marieke van der Linden and Jos Olders for their help in running and analyzing the experiment. This work was supported by a VICI grant from The Netherlands Organization for Scientific Research (to A.R.).
Abbreviations
- ACC
anterior cingulate cortex
- fMRI
functional MRI.
Footnotes
Conflict of interest statement: No conflicts declared.
Data deposition: The neuroimaging data have been deposited with the fMRI Data Center, www.fmridc.org (accession no. 2-2006-1227A).
Reproduced with permission from ref. 24 (Copyright 2002, Elsevier).
References
- 1.Stroop JR. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 2.MacLeod CM. Psychol Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 3.Baldo JV, Shimamura AP, Prinzmetal W. Percept Psychophys. 1998;60:427–437. doi: 10.3758/bf03206864. [DOI] [PubMed] [Google Scholar]
- 4.Turken UD, Swick D. Nat Neurosci. 1999;2:920–924. doi: 10.1038/13224. [DOI] [PubMed] [Google Scholar]
- 5.Posner MI, Raichle ME. Images of Mind. New York: Freeman; 1994. [Google Scholar]
- 6.Miller EK. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 7.Bush G, Luu P, Posner MI. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 8.Paus T. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 9.Vogt BA, Finch DM, Olson CR. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 10.Devinsky O, Morrell MJ, Vogt BA. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 11.Pardo JV, Pardo P, Janer KW, Raichle ME. Proc Natl Acad Sci USA. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RSJ, Dolan RJ. Neuropsychologia. 1993;31:907–922. doi: 10.1016/0028-3932(93)90147-r. [DOI] [PubMed] [Google Scholar]
- 13.Carter CS, Mintun M, Cohen JD. NeuroImage. 1995;2:264–272. doi: 10.1006/nimg.1995.1034. [DOI] [PubMed] [Google Scholar]
- 14.Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A. Cereb Cortex. 2001;11:837–848. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- 15.Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT. Hum Brain Mapp. 2005;25:6–21. doi: 10.1002/hbm.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derrfuss J, Brass M, Neumann J, Von Cramon DY. Hum Brain Mapp. 2005;25:22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neumann J, Lohmann G, Derrfuss J, Von Cramon DY. Hum Brain Mapp. 2005;25:165–173. doi: 10.1002/hbm.20133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald AW, Cohen JD, Stenger VA, Carter CS. Science. 2000;288:835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 20.Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JC. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 21.Carter CS, MacDonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Proc Natl Acad Sci USA. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holroyd CB, Coles MGH. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- 23.Roelofs A. Psychol Rev. 2003;110:88–125. doi: 10.1037/0033-295x.110.1.88. [DOI] [PubMed] [Google Scholar]
- 24.Roelofs A, Hagoort P. Cogn Brain Res. 2002;15:85–97. doi: 10.1016/s0926-6410(02)00218-5. [DOI] [PubMed] [Google Scholar]
- 25.Talairach J, Tournoux PA. A Coplanar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 26.MacLeod CM, MacDonald PA. Trends Cogn Sci. 2000;4:383–391. doi: 10.1016/s1364-6613(00)01530-8. [DOI] [PubMed] [Google Scholar]
- 27.George MS, Ketter TA, Parekh PI, Rosinsky N, Ring H, Casey BJ, Trimble MR, Horwitz B, Herscovitch P, Post RM. Hum Brain Mapp. 1993;1:194–209. doi: 10.1002/hbm.460010305. [DOI] [PubMed] [Google Scholar]
- 28.Swick D, Turken AU. Proc Natl Acad Sci USA. 2002;99:16354–16359. doi: 10.1073/pnas.252521499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milham MP, Banich MT. Hum Brain Mapp. 2005;25:328–335. doi: 10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markela-Lerenc J, Ille N, Kaiser S, Fiedler P, Mundt C, Weisbrod M. Cogn Brain Res. 2004;18:278–87. doi: 10.1016/j.cogbrainres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Cohen MS. NeuroImage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]