Abstract
Fatty acid synthesis in the central nervous system is implicated in the control of food intake and energy expenditure. An intermediate in this pathway, malonyl-CoA, mediates these effects. Malonyl-CoA is an established inhibitor of carnitine palmitoyltransferase-1 (CPT1), an outer mitochondrial membrane enzyme that controls entry of fatty acids into mitochondria and, thereby, fatty acid oxidation. CPT1c, a brain-specific enzyme with high sequence similarity to CPT1a (liver) and CPT1b (muscle) was recently discovered. All three CPTs bind malonyl-CoA, and CPT1a and CPT1b catalyze acyl transfer from various fatty acyl-CoAs to carnitine, whereas CPT1c does not. These findings suggest that CPT1c has a unique function or activation mechanism. We produced a targeted mouse knockout (KO) of CPT1c to investigate its role in energy homeostasis. CPT1c KO mice have lower body weight and food intake, which is consistent with a role as an energy-sensing malonyl-CoA target. Paradoxically, CPT1c KO mice fed a high-fat diet are more susceptible to obesity, suggesting that CPT1c is protective against the effects of fat feeding. CPT1c KO mice also exhibit decreased rates of fatty acid oxidation, which may contribute to their increased susceptibility to diet-induced obesity. These findings indicate that CPT1c is necessary for the regulation of energy homeostasis.
Keywords: acetyl-CoA carboxylase, fatty acid synthase, food intake, malonyl-CoA, obesity
Body weight is maintained by regulating food intake and energy expenditure. This balance is monitored by the central nervous system (CNS) in response to cytokine and endocrine signals, including leptin, ghrelin, obestatin, insulin, cholecystokinin, and peptide YY secreted by peripheral tissues. Concomitantly, parallel pathways in the CNS regulate energy balance by monitoring the availability of neuronal energy-rich metabolic substrates. Integration of these signals occurs in the hypothalamus and, ultimately, in higher brain centers where feeding behavior and energy expenditure are adjusted. Two primary indicators of energy surplus, glucose and fatty acids, are also monitored by subsets of hypothalamic neurons that modulate feeding behavior and energy expenditure (1). Fatty acids (2) and de novo fatty acid synthesis from glucose (3) are known to mediate these effects. Indeed, food intake and body weight have been shown to be altered by manipulating the activities of the enzymes involved in fatty acid synthesis, e.g., fatty acid synthase (FAS) (3), malonyl-CoA decarboxylase (4, 5), acetyl-CoA carboxylase (ACC) (6, 7), stearoyl-CoA desaturase (8, 9), and 5′-AMP kinase (10, 11).
Inhibition of FAS in the CNS, for example, reduces body weight by rapidly provoking a reduction in food intake and an increase in peripheral energy expenditure (3, 12). This inhibition can reverse the weight gain caused by diet-induced obesity (13, 14) or mutations in leptin (ob/ob) or its receptor (db/db) (3, 15), suggesting that it acts independently of STAT3, which is known to be essential for leptin’s action (16, 17). Inhibition of FAS increases the level of its substrate, malonyl-CoA, in the hypothalamus (18). The formation of malonyl-CoA from acetyl-CoA catalyzed by ACC is the primary regulatory step in fatty acid synthesis. There is now compelling evidence that malonyl-CoA is a signaling mediator of energy balance in the CNS and is physiologically regulated by fasting and refeeding (18). However, neither the target nor the molecular mechanism of malonyl-CoA’s action in the CNS has been elucidated.
A known target of malonyl-CoA in liver and muscle is carnitine palmitoyltransferase-1 (CPT1; palmitoyl-CoA:l-carnitine O-palmitoyltransferase, EC 2.3.1.21), which catalyzes the rate-limiting step of β-oxidation by translocating fatty acids across the mitochondrial membranes. Malonyl-CoA inhibits CPT1 action, thus blocking the oxidation of fatty acids during times of fatty acid synthesis and energy surplus (19–21). Pharmacologic inhibition of CPT1 in the CNS inhibits food intake, suggesting malonyl-CoA may suppress food intake through a CNS-expressed CPT1 (22). Recently, it was shown that mammals express another CPT1, i.e., CPT1c, which is brain-specific and has high homology to liver CPT1a and muscle CPT1b (23). CPT1c is expressed exclusively in the CNS with high local expression in areas critical for the regulation of energy homeostasis (ref. 22; Y.D., M.J.W., and M.D.L, unpublished results). CPT1c binds malonyl-CoA but, unlike CPT1a, is unable to catalyze acyl transfer from fatty acyl-CoAs to carnitine. To assess the physiological role of this putative enzyme in energy homeostasis, we produced CPT1c knockout (KO) mice. We found that disruption of the CPT1c gene leads to decreased food intake and body weight. Paradoxically, CPT1c KO mice are exceptionally susceptible to diet-induced obesity on a high-fat diet.
Results
CPT1c Binds Malonyl-CoA but Does Not Catalyze Fatty Acyl Transfer to Carnitine.
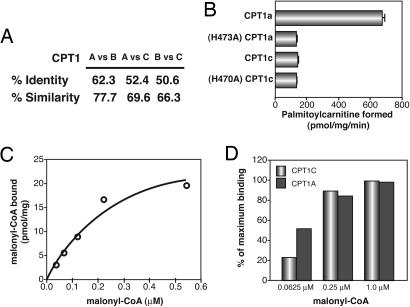
CPT1c was identified and cloned based on homology searching of EST databases with known CPT sequences (23). CPT1c is apparently a relatively recent gene duplication, because it has been found only in mammals. CPT1c protein was shown to be expressed exclusively in the brain (23), whereas CPT1a had limited expression in the brain, and CPT1b was not expressed (ref. 22; Y.D. and M.D.L, unpublished data). CPT1c has a high degree of primary amino acid similarity and identity to CPT1a and CPT1b (Fig. 1A). Based on this high degree of sequence similarity and in silico modeling, it was classified as a CPT1. However, whereas CPT1a was able to catalyze palmitoyl transfer from palmitoyl-CoA to carnitine in vitro, CPT1c and the inactive mutants of CPT1a (H473A) and CPT1c (H470A) were not (Fig. 1B). This finding agrees with previous data using medium, long, and very long chain acyl-CoAs and yeast-expressed CPT1a and CPT1c (23). We have extended the analysis to test acyl-CoA thioesters of various chain lengths and saturations. Acyl-CoAs were enzymatically synthesized from the corresponding free fatty acid by using Pseudomonas acyl-CoA synthetase. We expressed the CPT1 isoforms and isolated mitochondria from mammalian cells to more closely model their in vivo environment. All were tested for acyl transfer to carnitine catalyzed by CPT1a and CPT1c. We have been unable to detect transfer to carnitine in vitro from any acyl-CoA tested by using CPT1c (Table 1).
Fig. 1.
Comparison of amino acid sequence and functional properties of CPT1a and CPT1c. (A) Amino acid sequence identity and similarity comparison between CPT1a, CPT1b, and CPT1c. (B) Mitochondria from 293 T cells transfected with expression vectors for CPT1a, CPT1a (H473A), CPT1c, or CPT1c (H470A) were used in carnitine acyltransferase assays with palmitoyl-CoA (75 μM) as a substrate. “mg” refers to mitochondrial protein. (C) Concentration dependence of malonyl-CoA binding to CPT1c. (D) Comparison of malonyl-CoA binding to CPT1a and CPT1c.
Table 1.
Carnitine acyltransferase activity using a diverse array of fatty acyl-CoA derivatives
| Fatty acid | CPT1c | CPT1a |
|---|---|---|
| 6:0 | − | − |
| 12:0 | − | ++ |
| 12:1 (11) | − | ++ |
| 13:1 (12) | − | ++ |
| 14:0 | − | ++ |
| 16:0 | − | +++ |
| 16:1 (7) | − | +++ |
| 17:0 | − | +++ |
| 18:1 (9) | − | +++ |
| 18:3 (9, 12, 15) | − | ++ |
| 20:3 (11, 14, 17) | − | − |
| 20:4 (8, 11, 14, 17) | − | − |
| 20:5 (5, 8, 11, 14, 17) | − | − |
| 22:5 (7, 10, 13, 16, 19) | − | − |
| 14:1 (9) | − | +++ |
| 14:1 (9, trans) | − | ++ |
| 15:1 (10) | − | +++ |
| 18:2 (9, 12) | − | + |
| 18:3 (6, 9, 12) | − | +++ |
| 20:3 (8, 11, 14) | − | − |
| 20:4 (5, 8, 11, 14) | − | − |
| 16:1 (9, trans) | − | +++ |
| 17:1 (10) | − | +++ |
| 18:1 (11) | − | ++ |
| 18:1 (11, trans) | − | +++ |
| 18:1 (9) 12-OH | − | − |
| 20:4 (5, 8, 11, 14) | − | − |
| 16:1 (9, trans) | − | +++ |
| 17:1 (10) | − | +++ |
| 18:1 (11) | − | ++ |
| 18:1 (11, trans) | − | +++ |
| 18:1 (9) 12-OH | − | − |
| 24:1 (15) | − | − |
| 20:3 (5, 8, 11) | − | − |
| 18:1 (6) | − | + |
| 18:1 (6, trans) | − | +++ |
| 20:1 (8) | − | − |
| 20:1 (5) | − | − |
| 10:0 2-OH | − | − |
| 12:0 2-OH | − | − |
| 14:0 2-OH | − | − |
| 16:0 2-OH | − | ++ |
| 11:0 3-OH | − | ++ |
| 14:0 3-OH | − | ++ |
| 18:0 12-OH | − | ++ |
| 18:0 15-OH | − | ++ |
| 12:00 9-Me | − | ++ |
| 12:00 10-Me | − | ++ |
| 18:00 16-Me | − | ++ |
| 10:00 di-COOH | − | − |
| 15:00 di-COOH | − | − |
| α-Lipoic acid | − | − |
+, >0.2 nmol/mg per min at 60 μM; ++, 0.2–0.4 nmol/mg per min at 30 μM; +++, >0.4 nmol/mg per min at 30 μM; −, not detectable.
Hypothalamic malonyl-CoA is known to mediate food intake and energy expenditure. The molecular target of malonyl-CoA in the hypothalamus is not known, but CPT1c is a provocative candidate, given that it is brain-specific and is highly expressed in regions of the hypothalamus known to mediate energy homeostasis (ref. 23; Y.D. and M.D.L., unpublished data). Malonyl-CoA indeed binds to CPT1c with a Kd similar to that of CPT1a (Fig. 1 C and D) (23). Importantly, the CPT1c Kd (≈0.3 μM) is within the dynamic range of hypothalamic (malonyl-CoA) in fasted and refed states (18). This finding indicates that malonyl-CoA has the ability to interact with CPT1c and identifies CPT1c as a potential malonyl-CoA target in control of energy homeostasis.
Disruption of the cpt1c Gene Results in Lower Body Weight and Decreased Food Intake.
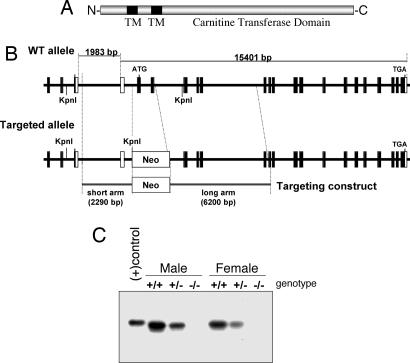
To understand the physiological role of CPT1c in energy homeostasis, we used homologous recombination in ES cells to produce a targeted KO of exons 1 and 2, encoding the translation initiation site and the first transmembrane domain of CPT1c, and replaced this genomic region with a neomycin-resistance cassette (Fig. 2A and B). After selection in vitro, targeted ES cells were injected into C57BL/6 blastocysts. Resulting chimeric males were bred to C57BL/6 WT mice, and founders were identified by PCR genotyping. Intercrossing heterozygous animals resulted in the birth and weaning of CPT1c KO mice at the expected Mendelian ratio with no apparent developmental abnormalities. Western blot analysis of CPT1c expression in whole brain revealed the KO was complete and that the heterozygotes exhibited approximately half the expression of WT littermates (Fig. 2C).
Fig. 2.
Generation of CPT1c KO mice. (A) Predicted domain structure of CPT1c. TM, transmembrane domain. (B) Exons 1 and 2 containing the initiation codon and first transmembrane domain were targeted by homologous recombination in ES cells. Neo, neomycin-resistance cassette. (C) Western blots of whole-brain lysates of CPT1c WT and KO mice. KO mice expressed no detectable CPT1c, and heterozygous mice exhibited approximately half the expression of WT mice. “(+)control” refers to a FLAG-tagged CPT1c.
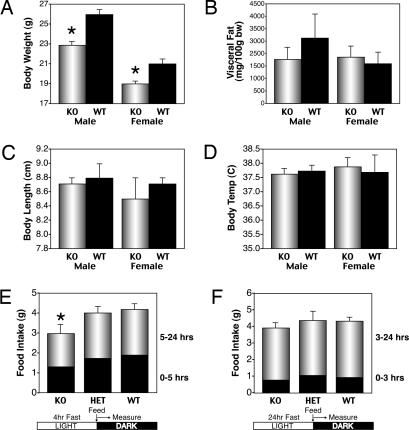
Animals were fed a standard laboratory chow, and, at 12–15 weeks, whole-body measurements of body weight, organ weight, visceral fat weight, body size, temperature, and food intake were made. KO males and females weighed significantly less (≈15%) than WT littermates (Fig. 3A). KO males tended to have less visceral fat, consistent with their smaller size (Fig. 3B). Male and female KO animals and WT littermates were equivalent in body length, indicating normal growth (Fig. 3C). Organ size measured as percent of body weight was normal for brain, pituitary, heart, lungs, thymus, liver, and spleen (data not shown). Body temperature did not differ consistently between groups (Fig. 3D).
Fig. 3.
CPT1c KO mice are hypophagic and have a lower body weight than WT mice. (A) Body weight was significantly lower in CPT1c KO males (∗, P < 0.0001; n = 10 per group) and females (∗, P < 0.005; n = 10 per group). (B) CPT1c KO male mice have a trend toward increased visceral adipose tissue (n = 6 per group). The CPT1c KO does not affect body length (C) or temperature (D) (n = 6 per group). (E) CPT1c KO mice exhibit lower food intake than WT or heterozygous littermates (∗, P < 0.05; n = 6 per group) after a short (4-h) fast just before the dark cycle. (F) Effect of CPT1c KO on food intake after a 24-h fast. Error bars indicate SEM.
Food intake of individually housed mice was measured under several conditions. In one experiment, mice were fasted for 4 h at the end of the light cycle, then given food at the beginning of the dark cycle, and food consumption was measured for 24 h. Food intake in KO animals was less than (by ≈25%) WT littermates (Fig. 3E). The suppression of ad libitum food intake is the most probable cause of the lower body weight in CPT1c KO mice. However, when mice were fasted for 24 h before, were given food as they entered the dark cycle, and food intake was measured for 24 h, little difference in food intake was observed, although there was a trend toward decreased food intake (Fig. 3F).
CPT1c KO Mice Are Susceptible to a High-Fat Diet.
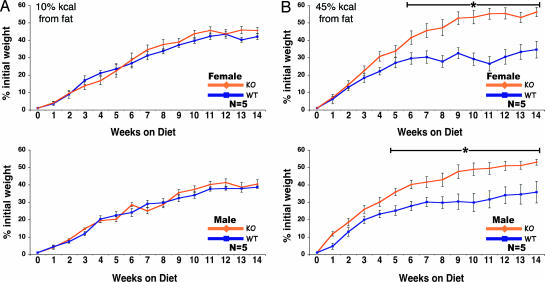
Because CPT1c KO animals exhibit reduced food intake and lower body weight on normal laboratory chow, we sought to determine whether these decreases would be protective against weight gain on a high-fat diet. We produced KO and WT littermate animals by in vitro fertilization to ensure that all animals in the study (n = 5 per group) were age, sex, and littermate matched. At ≈15 weeks of age, group-housed animals (five per cage) were switched to diets in which either 10% (control) or 45% (high-fat) of the calories were from fat. The dietary fat was derived from lard, which is high in nonessential monounsaturated fatty acids.
Male and female KO mice initially weighed less than their WT littermates. Male and female KO animals on the control diet consistently weighed less (≈2 g) than their WT littermates for the subsequent 14-week experiment. They also had an equivalent weight gain on the control diet (Fig. 4A). Unexpectedly, on the high-fat diet, both male and female KO mice began to rapidly gain weight (Fig. 4B). Total body weight of the KO mice was initially lower (week 0), equivalent at week 5, and greater than WT after 5 weeks. Weight gain was significantly greater in both KO male and female mice. These results indicate that CPT1c is protective against the effects of a high-fat diet on weight gain.
Fig. 4.
CPT1c KO mice are more susceptible to the effect of a high-fat diet. Male and female WT and CPT1c KO mice (n = 5 per group) were fed a control diet (10% of total kcal from fat; 1 kcal = 4.18 kJ) (A) or a high-fat diet (45% of total kcal from fat) (B) for 14 weeks. Animals were weighed weekly at 1400 hours. Initial body weights (average grams) were as follows: male WT, 25.9 g; male KO, 22.9 g; female WT, 20.9 g; and female KO, 19.0 g (∗, P < 0.05).
An independent experiment showed that individually housed KO mice on a high-fat diet had lower food intake than WT mice, suggesting that energy expenditure was altered in the CPT1c KO animals. This phenotype suggests a specific interaction of CPT1c with dietary fat. Indeed, this phenotype (eating less while gaining more weight) is opposite to that of stearoyl-CoA desaturase 1 KO (9) and ACCβ KO (6, 7) mice, both of which eat more and are resistant to diet-induced obesity. CPT1c may promote the metabolism of a fatty acid derivative, perhaps monounsaturated fatty acid, that is important for body weight regulation.
Effect of Disruption of CPT1c on Plasma Constituents.
At the end of the 14-week feeding study described above (Fig. 4), mice were fasted for 14 h, and plasma was collected and analyzed for various blood constituents, including nonesterified fatty acids, triglycerides, phospholipids, cholesterol, insulin, and glucose (Table 2). CPT1c KO females, but not males, had a significantly higher triglyceride level but were in the normal range.
Table 2.
Metabolic parameters in WT and CPT1c KO mice fed a low-fat of high-fat diet for 14 weeks
| Metabolite | 10% of kcal from fat diet |
45% of kcal from fat diet |
||||||
|---|---|---|---|---|---|---|---|---|
| Male |
Female |
Male |
Female |
|||||
| KO (n = 5) | WT (n = 5) | KO (n = 5) | WT (n = 5) | KO (n = 5) | WT (n = 5) | KO (n = 5) | WT (n = 5) | |
| Glucose, mg/dl | 103 ± 5.5 | 108 ± 4.2 | 101 ± 7.3 | 104 ± 7.3 | 156 ± 20.9 | 123 ± 13.4 | 134 ± 6.6* | 101 ± 7.2 |
| Insulin, ng/liter | 1.9 ± 0.16 | 1.9 ± 0.09 | 1.8 ± 0.11 | 1.9 ± 0.10 | 2.3 ± 0.14† | 1.9 ± 0.10 | 1.9 ± 0.11 | 1.8 ± 0.07 |
| Nonesterified fatty acid, mg/dl | 1.15 ± 0.09 | 1.11 ± 0.11 | 1.00 ± 0.05 | 0.84 ± 0.09 | 1.05 ± 0.13 | 0.91 ± 0.07 | 0.93 ± 0.05 | 0.88 ± 0.09 |
| Triglycerides, mg/dl | 60.6 ± 6.3 | 59.3 ± 4.6 | 90.1 ± 9.2‡ | 31.2 ± 4.6 | 81.3 ± 7.4 | 68.5 ± 6.6 | 49.7 ± 4.2 | 84.5 ± 23.8 |
| Phospholipids, mg/dl | 279 ± 16.6 | 300 ± 15.7 | 215 ± 10.7 | 200 ± 15.5 | 320 ± 23.5§ | 233 ± 30.9 | 230 ± 7.9† | 189 ± 13.3 |
| Cholesterol, mg/dl | 34.7 ± 3.7 | 38.8 ± 3.0 | 24.4 ± 1.8 | 19.4 ± 2.3 | 44.3 ± 4.7 | 33 ± 6.3 | 28.1 ± 1.4 | 23.5 ± 3.2 |
Comparison of WT vs. KO by using Student’s t test:
∗, P < 0.009;
†, P < 0.04;
‡, P < 0.001;
§, P = 0.054.
Both male and female KO mice on the high-fat diet exhibited markedly increased plasma phospholipids. Plasma phospholipids are most likely a result of hepatic steatosis (nonalcoholic fatty liver disease), which was evident at necropsy in both male and female KO animals on the high-fat diet. Both male and female KO mice showed a trend toward insulin resistance, because males had a higher blood insulin concentration, and females had a higher blood glucose concentration.
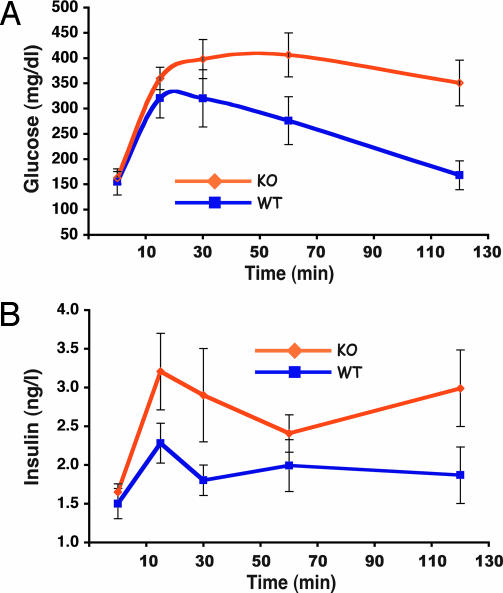
To access the glucose and insulin dynamics, i.p. glucose tolerance tests were conducted on fasted WT and KO male mice at 17 weeks. KO mice exhibited increased and prolonged glucose clearance accompanied by an increase in plasma insulin (Fig. 5 A and B). This result demonstrates that the CPT1c KO mice were mildly insulin-resistant. This finding was consistent with an increased amount of adipose tissue in KO animals and is most likely secondary to increased adiposity, because KO animals on the control diet exhibited normal glucose and insulin levels (Table 2).
Fig. 5.
CPT1c KO mice exhibit mild insulin resistance when fed a high-fat diet. WT and CPT1c KO mice fed a high-fat diet for 17 weeks were subjected to an i.p. glucose tolerance test. CPT1c KO mice exhibited an elevated and prolonged plasma glucose level (A) and concomitant elevated plasma insulin level (B) indicative of insulin resistance (n = 5 per group).
Fasted CPT1c KO Mice Exhibit Reduced Whole-Body Fatty Acid Oxidation.
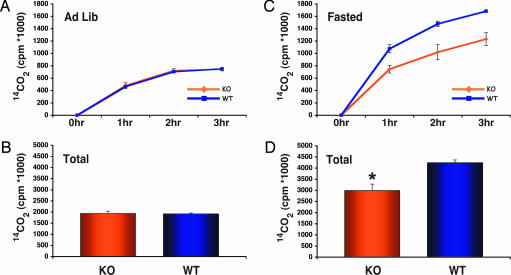
When the concentration of hypothalamic malonyl-CoA rises, whole-body fatty acid oxidation increases due largely to increased β-oxidation in skeletal muscle (12). These and other findings show that the “malonyl-CoA signal” is rapidly transmitted from the CNS to skeletal muscle by way of the sympathetic nervous system. Because CPT1c was unable to catalyze acyl transfer to carnitine from fatty acyl-CoAs in vitro (Fig. 1A and Table 1), we performed in vivo whole-body fatty acid oxidation studies with WT and CPT1c KO mice to determine whether peripheral signaling is affected by the brain-specific CPT1c. Mice were fasted for 24 h as previously described and then given injections of 14C-labeled fatty acid (12). Expired 14CO2 was trapped in NaOH and quantified. In the ad libitum state, WT and KO animals had equivalent rates of fatty acid oxidation (Fig. 6 A and B). In the fasted state, however, oxidation increased substantially in both WT and KO animals, with KO animals exhibiting a markedly lower rate of fatty acid oxidation (Fig. 6 C and D). This finding is indicative of decreased energy expenditure that may contribute to the increased weight gain observed in KO animals on the high-fat diet.
Fig. 6.
CPT1c KO mice exhibit lower fasting whole-body fatty acid oxidation. WT and CPT1c KO weight- and sex-matched mice were tested for their ability to β-oxidize [1-14C]oleic acid to 14CO2 at 1, 2, and 3 h after i.p. injection of labeled fatty acid. (A and C) Rate of 14CO2 production. (B and D) Total 14CO2 production. (A and B) In the ad libitum fed state, WT and CPT1c KO mice exhibited equivalent rates of fatty acid oxidation. (C and D) In the fasted state (24 h), CPT1c KO mice had significantly decreased fatty acid oxidation rates at 1, 2, and 3 h (C) and decreased total fasting fatty acid oxidation (D) (∗, P < 0.007).
Discussion
The regulation and maintenance of energy homeostasis is vital for normal physiological function and survival. An imbalance in energy regulation (obesity and anorexia) often leads to multiple pathological sequelae, such as type 2 diabetes, coronary heart disease, and cancer. Environmental factors, such as stress, food availability, and diet composition, and inherited factors impact body weight. The CNS, notably the hypothalamus, plays a major role in energy homeostasis by sensing and responding to peripheral physiological cues (24, 25).
One mechanism for sensing energy status is through changes in the levels of intermediates in the fatty acid biosynthetic pathway in the CNS (1). The inhibition of FAS in the CNS causes a rapid suppression of food intake and an increase in peripheral energy expenditure, resulting in substantial weight loss in obese rodents (14). This effect is reversed by inhibition of ACC, the enzyme that produces malonyl-CoA, the substrate for FAS (3). A large body of evidence indicates that malonyl-CoA serves as an intermediary in a signaling circuit that regulates feeding behavior (1). Malonyl-CoA in the hypothalamus is regulated by fasting and refeeding (12), Moreover, lowering malonyl-CoA by the expression of malonyl-CoA decarboxylase in the hypothalamus causes hyperphagia and weight gain (4, 5) and reverses the effect of FAS inhibitors (4). The identification of downstream targets of malonyl-CoA would greatly enhance our understanding of the molecular mechanism of homeostatic control of energy balance. Previously, it was shown that inhibiting CPT1 function in the hypothalamus results in the suppression of food intake (22). Malonyl-CoA is an established physiological inhibitor of CPT1a (liver) and CPT1b (muscle), thus CPT1 is a logical target of the inhibitor (19–21). However, the CNS, notably the hypothalamus (Y.D., M.J.W., and M.D.L., unpublished results), expresses a low level of CPT1a and no CPT1b. However, a previously unrecognized brain-specific CPT1, i.e., CPT1c, was recently discovered.
We and others have shown that, unlike CPT1a and CPT1b, CPT1c does not catalyze the acyltransferase activity by using the prototypic substrates, i.e., fatty acyl-CoA derivatives and carnitine (ref. 23 and Table 1). However, CPT1c does bind malonyl-CoA (Fig. 1B). We have further demonstrated that CPT1c, unlike CPT1a, does not support fatty acid oxidation by cells transfected with the respective genes in cell culture (M.J.W. and M.D.L., unpublished results). This finding suggests that CPT1c may require a CNS neuron-specific covalent modification or allosteric activation that is absent in our heterologous system. Alternatively, CPT1c may use a unique acyl donor or acceptor substrate. We have tested numerous acyl-CoA thioesters of various chain length and saturation as acyl donors, none of which served as substrates (Table 1). It should be noted that the putative carnitine-binding site is conserved at the primary amino acid sequence level, suggesting that carnitine or a carnitine-like molecule act as the acyl acceptor (26). Thus, the reaction catalyzed by CPT1c remains unresolved.
The targeted KO of CPT1c results in a complex metabolic phenotype. CPT1c KO animals exhibit decreased food intake, resulting in a lower body weight (Fig. 3). This result is consistent with the hypothesis that CPT1c is a target of malonyl-CoA in the CNS that mediates control of food intake. Increasing malonyl-CoA concentration in the CNS results in food-intake suppression and weight loss (18). It follows that CPT1c activity enhances food intake and may be suppressed by malonyl-CoA, although further rigorous experimentation is required. CPT1c KO animals are not, however, resistant to a high-fat diet. They, in fact, are more susceptible. KO animals gain substantially more weight than WT littermates. On an even higher-fat diet (60% of kcal from fat) the difference is greater, and heterozygotes, which exhibit approximately half the expression of CPT1c of WT animals, have an intermediate weight gain (data not shown). Food intake in KO mice remains lower than WT mice on a high-fat diet, suggesting that peripheral energy expenditure is affected. Fasting fatty acid oxidation is lower in KO animals, which may account for the increased susceptibility to a high-fat diet.
These results highlight the interaction of fatty acids and the regulation of energy expenditure. Evidence that fatty acid metabolism in the CNS regulates energy homeostasis is now overwhelming. AMP kinase activity in the hypothalamus has been shown to modulate food intake and body weight and is physiologically regulated by fasting and refeeding (10, 11). AMP kinase activity impacts fatty acid biosynthesis by phosphorylating and inactivating ACC, thus, lowering malonyl-CoA concentration and thereby fatty acid biosynthesis (27). Elevating hypothalamic malonyl-CoA concentration lowers food intake (3, 18), whereas lowering malonyl-CoA concentration increases food intake and obesity (4, 5). Mice with a targeted KO of ACCβ exhibit increased food intake while remaining lean due to an increased energy expenditure (6, 7). Stearoyl-CoA desaturase 1 (which catalyzes the rate-limiting step of monounsaturated fatty acid synthesis) KO mice also consume more food while burning more energy and are obesity-resistant (8, 9). CPT1c KO mice have the inverse phenotype, i.e., eating less and gaining more. We suggest that CPT1c metabolizes a fatty acid product in the CNS that has weight-modifying capabilities.
These findings raise important questions. What is the origin of fatty acids in the hypothalamus, and what is the molecular mechanism by which they act to alter food intake and energy homeostasis? It is widely recognized that free fatty acids do not readily gain access to the CNS and that the CNS does not use fatty acids as a major fuel source. Rather, glucose is used almost exclusively in the fed state, whereas ketones (derived from fatty acids) are a major fuel source in the CNS during prolonged fasting. Why, then, are fatty acids in the CNS so intimately involved in energy homeostasis? Furthermore, blood levels of fatty acids are elevated during times of fasting, the inverse of what would be expected for an appetite-suppressing molecule. Clearly, further studies on the metabolism/role of fatty acids in the CNS on energy homeostasis are needed to understand how these energy-rich molecules affect how the brain senses and responds to peripheral energy sources.
Materials and Methods
Animals.
Mice with a targeted KO of the cpt1c gene were generated by standard methodology in ES cells. Exons 1 and 2, encompassing the translation initiation codon and first transmembrane domain, were replaced by a neomycin-resistance cassette. Genomic DNA was extracted from mouse tail tips by using a DNeasy 96 tissue kit (Qiagen, Valencia, CA). Real-time quantitative PCR was performed by using the PRISM 7900 Sequence Detection System (Applied Biosystems) with SYBR green PCR master mix (Applied Biosystems) according to the manufacturer’s recommendations. The copy number of CPT1c and the neomycin-resistance gene in genomic DNA were quantified and normalized to G3PDH. Primers were as follows: CPT1c, 5′-AGAAGTAGAGCTCAGCTCGCCA and 5′-CCAGAGATGCCTTTTCCAGGAG; neomycin-resistance cassette, 5′-CAGCTGTGCTCGACGTTGTC and 5′-CCAATAGCAGCCAGTCCCTTC; G3PDH, 5′-GGAGCGAGACCCCACTAACA and 5′-ACATACTCAGCACCGGCCTC. Genotyping showed the neomycin-resistance cassette to CPT1c ratio to be 0:2, 1:1, and 2:0 for WT, heterozygous, and homozygous KO mice, respectively.
Mice were fed a standard laboratory chow (Harlan 2018; Harlan Teklad, Madison, WI) after weaning. For longitudinal diet studies, age- and sex-matched littermates where produced en mass through in vitro fertilization. The diet-induced susceptibility studies were conducted with 10% of kcal from fat (D12450B; Research Diets, New Brunswick, NJ) or 45% of kcal from fat (D12451; Research Diets) diets for 14 weeks. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and under the approval of the Johns Hopkins Medical School Animal Care and Use Committee.
Malonyl-CoA Binding.
CPT1a and CPT1c expression vectors (pcDNA3.1) were used to transfect HEK293T cells. After 48 h, cells were washed with PBS, homogenized in 0.25 M mannitol/5 mM Hepes (pH 7)/1 mM EGTA and centrifuged at 800 × g for 6 min. The supernatant was centrifuged at 8,000 × g for 10 min, and the crude mitochondrial pellet was resuspended in the homogenization buffer. Binding assays were performed at 4°C for 20 min in 90 mM Hepes (pH 7.4)/60 mM KCl/5 mM MgCl2/1 mM DTT/1% (wt/vol) defatted BSA containing 0.8–1 mg of mitochondrial protein and [2-14C]malonyl-CoA (CFA570; Amersham Pharmacia) in the presence or absence of 100 μM unlabeled malonyl-CoA. After centrifugation at 15,000 × g for 10 min at 4°C, the supernatants were aspirated, and 0.25 ml of 1 M KOH was added to solubilize the pellets at 50°C for 30 min, after which the samples were counted. Specific binding equals total [14C]malonyl-CoA bound minus binding in the presence of 100 μM unlabeled malonyl-CoA.
Immunoblotting.
CPT1c immunoblotting was done by using antibodies produced in rabbits against the peptide CKTVDPNTPTSSTNL. Antibodies were affinity purified from rabbit serum by using this peptide. The antibody was tested against epitope-tagged mCPT1a and mCPT1c constructs and shown to be specific for mCPT1c. A horseradish peroxidase-conjugated secondary anti-rabbit antibody was used and visualized by using SuperSignal chemiluminescent substrate (Pierce).
Analysis of Plasma Constituents.
Blood samples were taken from cut tail tips of conscious fasted (14 h) mice. Plasma was collected for measurement of glucose (glucose oxidase method, QuantiChrom; Bioassay Systems, Hayward, CA), insulin (double antibody rat RIA; Linco Research, St. Charles, MO), triglycerides (enzymatic method; Sigma), nonesterified fatty acids (enzymatic method, NEFA-C; Wako Pure Chemical, Osaka), free cholesterol, and phospholipids (Wako Pure Chemical).
Glucose-Tolerance Test.
Intraperitoneal glucose tolerance tests were performed as described in ref. 17.
Carnitine Fatty Acyltransferase Activity.
Acyl-CoA esters were produced from free fatty acids by using Pseudomonas acyl-CoA synthetase. Mitochondrial pellets were prepared from HEK293T cells transfected or not with CPT1c or CPT1a expression vectors, as described in Fig. 1. Acyl-CoA thioesters were incubated for 10 min at 30°C with 200 μl of assay buffer [150 mM KCl/20 mM Hepes (pH 7.4) containing 5 mM MgCl2, 1 mM EGTA, 5 mM ATP, 1% free fatty acid-free BSA (Sigma), 1 mM DTT, 4 μg/ml rotenone, and 2 μg/ml antimycin A], 100 μg of crude mitochondria, and l-[methyl-3H]carnitine (Amersham Pharmacia) diluted with unlabeled carnitine. Reaction mixtures were then extracted with 1-butanol for the measurement of the acyl-[3H]carnitine formed.
Measurement of Fatty Acid Oxidation.
Whole-animal fatty acid oxidation was measured as described in ref. 12.
Acknowledgments
This work was supported by Astellas Pharma Inc., Tsukuba, Japan.
Abbreviations
- ACC
acetyl-CoA carboxylase
- CPT
carnitine palmitoyltransferase
- FAS
fatty acid synthase
- KO
knockout.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Dowell P., Hu Z., Lane M. D. Annu. Rev. Biochem. 2005;74:515–534. doi: 10.1146/annurev.biochem.73.011303.074027. [DOI] [PubMed] [Google Scholar]
- 2.Obici S., Feng Z., Morgan K., Stein D., Karkanias G., Rossetti L. Diabetes. 2002;51:271–275. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- 3.Loftus T. M., Jaworsky D. E., Frehywot G. L., Townsend C. A., Ronnett G. V., Lane M. D., Kuhajda F. P. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 4.Hu Z., Dai Y., Prentki M., Chohnan S., Lane M. D. J. Biol. Chem. 2005;280:39681–39683. doi: 10.1074/jbc.C500398200. [DOI] [PubMed] [Google Scholar]
- 5.He W., Lam T. K., Obici S., Rossetti L. Nat. Neurosci. 2006;9:227–233. doi: 10.1038/nn1626. [DOI] [PubMed] [Google Scholar]
- 6.Abu-Elheiga L., Matzuk M. M., Abo-Hashema K. A., Wakil S. J. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Elheiga L., Oh W., Kordari P., Wakil S. J. Proc. Natl. Acad. Sci. USA. 2003;100:10207–10212. doi: 10.1073/pnas.1733877100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ntambi J. M., Miyazaki M., Stoehr J. P., Lan H., Kendziorski C. M., Yandell B. S., Song Y., Cohen P., Friedman J. M., Attie A. D. Proc. Natl. Acad. Sci. USA. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen P., Miyazaki M., Socci N. D., Hagge-Greenberg A., Liedtke W., Soukas A. A., Sharma R., Hudgins L. C., Ntambi J. M., Friedman J. M. Science. 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 10.Andersson U., Filipsson K., Abbott C. R., Woods A., Smith K., Bloom S. R., Carling D., Small C. J. J. Biol. Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 11.Minokoshi Y., Alquier T., Furukawa N., Kim Y. B., Lee A., Xue B., Mu J., Foufelle F., Ferre P., Birnbaum M. J., et al. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 12.Cha S. H., Hu Z., Chohnan S., Lane M. D. Proc. Natl. Acad. Sci. USA. 2005;102:14557–14562. doi: 10.1073/pnas.0507300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar M. V., Shimokawa T., Nagy T. R., Lane M. D. Proc. Natl. Acad. Sci. USA. 2002;99:1921–1925. doi: 10.1073/pnas.042683699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thupari J. N., Kim E. K., Moran T. H., Ronnett G. V., Kuhajda F. P. Am. J. Physiol. 2004;287:E97–E104. doi: 10.1152/ajpendo.00261.2003. [DOI] [PubMed] [Google Scholar]
- 15.Cha S. H., Hu Z., Lane M. D. Biochem. Biophys. Res. Commun. 2004;317:301–308. doi: 10.1016/j.bbrc.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Vaisse C., Halaas J. L., Horvath C. M., Darnell J. E., Jr, Stoffel M., Friedman J. M. Nat. Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 17.Gao Q., Wolfgang M. J., Neschen S., Morino K., Horvath T. L., Shulman G. I., Fu X. Y. Proc. Natl. Acad. Sci. USA. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Z., Cha S. H., Chohnan S., Lane M. D. Proc. Natl. Acad. Sci. USA. 2003;100:12624–12629. doi: 10.1073/pnas.1834402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGarry J. D., Foster D. W. Annu. Rev. Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- 20.McGarry J. D., Mannaerts G. P., Foster D. W. J. Clin. Invest. 1977;60:265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGarry J. D., Leatherman G. F., Foster D. W. J. Biol. Chem. 1978;253:4128–4136. [PubMed] [Google Scholar]
- 22.Obici S., Feng Z., Arduini A., Conti R., Rossetti L. Nat. Med. 2003;9:756–761. doi: 10.1038/nm873. [DOI] [PubMed] [Google Scholar]
- 23.Price N., van der Leij F., Jackson V., Corstorphine C., Thomson R., Sorensen A., Zammit V. Genomics. 2002;80:433–442. doi: 10.1006/geno.2002.6845. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz M. W., Porte D., Jr Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz M. W., Woods S. C., Porte D., Jr., Seeley R. J., Baskin D. G. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 26.Jogl G., Tong L. Cell. 2003;112:113–122. doi: 10.1016/s0092-8674(02)01228-x. [DOI] [PubMed] [Google Scholar]
- 27.Hardie D. G. Rev. Endocr. Metab. Disord. 2004;5:119–125. doi: 10.1023/B:REMD.0000021433.63915.bb. [DOI] [PubMed] [Google Scholar]