Abstract
Gaucher disease (GD) is a lysosomal storage disorder due to an inherited deficiency in the enzyme glucosylceramidase (GCase) that causes hepatosplenomegaly, cytopenias, and bone disease as key clinical symptoms. Previous mouse models with GCase deficiency have been lethal in the perinatal period or viable without displaying the clinical features of GD. We have generated viable mice with characteristic clinical symptoms of type 1 GD by conditionally deleting GCase exons 9–11 upon postnatal induction. Both transplantation of WT bone marrow (BM) and gene therapy through retroviral transduction of BM from GD mice prevented development of disease and corrected an already established GD phenotype. The gene therapy approach generated considerably higher GCase activity than transplantation of WT BM. Strikingly, both therapeutic modalities normalized glucosylceramide levels and practically no infiltration of Gaucher cells could be observed in BM, spleen, and liver, demonstrating correction at 5–6 months after treatment. The findings demonstrate the feasibility of gene therapy for type 1 GD in vivo. Our type 1 GD mice will serve as an excellent tool in the continued efforts toward development of safe and efficient cell and gene therapy for type 1 GD.
Gaucher disease (GD) is a lysosomal storage disorder caused by an inherited deficiency in glucosylceramidase (GCase) (1–3). The defective enzyme cannot degrade sufficient glucosylceramide (GluCer), which therefore accumulates in macrophages throughout the body, producing Gaucher cells, the hallmark feature of GD (2). As a result of the massive lipid accumulation, patients with GD exhibit hepatosplenomegaly, cytopenias, and bone disease (2). The disorder is usually divided into three different clinical types based on the absence (type 1) or presence and severity (types 2 and 3) of CNS involvement (2), although the distinction between these subtypes is becoming more diffuse (4).
Enzyme replacement therapy is effective in most patients with type 1 GD (5–9) and is the treatment of choice for the systemic disease. Allogenic BM transplantation (BMT) has proven therapeutic especially for type 1 GD (10, 11), but it has disadvantages with regard to the availability of suitable donors and risks involved with the transplantation procedure. Other emerging therapeutic approaches include substrate inhibitors (12, 13) and chemical chaperones (14); however, although exciting and novel, these strategies do not cure the disorder. A curative alternative could be transplantation of genetically modified autologous hematopoietic stem cells (HSC). However, lack of a suitable animal model has been the main obstacle in developing gene therapy for GD. During the past 15 years, several attempts to generate mouse models for symptomatic type 1 GD have been made, but the mice have either died in the perinatal period or been viable without clinical signs of GD (15–22). GCase-deficient mice, generated through targeted insertion of the neomycin resistance gene into exons 9 and 10 of the GCase gene died within 24 h of birth, likely because of a disturbance in the skin barrier function (16, 23). Further attempts to generate a mouse model for type 1 GD were made by introducing common mutations found in GD patients (24) into the mouse genome by knockin strategies. However, the resulting mice either died within hours of birth or exhibited only limited accumulation of GluCer (levels two to four times higher than WT) and no clinical symptoms of GD (22).
The aim of this study was to generate mice with symptomatic type 1 GD for use in the development of cell and gene therapy for the disorder. The Mx1/Cre-loxP system used here (25) enabled deletion of GCase exons 9–11 after birth, thereby maintaining GCase activity during development and avoiding disturbance of the skin barrier formation (23). Enzyme deficiency was most prominent in the hematopoietic organs (typically involved in GD), with minimal deficiency in the brain, consistent with the reported variations in efficiency of exon deletion in various tissues (25). The mice, hereafter referred to as GD mice, were viable, and thorough characterization revealed severely reduced GCase activity, a great increase in GluCer, and massive infiltration of Gaucher cells in the BM, liver, and spleen 12 months after induction of disease. In addition, the mice exhibited splenomegaly and microcytic anemia. Both BMT and transplantation of retrovirally corrected autologous BM cells could prevent the development of disease as well as correct an already established phenotype 5–6 months after transplantation. Our study demonstrates that the GD mouse provides an excellent opportunity to evaluate treatments for type 1 GD. In addition, the findings presented here demonstrate in vivo the feasibility of gene therapy for type 1 GD.
Results
Homozygous Null Embryos Exhibit Profound Deficiency of GCase and Die Shortly After Birth.
To disrupt the GCase gene, GCase exons 9–11 were conditionally deleted. The choice of exons to target was based on previous reports demonstrating that most human GD mutations cluster toward the 3′ end of the gene (3). The null (Fig. 5A, which is published as supporting information on the PNAS web site) and the floxed (Fig. 1A) ES cell lines were derived from targeted ES cells and were verified by PCR (data not shown). To confirm that deletion of GCase exons 9–11 disrupted GCase activity, homozygous null embryos were obtained from heterozygous null females 14.5 days after coitum. The genotype was verified by PCR. Fetal livers from homozygous null embryos were completely deficient in GCase activity, demonstrating the effectiveness in targeting GCase exons 9–11 (Fig. 5 B and C). Fetal livers from heterozygotes had approximately half the activity of the WT fetal livers. Newborn homozygous pups had the same lethal skin phenotype as has been reported in previous mouse models of GD (16–18) and died within the first day of life (Fig. 5D). The heterozygous null mice appeared healthy and were indistinguishable from WT mice.
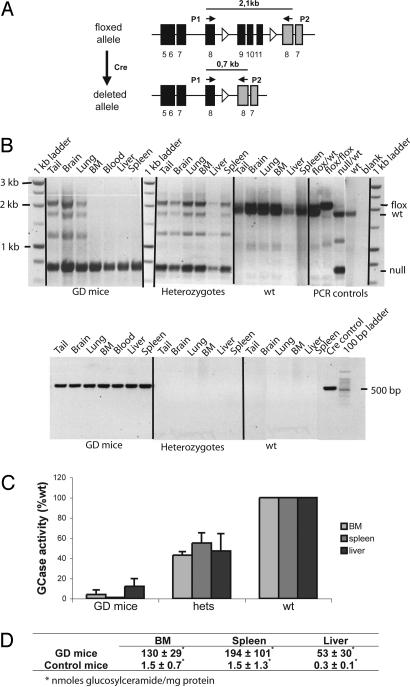
Fig. 1.
Induction of Cre causes excision of exons 9–11 of the GCase gene with concomitant enzyme deficiency and substrate accumulation in the BM, spleen, and liver. (A) Schematic view of the Cre-mediated deletion of GCase exons 9–11. PCR was used to screen for deletion (primers: P1 and P2). black bars, GCase exons; gray bars, metaxin exons; open triangles, loxP sites. (B) (Upper) PCR confirmed successful deletion of exons 9–11 in the BM, blood, liver, and spleen of GD mice. (Lower) PCR verified the presence of Cre in the GD mice. Bands were visualized by ethidium bromide. (C) The enzyme activity was practically absent (spleen) or severely reduced (BM and liver) in GD mice compared with WT (tissues from three to six individual GD mice; age, 6.5–12 months). Heterozygotes (hets) had approximately half of the enzyme activity of WT (tissues from three to six individual heterozygotes; age, 6.5–12 months). WT GCase activity was set to 100%, and activity in the GD mice and heterozygotes is presented as the percentage of WT. Error bars represent standard deviation. (D) BM, spleen, and liver from 12-month-old GD mice show a massive accumulation of GluCer (n = 3). WT and heterozygotes were pooled (depicted control, n = 4; age, 8–12 months), because they were indistinguishable from each other with respect to GluCer accumulation. Results are shown as nanomoles of GluCer per milligram of protein.
Postnatal Induction of Genetic Deletion Causes Severe Enzyme Deficiency and Accumulation of GluCer in the BM, Spleen, and Liver.
GCase exons 9–11 were deleted after birth through polyinosinic–polycytidylic acid-mediated induction of Cre recombinase expression (25). Enzyme deficiency during embryogenesis could therefore be avoided, as could the lethal skin phenotype that most likely is the cause of the rapid demise of homozygous null mice (23). We have previously shown that the Mx1 promoter mediates Cre expression upon induction and that the subsequent Cre-loxP-mediated deletion occurs with high efficiency in HSC (26, 27) and, therefore, in practically all subsequent progeny cells (including macrophages). In addition, the Mx1 promoter is active only to a limited extent in the brain (25), and the choice of this promoter therefore enabled the generation of a mouse with hematopoietic organ involvement but without CNS, signs corresponding to type 1 GD patients. All mice received a series of five polyinosinic–polycytidylic acid injections starting within the first week of life to induce excision of “floxed” exons. Therefore, the age of a mouse roughly corresponded to the duration of the enzyme deficiency in that mouse. The pups tolerated the treatment well. Complete excision of exons 9–11 in blood, BM, liver, and spleen was confirmed by PCR analysis (Fig. 1B Upper). The Cre PCR (Fig. 1B Lower) confirmed presence of Cre gene in all tissues analyzed from the GD mice but not in the controls (heterozygotes or WT). In the absence of Cre, there was no excision of exons 9–11 (heterozygotes and WT) (Fig. 1B Upper). Tissue samples from tail, brain, and lung from GD mice showed the presence of the floxed allele as expected from previous studies on Mx1 promoter activity in different tissues (25–27). GCase activity was absent in spleen and severely reduced in BM and liver of the GD mice (Fig. 1C). The heterozygotes had approximately half the enzyme activity of the WT mice, although they were phenotypically indistinguishable from each other. The GD mice had high levels of GluCer in BM, spleen, and liver compared with control tissues (Fig. 1D). The lung also had elevated levels of GluCer (data not shown), but, as expected, the GD mice did not accumulate substrate in the brain (data not shown).
Extensive Infiltration of Gaucher Cells in the Hematopoietic Tissues in Mice with Induced GCase Deficiency.
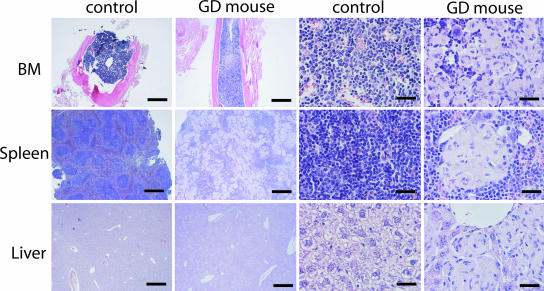
Histopathological analysis of the BM, spleen, liver, thymus, and lymph nodes from 12-month-old GD mice revealed massive infiltration of Gaucher cells (Fig. 2; thymus and lymph nodes are not shown). The Gaucher cells were multinucleated in most cases, and the cytoplasm had the typical appearance of “wrinkled tissue paper” due to the large amounts of accumulated GluCer. Gaucher cells were not present in the brain, kidney, or skin of the GD mice (data not shown). Heterozygous and WT mice (depicted control) were indistinguishable from each other and lacked Gaucher cells in all tissues (Fig. 2). Apart from the massive infiltration of Gaucher cells in the spleen, the normal splenic architecture was disrupted, and demarcation of the red and white pulp was no longer evident (Fig. 2). Inflammatory cells were also present throughout the spleen. Careful pathological examination of femurs and tibia failed to show any signs of bone disease.
Fig. 2.
Infiltration of Gaucher cells in the BM, spleen, and liver after conditional deletion of GCase exons 9–11. Columns labeled “GD mouse” show specimens from 12-month-old GD mice with massive infiltration of GluCer-engorged multinucleated cells in BM, spleen, and liver. Columns labeled “control” show tissues from heterozygous and WT mice lacking Gaucher cells. Results were obtained through hematoxylin and eosin and periodic acid Schiff staining. (Scale bars: first and second columns, 1,000 μm; third and fourth columns, 50 μm.)
Induced GCase Deficiency Causes Splenomegaly and Microcytic Anemia.
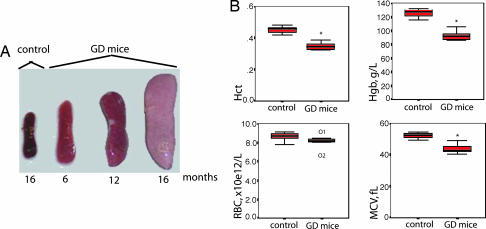
The GD mouse had a normal life span and did not show any gross signs of CNS abnormalities. Sixteen-month-old GD mice (≈16 months after disease induction) had up to 10-fold increase in spleen size compared with control (Fig. 3A). Upon macroscopic examination, spleen infarcts could be seen, which is common in patients with GD. The liver was pale and slightly enlarged but to a lesser extent than the degree of enlargement seen in the spleen (data not shown). Hematocrit, hemoglobin, and mean red blood cell volume values were significantly reduced in the GD mice compared with control (Fig. 3B), although there was no difference in red or white blood cell count (Fig. 3B and data not shown).
Fig. 3.
Induced GCase deficiency causes splenomegaly and microcytic anemia. (A) Spleen size increased over time, and the spleen from a representative 16-month-old GD mouse was ≈10-fold larger compared with control (heterozygotes and WT). White areas in the spleens show splenic infarcts. (B) GD mice had a significant reduction in hematocrit (Hct) (n = 5) (Upper Left), hemoglobin (Hgb) (n = 5) (Upper Right), and mean red blood cell volume (MCV) (n = 7) (Lower Right) compared with control (heterozygoyes and WT; n = 7–8); there was no significant difference in the number of red blood cells between GD mice (n = 7) and control mice (n = 8) (Lower Left). The findings are presented as box plots, where the thick black line represents median values, the box represents 50% of values, and the whiskers represent the range of values. ∗, P = 0,003 (Mann–Whitney U test); O1 and O2, outliers 1 and 2.
BMT Can Prevent or Correct the Phenotype of GD Mice.
To ask whether BMT could prevent disease development and correct an established GD phenotype, two different experimental approaches were used in which the age of the recipient GD mice differed (Fig. 6 A and B, which is published as supporting information on the PNAS web site). At 1.5 months after induction of the genetic deletion, the GD mice are not expected to have substantial GD symptoms. We therefore asked whether BMT could prevent disease development by transplanting WT BM to GD mice 1.5 months after genetic deletion. Five months after BMT, GCase activity was increased in BM, spleen, and liver of recipient GD mice (Fig. 6C). The increase in GCase activity was sufficient to prevent elevation of GluCer in the recipients (Fig. 6E). In addition, no Gaucher cells could be seen 5 months after transplantation (Fig. 6G). We next asked whether WT BMT could correct GD in the mice once manifestations of the disease had developed. GD was allowed to proceed for 7.5 months before transplantation of WT BM. Five and a half months later, when the mice were 13 months old, the therapeutic consequences were evaluated. GCase activity was increased in BM, spleen, and liver of recipient GD mice (Fig. 6D). The increase in GCase activity was sufficient to normalize GluCer levels in the recipients (Fig. 6F). Four of six mice were completely devoid of Gaucher cells in BM, spleen, and liver, and only a few scattered Gaucher cells were seen in spleen and liver in the two remaining mice (representative findings from a mouse with few scattered Gaucher cells are shown in Fig. 6G). Given longer time to recover, it is likely that these mice would have been cleared of Gaucher cells as well, and the slight variability in outcome could be due to differences in disease severity in different mice at the time of transplantation. These findings suggest that BMT cannot only prevent disease development but also correct an established GD phenotype in the GD recipient mice.
Gene Therapy Can Correct the Phenotype of GD Mice.
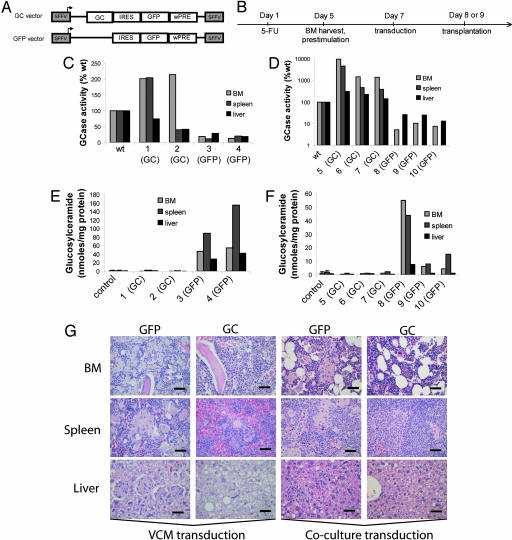
The GD mice were used in proof of principle experiments to investigate the feasibility of gene therapy for type 1 GD. A retroviral vector with the spleen focus-forming virus promoter driving GCase expression (GC vector) was used. The control vector (GFP vector) was identical to the GC vector with the exception of the GCase gene (Fig. 4A). The experimental rationale involved two different transduction protocols (Fig. 4B). To ask whether gene therapy could correct the GD phenotype once it had developed, recipients were treated with transduced HSC 4.5 to 6 months after disease induction. The findings presented in Fig. 4 C and E are from transplantations with BM cells transduced through incubation with virus-containing medium (VCM). Using this transduction protocol, transduction efficiencies (as measured by the percentage of GFP) ranged from 14% to 39% in BM cells before transplantation and from 4% to 79% in blood 5 months after transplant. The findings presented in Fig. 4 D and F are from transplantations with BM cells transduced through coculture with virus producer cell lines. By using this method, transduction efficiencies ranged from 48% to 75% in BM cells before transplantation and 5.6% to 56% in blood 5 months after transplantation. The findings are presented individually for each mouse. Five months after transplant, there was a robust increase in enzyme activity in BM, spleen, and liver from all mice treated with GC vector, regardless of the transduction protocol used (mice 1, 2, and 5–7) (Fig. 4 C and D). The increase in GCase activity completely normalized substrate levels in BM, spleen, and liver 5 months after transplantation (mice 1, 2, and 5–7) (Fig. 4 E and F), whereas mice treated with the GFP control vector continued to accumulate GluCer in these tissues (mice 3, 4, and 8–10) (Fig. 4 E and F). In addition, Gaucher cells were practically eliminated 5 months after transplantation in mice treated with GC vector, compared with GFP-treated mice (Fig. 4G). These data demonstrate that gene therapy can correct the GD phenotype in the GD mice 5 months after transplantation.
Fig. 4.
Gene therapy can correct the disease phenotype in the GD mice. (A) GC vector (GC) and GFP vector (GFP) design (details are given in Supporting Materials and Methods) (B) Experimental design (Supporting Materials and Methods). BM cells transduced through VCM incubation or in coculture with vector producer cell lines were transplanted into lethally irradiated recipient GD mice (4.5–6 months after GD induction). Results are presented individually for each mouse. (C) VCM transduction. The relative GCase activity in BM, spleen, and liver was increased in the GD mice treated with GC vector (mice 1 and 2) compared with tissues from GFP-treated GD mice (mice 3 and 4). (D) Coculture transduction. A robust increase in the relative GCase activity was demonstrated in BM, spleen, and liver in the GD mice treated with GC vector (mice 5–7) compared with tissues from GFP-treated GD mice (mice 8–10). The y axis is in log-scale. (C and D) WT GCase activity was set to 100%, and activity in individual GD mice (GC vector- or GFP vector-treated) is presented as the percentage of WT. (E) GC-treated GD mice (VCM transduction mice 1 and 2) had normal levels of GluCer in BM, spleen, and liver compared with GFP-treated mice (mice 3 and 4). Control levels (WT and heterozygous tissue, n = 5; age, 8–12 months) are included for comparison. (F) GC vector-treated GD mice (coculture transduction, mice 5–7) had normal levels of GluCer in BM, spleen, and liver compared with GFP vector-treated mice (mice 8–10). Control levels (WT and heterozygous tissue, n = 5; age, 8–12 months) are included for comparison. (E and F) Results are shown as nanomoles of GluCer per milligram of protein. Error bars represent standard deviation. (G) Gaucher cells were practically eliminated in BM, spleen, and liver 5 months after transplantation in mice treated with GluCer, whereas GFP-treated mice maintained the massive infiltration of Gaucher cells. The first and second columns are representative of the VCM incubation transduction, and the third and fourth columns are representative of the coculture transduction (hematoxylin and eosin and periodic acid Schiff staining). (Scale bars: 50 μm.)
Discussion
In this study we report a mouse model for type 1 GD. The GD mouse generated here exhibits a severe phenotype with clinical signs that are typically seen in patients with a serious and debilitating form of type 1 GD. By using this model, we also show that gene therapy can correct GD in vivo. The mouse model therefore opens new avenues both for the evaluation of existing therapies and for the development of novel therapeutic approaches for the disorder.
Our findings demonstrate that both transplantation of WT BM and gene therapy through retroviral transduction of BM from the GD mouse could prevent disease development and, more importantly, could correct an already established GD phenotype. Both therapeutic modalities resulted in increased enzyme activities, normal GluCer levels, and practically no infiltration of Gaucher cells in BM, spleen, and liver 5–6 months after treatment. It is noteworthy that this correction was seen in all GC vector-treated mice, despite the relatively low gene marking (Fig. 4). In addition, the BMT and gene therapy experiments demonstrated that achievement of GCase activity comparable with WT levels was not necessary for a favorable therapeutic outcome. The coculture transduction resulted in a much higher increase in GCase activity than gene therapy through VCM transduction and ordinary BMT. The magnitude of increase in GCase activity in the coculture transduction varied between the mice, but, overall, the enzyme activity was well above WT levels in all tissues analyzed. A possible reason for the high enzyme activity may be that the coculture-transduced BM cells obtained multiple copies of the GC vector. Additionally/alternatively, insertional activation of genes could have promoted a selective advantage for single clones as was seen in murine BMT models (28) and in the recently published gene therapy trial for X-linked chronic granulomatous disease (29).
Previous preclinical studies have shown that transduction of hematopoietic progenitor cells (murine and from GD patients) with gammaretroviral vectors containing the GCase gene could correct the enzyme deficiency in the granulocyte-macrophage progeny cells (30–32). Similarly, WT murine HSC could be genetically modified through retroviral transduction to produce high levels of GCase in HSC-derived macrophages in vivo (33, 34). These data constituted the foundation for a clinical gene therapy trial involving three type 1 GD patients. The GCase gene was transferred into autologous CD34+ hematopoietic cells using gammaretroviral vectors, and the treated cells were infused intravenously without any BM ablation regimen (35). However, gene marking was only seen at low levels for 3 months, after which no trace of gene delivery could be detected. It was concluded that HSC-based gene therapy for GD was not likely to be effective without full or partial ablation of the BM before transplantation. Partial ablation techniques were not well developed in a clinical setting during the 1990s, and further clinical gene therapy trials for GD were put on hold because scientists were lacking a suitable animal model for type 1 GD and because of the availability of relatively effective and risk-free enzyme replacement therapy.
Recent success in clinical gene therapy for genetic disease demonstrates the feasibility of this approach in treating patients with X-linked SCID (36), adenosine-deaminase-deficient SCID (37), and chronic granulomatous disease (29). The findings presented here demonstrate the feasibility of gene therapy for type 1 GD in vivo, and the mouse model will serve as an excellent tool in the continued effort toward development of safe and efficient cell and gene therapy for type 1 GD.
Materials and Methods
Generation of the GD Mouse.
A detailed description of how the GD mouse was generated is available in Supporting Materials and Methods, which is published as supporting information on the PNAS web site. Screening of adult mice after polyinosinic–polycytidylic acid-induced exon deletion and embryos was done by PCR with the following primers: 5′-GTACGTTCATGGCATTGCTGTTCACT-3′ (P1 in Fig. 1A) and 5′-ATTCCAGCTGTCCCTCGTCTCC-3′ (P2 in Fig. 1A; see also Figs. 1B and 5 A and B). PCR primers 5′-ACGAGTGATGAGGTTCGCAA-3′ and 5′-AGCGTTTTCGTTCTGCCAAT-3′ verified the presence of the Cre gene. The platinum TaqDNA polymerase (Invitrogen, Carlsbad, CA) was used in the PCR screening of the mice. Mice were maintained in individually ventilated cages with food and water ad libitum in the animal facility at Lund University Biomedical Center. Breeding and experimental procedures were approved by the Committee for Animal Ethics in Malmö/Lund, Sweden.
Vector Construction and Production.
The GCase-containing vector is referred to as GC vector, and the control vector (which is identical to GluCer only without the GCase gene) is referred to as the GFP vector throughout the text. Details concerning the construction and production of the retroviral vectors are given in Supporting Materials and Methods.
Transduction and Transplantations.
Prestimulated BM cells were transduced through VCM incubation or through coculture with GluCer or GFP virus producer cell lines. Details on the prestimulation, transduction, and transplantation procedures are given in Supporting Materials and Methods.
Tissue and Cell Preparations.
Mice were anesthetized (isofluorane; Abbott Scandinavia, Solna, Sweden) before orbital blood sampling. Blood was kept in 2,000 units/ml heparin (Leo Pharma, Ajax, ON, Canada) until analysis. The mice were killed by CO2, and the liver, spleen, and BM were removed and weighed. For evaluation of substrate accumulation, tissue pieces were submerged in liquid nitrogen directly after removal from the mice. Tissue and harvested BM cells destined for enzyme activity analysis were kept in PBS (2% FBS). For histopathology, tissue samples were fixed in PBS supplemented with 4% paraformaldehyde, embedded in paraffin, and sectioned.
FACS.
The transduction efficiency (percentage of GFP) was analyzed in BM cells before transplantation and in peripheral blood 5 months after transplantation on a FACSCalibur (BD Biosciences, Franklin Lakes, NJ). Results were analyzed with FlowJo software (Tree Star Software, San Carlos, CA).
Enzyme Assay.
Cells were lysed by three freeze–thaw cycles. Aliquots of the lysate where incubated for 40 min at 37°C with or without 400 μM conduritol B epoxide (38) (Sigma–Aldrich, St. Louis, MO). GCase activities were determined flourimetrically after 2 h of incubation at 37°C with 15 mM 4-methylumbelliferyl-d-glucuronide trihydrate (Sigma–Aldrich) (30). In all assays, control cells were analyzed, and the level in these was set as 100%.
Hematological Analysis, Substrate Accumulation Analysis, and Histopathological Studies.
Hematological analysis was performed on a Sysmex (Kobe, Japan) automated cell counter. The GluCer content was determined as previously described (39). Fixed, sectioned tissue was stained with hematoxylin and eosin or periodic acid Schiff for microscopic examination.
Supplementary Material
Acknowledgments
We thank Lottie Jansson-Sjöstrand, Jonas Larsson, Per Levéen, Britt-Marie Rynmark, Johan Flygare, Jennifer Moody, Ulrika Blank, Mattias Magnusson, Ann Brun, Eva Gynnstam, and Lilian Wittman for expert help and guidance throughout this study. This work was supported by the Swedish Cancer Society, the European Commission (the INHERINET and CONSERT consortiums), the Swedish Gene Therapy Program, the Swedish Medical Research Council, the Swedish Children Cancer Foundation, a clinical research award from Lund University Hospital, and the Joint Program on Stem Cell Research supported by the Juvenile Diabetes Research Foundation and the Swedish Medical Research Council. The Lund Stem Cell Center is supported by a Center of Excellence grant in life sciences from the Swedish Foundation for Strategic Research.
Abbreviations
- BM
bone marrow
- BMT
BM transplantation
- HSC
hematopoietic stem cell
- GCase
glucosylceramidase
- GluCer
glucosylceramide
- GD
Gaucher disease
- VCM
virus-containing medium.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Brady RO, Kanfer JN, Bradley RM, Shapiro D. J Clin Invest. 1966;45:1112–1115. doi: 10.1172/JCI105417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beutler E, Grabowski GA. In: The Metabolic & Molecular Bases of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Vol 3. New York: McGraw–Hill; 2001. pp. 3635–3668. [Google Scholar]
- 3.Grabowski GA, Horowitz M. Baillieres Clin Haematol. 1997;10:635–656. doi: 10.1016/s0950-3536(97)80032-7. [DOI] [PubMed] [Google Scholar]
- 4.Sidransky E. Mol Genet Metab. 2004;83:6–15. doi: 10.1016/j.ymgme.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Barton NW, Furbish FS, Murray GJ, Garfield M, Brady RO. Proc Natl Acad Sci USA. 1990;87:1913–1916. doi: 10.1073/pnas.87.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barton NW, Brady RO, Dambrosia JM, Di Bisceglie AM, Doppelt SH, Hill SC, Mankin HJ, Murray GJ, Parker RI, Argoff CE, et al. N Engl J Med. 1991;324:1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- 7.Figueroa ML, Rosenbloom BE, Kay AC, Garver P, Thurston DW, Koziol JA, Gelbart T, Beutler E. N Engl J Med. 1992;327:1632–1636. doi: 10.1056/NEJM199212033272304. [DOI] [PubMed] [Google Scholar]
- 8.Weinreb NJ, Charrow J, Andersson HC, Kaplan P, Kolodny EH, Mistry P, Pastores G, Rosenbloom BE, Scott CR, Wappner RS, Zimran A. Am J Med. 2002;113:112–119. doi: 10.1016/s0002-9343(02)01150-6. [DOI] [PubMed] [Google Scholar]
- 9.Beutler E. PLoS Med. 2004;1:e21. doi: 10.1371/journal.pmed.0010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ringden O, Groth CG, Erikson A, Granqvist S, Mansson JE, Sparrelid E. Transplantation. 1995;59:864–870. [PubMed] [Google Scholar]
- 11.Young E, Chatterton C, Vellodi A, Winchester B. J Inherit Metab Dis. 1997;20:595–602. doi: 10.1023/a:1005367328003. [DOI] [PubMed] [Google Scholar]
- 12.Cox T, Lachmann R, Hollak C, Aerts J, van Weely S, Hrebicek M, Platt F, Butters T, Dwek R, Moyses C, Gow I, Elstein D, Zimran A. Lancet. 2000;355:1481–1485. doi: 10.1016/S0140-6736(00)02161-9. [DOI] [PubMed] [Google Scholar]
- 13.Elstein D, Hollak C, Aerts JM, van Weely S, Maas M, Cox TM, Lachmann RH, Hrebicek M, Platt FM, Butters TD, Dwek RA, Zimran A. J Inherit Metab Dis. 2004;27:757–766. doi: 10.1023/B:BOLI.0000045756.54006.17. [DOI] [PubMed] [Google Scholar]
- 14.Sawkar AR, Cheng WC, Beutler E, Wong CH, Balch WE, Kelly JW. Proc Natl Acad Sci USA. 2002;99:15428–15433. doi: 10.1073/pnas.192582899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sidransky E, Ginns EI. Baillieres Clin Haematol. 1997;10:725–737. doi: 10.1016/s0950-3536(97)80036-4. [DOI] [PubMed] [Google Scholar]
- 16.Tybulewicz VL, Tremblay ML, LaMarca ME, Willemsen R, Stubblefield BK, Winfield S, Zablocka B, Sidransky E, Martin BM, Huang SP. Nature. 1992;357:407–410. doi: 10.1038/357407a0. [DOI] [PubMed] [Google Scholar]
- 17.Bornstein P, McKinney CE, LaMarca ME, Winfield S, Shingu T, Devarayalu S, Vos HL, Ginns EI. Proc Natl Acad Sci USA. 1995;92:4547–4551. doi: 10.1073/pnas.92.10.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Suzuki K, Reed JD, Grinberg A, Westphal H, Hoffmann A, Doring T, Sandhoff K, Proia RL. Proc Natl Acad Sci USA. 1998;95:2503–2508. doi: 10.1073/pnas.95.5.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizukami H, Mi Y, Wada R, Kono M, Yamashita T, Liu Y, Werth N, Sandhoff R, Sandhoff K, Proia RL. J Clin Invest. 2002;109:1215–1221. doi: 10.1172/JCI14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beutler E, West C, Torbett BE, Deguchi H. Mol Med. 2002;8:247–250. [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall J, McEachern KA, Kyros JA, Nietupski JB, Budzinski T, Ziegler RJ, Yew NS, Sullivan J, Scaria A, van Rooijen N, Barranger JA, Cheng SH. Mol Ther. 2002;6:179–189. doi: 10.1006/mthe.2002.0650. [DOI] [PubMed] [Google Scholar]
- 22.Xu YH, Quinn B, Witte D, Grabowski GA. Am J Pathol. 2003;163:2093–2101. doi: 10.1016/s0002-9440(10)63566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holleran WM, Ginns EI, Menon GK, Grundmann JU, Fartasch M, McKinney CE, Elias PM, Sidransky E. J Clin Invest. 1994;93:1756–1764. doi: 10.1172/JCI117160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beutler E. Science. 1992;256:794–799. doi: 10.1126/science.1589760. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 26.Leveen P, Larsson J, Ehinger M, Cilio CM, Sundler M, Sjostrand LJ, Holmdahl R, Karlsson S. Blood. 2002;100:560–568. doi: 10.1182/blood.v100.2.560. [DOI] [PubMed] [Google Scholar]
- 27.Larsson J, Blank U, Helgadottir H, Bjornsson JM, Ehinger M, Goumans MJ, Fan X, Leveen P, Karlsson S. Blood. 2003;102:3129–3135. doi: 10.1182/blood-2003-04-1300. [DOI] [PubMed] [Google Scholar]
- 28.Kustikova O, Fehse B, Modlich U, Yang M, Dullmann J, Kamino K, von Neuhoff N, Schlegelberger B, Li Z, Baum C. Science. 2005;308:1171–1174. doi: 10.1126/science.1105063. [DOI] [PubMed] [Google Scholar]
- 29.Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, Glimm H, Kuhlcke K, Schilz A, Kunkel H, et al. Nat Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 30.Fink JK, Correll PH, Perry LK, Brady RO, Karlsson S. Proc Natl Acad Sci USA. 1990;87:2334–2338. doi: 10.1073/pnas.87.6.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nolta JA, Sender LS, Barranger JA, Kohn DB. Blood. 1990;75:787–797. [PubMed] [Google Scholar]
- 32.Nolta JA, Yu XJ, Bahner I, Kohn DB. J Clin Invest. 1992;90:342–348. doi: 10.1172/JCI115868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Correll PH, Colilla S, Dave HP, Karlsson S. Blood. 1992;80:331–336. [PubMed] [Google Scholar]
- 34.Ohashi T, Boggs S, Robbins P, Bahnson A, Patrene K, Wei FS, Wei JF, Li J, Lucht L, Fei Y, et al. Proc Natl Acad Sci USA. 1992;89:11332–11336. doi: 10.1073/pnas.89.23.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunbar CE, Kohn DB, Schiffmann R, Barton NW, Nolta JA, Esplin JA, Pensiero M, Long Z, Lockey C, Emmons RV, et al. Hum Gene Ther. 1998;9:2629–2640. doi: 10.1089/hum.1998.9.17-2629. [DOI] [PubMed] [Google Scholar]
- 36.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basileqq G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL, Bousso P, Deist FL, Fischer A. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 37.Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A, Morecki S, Andolfi G, Tabucchi A, Carlucci F, et al. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 38.Grabowski GA, Osiecki-Newman K, Dinur T, Fabbro D, Legler G, Gatt S, Desnick RJ. J Biol Chem. 1986;261:8263–8269. [PubMed] [Google Scholar]
- 39.Kyllerman M, Conradi N, Mansson JE, Percy AK, Svennerholm L. Acta Paediatr Scand. 1990;79:448–453. doi: 10.1111/j.1651-2227.1990.tb11492.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.