Abstract
L-type Ca2+ channels play a critical role in regulating Ca2+-dependent signaling in cardiac myocytes, including excitation-contraction coupling; however, the subcellular localization of cardiac L-type Ca2+ channels and their regulation are incompletely understood. Caveolae are specialized microdomains of the plasmalemma rich in signaling molecules and supported by the structural protein caveolin-3 in muscle. Here we demonstrate that a subpopulation of L-type Ca2+ channels is localized to caveolae in ventricular myocytes as part of a macromolecular signaling complex necessary for β2-adrenergic receptor (AR) regulation of ICa,L. Immunofluorescence studies of isolated ventricular myocytes using confocal microscopy detected extensive colocalization of caveolin-3 and the major pore-forming subunit of the L-type Ca channel (Cav1.2). Immunogold electron microscopy revealed that these proteins colocalize in caveolae. Immunoprecipitation from ventricular myocytes using anti-Cav1.2 or anti-caveolin-3 followed by Western blot analysis showed that caveolin-3, Cav1.2, β2-AR (not β1-AR), G protein αs, adenylyl cyclase, protein kinase A, and protein phosphatase 2a are closely associated. To determine the functional impact of the caveolar-localized β2-AR/Cav1.2 signaling complex, β2-AR stimulation (salbutamol plus atenolol) of ICa,L was examined in pertussis toxin-treated neonatal mouse ventricular myocytes. The stimulation of ICa,L in response to β2-AR activation was eliminated by disruption of caveolae with 10 mM methyl β-cyclodextrin or by small interfering RNA directed against caveolin-3, whereas β1-AR stimulation (norepinephrine plus prazosin) of ICa,L was not altered. These findings demonstrate that subcellular localization of L-type Ca2+ channels to caveolar macromolecular signaling complexes is essential for regulation of the channels by specific signaling pathways.
Keywords: caveolae, electrophysiology, ventricular myocyte
Cardiac voltage-gated L-type Ca2+ channels play critical roles in cellular processes modulated by intracellular Ca2+ such as excitation-contraction coupling and gene expression. In adult mammalian ventricular myocytes, L-type Ca2+ channels have been localized to both surface and T-tubular sarcolemma (1, 2). A significant fraction of the L-type Ca2+ channels are targeted to junctional complexes composed of sarcolemmal L-type Ca2+ channels in close apposition with the intracellular Ca2+ release channels of the sarcoplasmic reticulum. However, there are also L-type Ca2+ channels present outside of junctional complexes in cardiac muscle that may have significantly less impact on excitation-contraction coupling but could be essential for regulating other cellular functions (3, 4). Discriminating between different subpopulations of L-type Ca2+ channels in cardiac myocytes represents a critical challenge for fully understanding the role of L-type Ca2+ channels in cardiac physiology and pathophysiology.
Caveolae are small invaginations of the plasma membrane enriched in cholesterol and sphingolipids, which are defined by their principal structural protein, caveolin. Caveolin-3 (Cav-3) is the specific and predominant isoform that is expressed in muscle (5). In the heart, a variety of signaling molecules have been localized to caveolae (6–9). For example, β2-adrenergic receptor (AR) associates with Cav-3 in neonatal mouse cardiac myocytes (10–12). Several ion channels and exchangers have been localized to caveolae in cardiac myocytes, including the voltage-dependent Na channel, a voltage-dependent K channel (Kv1.5), the Na/Ca2+ exchanger, and the HCN4 pacemaker channel (13–16). Caveolae appear to be critically involved in the regulation of Ca2+-mediated signaling in a variety of cell types (17, 18). Nevertheless, it is not yet known whether L-type Ca2+ channels are present in caveolae. The purpose of the present study was to determine whether L-type (Cav1.2) Ca2+ channels are present in caveolae and how this localization impacts their regulation by β-ARs.
Results
Cav1.2 Channels and Components of the β-AR Signaling Cascade Are Present in Caveolae-Enriched Membranes.
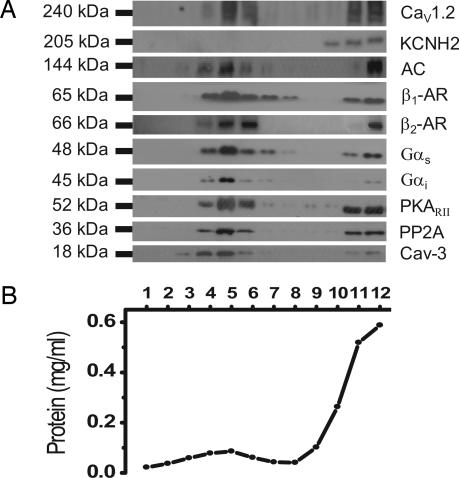
To probe for the presence of Cav1.2 channels and signaling molecules of the β-AR cascade in caveolar lipid rafts in ventricular myocytes, we used a detergent-free method of membrane preparation from mouse neonatal and adult ventricular myocytes followed by continuous sucrose density gradient separation. Western blot analysis of the density gradient fractions from neonatal mouse ventricular myocytes revealed the greatest enrichment of the marker protein for caveolae, Cav-3, in the lower-density fractions 4-6 (15-20% sucrose) as shown from a typical Western blot in Fig. 1A. In the same caveolar-enriched fractions, immunoreactivity for Cav1.2 was detected. In contrast, the pore-forming subunit of the delayed rectifier K+ channel KCNH2 (hERG) was not present in the caveolar lipid raft fractions and was detected only in higher-density fractions of the gradient. Next we probed the same membrane fractions for components of the β-AR/AC/protein kinase A (PKA) signaling cascade. An antibody to the β1-AR detected a 65-kDa band across a broad range of the gradient fractions. The β2-AR was more narrowly distributed, being found in the caveolin-rich fractions, similar to previous studies (19). In addition, we detected the presence of AC, G proteins (Gαs and Gαi), PKARII, and protein phosphatase 2A (PP2A) in the caveolar-enriched fractions. Fractionation of adult mouse ventricular myocytes revealed a similar distribution of proteins across the gradient compared with neonatal myocytes (data not shown). Therefore, the major component proteins required for the β-AR activation of PKA as well as the Cav1.2 subunit of the L-type Ca2+ channel are present in the caveolar-enriched membrane fractions.
Fig. 1.
Cav1.2 is enriched in caveolar membranes together with β2-AR, AC, Gαs, PKA, PP2A, and Cav-3. Caveolar membranes were fractionated from neonatal cardiomyocytes by homogenizing in sodium carbonate buffer and centrifugation to equilibrium in sucrose gradients. (A) One-milliliter fractions were collected from the top of the gradient and analyzed by SDS/PAGE and immunoblot analysis with antibodies to Cav1.2, KCNH2, AC, β1-AR, β2-AR, Gαs, Gαi, PKARII, PP2A, and Cav-3. (B) Protein recovery in each of the gradient fractions. Results are representative of data from six separate experiments.
Cav1.2 Channel and Cav-3 Are Colocalized in Ventricular Myocytes.
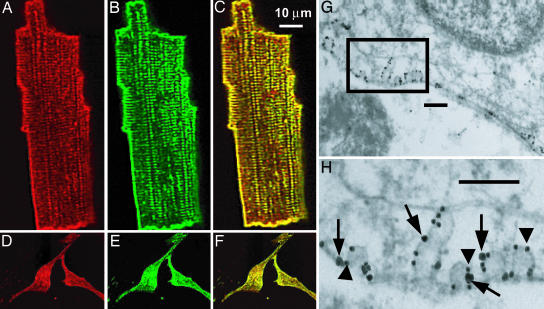
To investigate whether L-type Ca2+ channels colocalize with Cav-3, we used immunofluorescence microscopy. Fig. 2 shows indirect immunofluorescence images from adult canine ventricular myocytes and neonatal mouse ventricular myocytes. The antibody directed against Cav1.2 (red) and Cav-3 (green) demonstrated a prominent surface sarcolemma and T-tubule-staining pattern in adult canine ventricular myocytes (Fig. 2 A and B). In the neonatal mouse myocytes, the T-tubule system is absent; however; sarcolemmal punctate areas of Cav1.2 (red) and Cav-3 (green) staining are observed in these cells (Fig. 2 D and E). Merging the red and green images (Fig. 2 C and F) demonstrates significant areas of colocalization (yellow) for Cav-3 and Cav1.2. The colocalization is partial because some immunolabeling for Cav1.2 (red) does not overlap (see Fig. 6, which is published as supporting information on the PNAS web site). This finding suggests that Cav1.2 channels are present in the cells both in association with caveolae and in other membrane domains.
Fig. 2.
Cav1.2 and Cav-3 are colocalized in adult canine and neonatal mouse ventricular myocytes. Isolated adult canine ventricular myocytes (A-C) and neonatal mouse myocytes (D-F) were immunolabeled with anti-Cav1.2 (A and D) and anti-Cav-3 (B and E) antibodies. Both proteins are detected on the surface membrane and in adult myocytes also in punctuate areas consistent with T-tubule localization. (C and F) Merged images with yellow regions indicating colocalization of Cav1.2 and Cav-3. (G and H) Immunogold colocalization of the Cav1.2 subunit of L-type Ca2+ channel (large particle, arrows) and Cav-3 (small particle, arrowheads) in the caveolae in isolated mouse cardiomyocytes. (Scale bars: 200 nm.).
The spatial resolution of confocal microscopy is inadequate to definitively localize proteins to caveolae given the small size of caveolae (50-100 nm). Therefore, we used the technique of immunogold labeling combined with electron microscopy on isolated neonatal mouse myocytes to determine the localization of Cav-3 and Cav1.2 relative to caveolae. Isolated myocytes were fixed and immunogold colabeled with anti-Cav-3 and anti-Cav1.2 by using a silver enhancement technique. Transmission electron micrographs revealed two distinct populations of different-sized gold particles (Fig. 2 G and H). The small gold particles identify anti-Cav-3 (arrowheads), and large gold particles (the result of double silver enhancement) identify anti-Cav1.2 (arrows). The gold particle distribution was restricted to the membrane, indicative of specific recognition of membrane-associated proteins. Furthermore, the immunogold labeling for both proteins was present on surface membrane invaginations typical of caveolae. Some Cav1.2 labeling was also identified outside of caveolae, demonstrating that not all Cav1.2 channels localize to caveolae. In control samples from which the primary antibodies had been omitted, there were rare random gold-silver particles detected (data not shown). These data demonstrate that a subset of Cav1.2 channels is localized to caveolae in neonatal ventricular myocytes.
Coimmunoprecipitation of Cav1.2 and Cav-3 from Ventricular Myocytes.
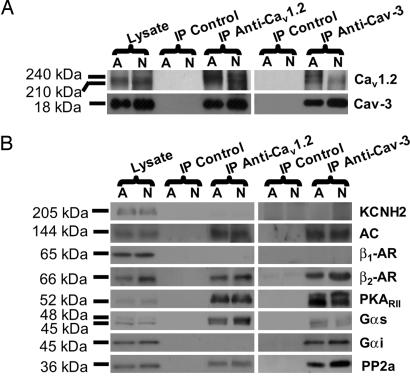
To determine whether Cav-3 and Cav1.2 are associated in ventricular myocytes, we performed immunoprecipitation experiments. Homogenates from neonatal and adult mouse ventricular myocytes were solubilized in Triton X-100 and N-octyl d-glucoside-containing buffer and subjected to immunoprecipitation with anti-Cav1.2, anti-Cav-3, or control mouse or rabbit IgG. Eluted proteins were analyzed by using Western blotting. Fig. 3A shows that anti-Cav1.2 immunoprecipitates Cav-3 in adult and neonatal mouse ventricular myocytes. Conversely, anti-Cav-3 immunoprecipitates Cav1.2 (full blots in Fig. 7, which is published as supporting information on the PNAS web site). Neither protein immunoprecipitated with control IgG. These results suggest that the Cav1.2 subunit associates with Cav-3 in ventricular myocytes.
Fig. 3.
Cav1.2 channels are associated with Cav-3 and components of β2-AR/AC/PKA signaling cascade in mouse hearts. Adult (A) and neonatal (N) mouse myocyte homogenates were subjected to immunoprecipitation with either anti-Cav1.2 or anti-Cav-3 antibodies, and the immunoprecipitates were analyzed by immunoblotting. Both Cav1.2 and Cav-3 are detected in the immunoprecipitates with either of the two antibodies, whereas control IgG does not immunoprecipitate the proteins (A), indicating an association between the two proteins. In B, immunoprecipitation of Cav1.2 or Cav-3 led to coprecipitation of AC, β2-AR, Gαs, PKARII, and PP2A but not β1-AR and KCNH2. A protein band for Gαi was detected only in the anti-Cav-3 immunoprecipitate. Results are representative of six different experiments.
Cav1.2 Is Part of a Caveolar β2-AR/AC/PKA Macromolecular Signaling Complex.
The L-type Ca2+ channel is potently regulated by β-AR signaling in the heart, so we evaluated whether the caveolar Cav1.2 channel is associated with components of the β-AR/AC/PKA cascade in the ventricle. Immunoprecipitation with anti-Cav1.2 was performed by using neonatal and adult mouse ventricular myocyte lysates, and Western blot analysis of eluted proteins was performed. We found that β2-AR, Gαs, AC, PKARII, and PP2A coimmunoprecipitated with anti-Cav1.2, but β1-AR and Gαi did not (Fig. 3B). Using anti-Cav-3 for immunoprecipitation, β2-AR, GαS, Gαi, AC, PKARII, and PP2A coimmunoprecipitated, but the β1-AR did not. We also probed immunoblots with anti-KCNH2 antibody because this protein was not present in caveolar-enriched membranes and could serve as a negative control. In agreement with this prediction, KCNH2 was detected in homogenates but did not coimmunoprecipitate with anti-Cav1.2 or anti-Cav-3. Comparison of the anti-Cav1.2 and anti-Cav-3 immunoprecipitates was remarkable for a difference in only one of the proteins probed, Gαi, which was identified only with anti-Cav-3. These results demonstrate that the caveolar Cav1.2 channels are part of a macromolecular signaling complex including components of the β2-AR/AC/PKA cascade.
Methyl β-Cyclodextrin (MβCD) Eliminates β2-AR Stimulation of ICa,L.
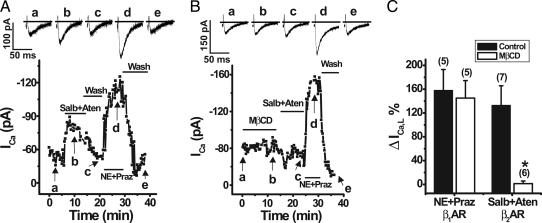
To investigate for a functional interaction between caveolar L-type Ca2+ channels and the associated β2-ARs, we performed whole-cell electrophysiology experiments on isolated neonatal mouse ventricular myocytes. We tested the effect of β2-AR stimulation on ICa,L using the β2-AR selective agonist salbutamol (Salb, 10 μM) and a β1-AR antagonist, atenolol (Aten, 10 μM), to ensure β2-AR-specific activation. In agreement with previous studies, we failed to see any effect of β2-AR stimulation on ICa,L in the mouse ventricular myocytes (data not shown) (20, 21). Given the presence of Gi in the caveolae and previous results showing that Gi can block β2-AR effects on ICa,L (20), we pretreated myocytes with pertussis toxin (PTX, 1 μg/ml) to inactivate Gi. Biochemical experiments confirmed that PTX treatment resulted in Gαi no longer immunoprecipitating with Cav-3 (Fig. 8A, which is published as supporting information on the PNAS web site). In PTX-treated myocytes, Salb plus Aten resulted in reversible stimulation of ICa,L, shown in a representative cell in Fig. 4A, and on average ICa,L was increased 133+ 33% (Fig. 4C). To determine whether caveolae are essential for this regulation, we subjected PTX-treated cells first to superfusion with 10 mM MβCD for 10 min, which disrupts caveolae by depleting cholesterol and thus disrupts the β2-AR/Cav1.2 macromolecular signaling complex (Fig. 8 B and C). We found that MβCD treatment completely eliminated the response to β2-AR stimulation of ICa,L with Salb and Aten (Fig. 4 B and C). Subsequent application of the adrenergic agonist norepinephrine (1 μM) and an α1-adrenergic antagonist, prazosin (1 μM), revealed that the β1-AR stimulation of ICa,L was unchanged in MβCD-treated cells relative to untreated cells (Fig. 4). These findings suggest that β2-AR regulation of L-type Ca2+ channels requires intact caveolae where β2-AR and Cav1.2 associate. In contrast, β1-AR regulation occurs independent of intact caveolae.
Fig. 4.
Caveolar disruption with MβCD eliminated β2-AR but not β1-AR stimulation of ICa,L in neonatal mouse ventricular myocytes. Perforated patch whole-cell voltage clamp recordings of ICa,L were performed by using a holding potential of −40 mV with 50-ms test pulses to +20 mV every 15 s in myocytes pretreated with PTX. (A) Peak ICa,L was reversibly increased by β2-AR activation with 10 μM Salb plus 10 μM Aten and by β1-AR activation with 1 μM norepinephrine (NE) plus 1 μM prazosin (praz) in a representative cell (whole-cell capacitance = 13.3 pF). (B) Application of 10 mM MβCD eliminated the β2-AR but not β1-AR stimulation of ICa,L in a representative myocyte (whole-cell capacitance = 22.0 pF). (C) Average effect of β1-AR and β2-AR stimulation on ICa,L in cells with and without MβCD treatment. The number of cells tested is shown in parentheses. ∗, P < 0.005, MβCD-treated relative to control.
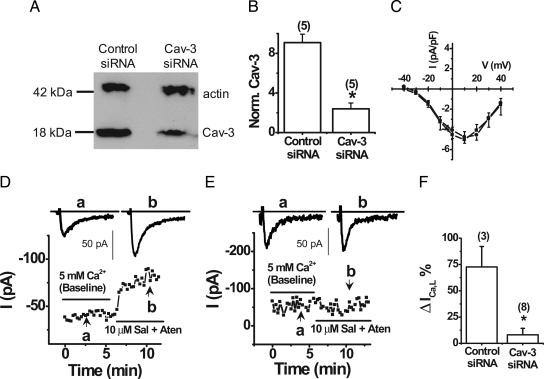
Small Interfering RNA (siRNA) Inhibition of Cav-3 Expression Eliminates β2-AR Stimulation of ICa,L.
Although acute MβCD treatment of neonatal myocytes caused caveolar disruption and resulted in the loss of β2-AR regulation of ICa,L, interpretation of the results could be complicated by MβCD-mediated cholesterol depletion impacting the regulation of Cav1.2 channels outside of caveolae. Because Cav-3 is essential for formation of caveolae in ventricular myocytes (22), we investigated the impact of specific inhibition of Cav-3 expression in neonatal myocytes using siRNA-mediated gene silencing. Lysates from transfected myocytes underwent immunoblotting with antibodies to Cav-3 and sarcomeric actin, a marker protein for myocytes (Fig. 5A). The actin signal provides a control to normalize for abundance of myocytes in a preparation and thus allows calculation of a normalized Cav-3 value. The normalized Cav-3 protein level decreased by 75+ 13% (Fig. 5 A and B). The average transfection efficiency was 64+ 7% (n = 5), which suggested a nearly complete knockdown of Cav-3 protein in the transfected cells. Immunofluorescence imaging confirmed that Cav-3 siRNA-transfected cells (GFP-expressing) exhibited nearly complete knockdown of Cav-3 (Fig. 9, which is published as supporting information on the PNAS web site).
Fig. 5.
siRNA-mediated Cav-3 inhibition eliminated β2-AR stimulation of ICa,L in neonatal mouse ventricular myocytes. (A) Representative Western blot of Cav-3 and sarcomeric actin expression in myocytes with transfected with control and Cav-3 siRNA. (B) Densitometric analysis of Cav-3 expression normalized to sarcomeric actin (n = 5). Perforated patch whole-cell voltage clamp recordings of ICa,L were performed by using a holding potential of −40 mV with 50-ms test pulses to +20 mV every 15 s in myocytes treated with PTX. (C) Average current-voltage relationship of control siRNA (n = 6, ■) and Cav-3 siRNA (n = 6, ●). (D) Peak ICa,L is increased by β2-AR activation with 10 μM Salb plus 10 μM Aten in a representative control siRNA-treated myocyte (whole-cell capacitance = 7.7 pF). (E) siRNA-mediated Cav-3 inhibition eliminated β2-AR stimulation of ICa,L in a representative myocyte (whole-cell capacitance = 11.9 pF). (F) Average effect of β2-AR stimulation on ICa,L in myocytes with and without Cav-3 siRNA inhibition. ∗, P < 0.001 relative to control.
We then performed whole-cell electrophysiology experiments on isolated PTX-treated neonatal mouse ventricular myocytes that were subjected to Cav-3 siRNA or control siRNA. Knockdown of Cav-3 did not affect average ICa,L current densities compared with control siRNA-treated myocytes (Fig. 5C). In myocytes that were transfected with control siRNA, Salb plus Aten resulted in an average β2-AR stimulation of ICa,L of 73+ 19% (Fig. 5 D and F). In contrast, siRNA-mediated Cav-3 knockdown completely eliminated the response to β2-AR stimulation of ICa,L with Salb and Aten (Fig. 5 E and F). These findings confirm that β2-AR regulation of L-type Ca2+ channels in mouse ventricular myocytes requires Cav-3 and thus intact caveolae where β2-AR and Cav1.2 associate.
Discussion
In the present study, we demonstrate that a subpopulation of L-type Ca2+ channels are localized to the caveolar membranes in ventricular myocytes based on membrane fractionation studies, coimmunoprecipitation of Cav1.2 and Cav-3, and immunogold electron microscopy. The caveolar L-type Ca2+ channels exist as part of a multiprotein signaling complex including Cav-3, β2-AR, AC, Gαs, PKARII, and PP2A. Whole-cell patch clamp studies revealed that intact caveolae are required for β2-AR but not β1-AR regulation of Cav1.2 channels in neonatal mouse ventricular myocytes. Therefore, localization of Cav1.2 channels to caveolar macromolecular signaling complexes are necessary for the regulation of these channels by β2-AR and potentially other signaling pathways.
Impact of Caveolar Localization on L-Type Ca2+ Channel Function.
The localization of ion channels to caveolae may modulate the function of the channels in multiple ways. For example, the precise lipid composition can potently regulate channel function, and dynamic changes in this composition underlie some forms of regulation. In addition, the caveolar localization of ion channels can provide compartmentalization of signaling networks, enabling rapid and specific regulation of the channels. Caveolar-localized signaling complexes composed of β2-AR, AC, Gαs, and Gαi have previously been observed in rat ventricular myocytes (9, 19), but the present study adds Cav1.2 and PP2A to the complex. Conversely, the molecules associated with Cav1.2 channels in the heart have not been extensively defined. Recent studies have identified AKAP15 and PKA as well as certain PKC isoforms in association with Cav1.2 in cardiac muscle (23, 24); however, whether these molecules are associated with the channels in caveolae is unknown. The present results add to the list of proteins known to be associated with Cav1.2 in the heart, including β2-AR, Gαs, AC, and PP2A. Studies in rat brain have identified a similar macromolecular signaling complex including β2-AR and Cav1.2 (25). In neurons, Cav1.2 is clustered with glutamate receptors in dendritic spines, which are devoid of morphological caveolae and constitute the postsynaptic sites of glutaminergic synapses. However, the postsynaptic site, and especially the postsynaptic density, constitutes a special subcompartment of the plasma membrane, which may resemble in several respects caveolae, including a dense protein scaffold network on the inside and the requirement of cholesterol for its integrity (26). Therefore, localization of β2-AR/Cav1.2 signaling complexes in various tissues may share certain preferred lipid environments, although the details of the associated scaffolding proteins and other signaling molecules may vary substantially.
The functional impact of the colocalization of Cav1.2 and β2-AR has been observed in previous studies in both cardiac myocytes and neurons, which have demonstrated highly localized regulation of L-type Ca2+channels by β2-AR stimulation (25, 27–29). In addition, single-channel studies using rat ventricular myocytes comparing the effects of β1-AR and β2-AR activation have identified differential patterns of stimulation of single L-type Ca2+ channel activity, which is consistent with distinct localization and regulation of channels by β1-AR and β2-ARs (30). The present work demonstrates that the underlying basis for the localized regulation of ICa,L in the heart is the colocalization of the β2-AR/AC/PKA signaling molecules in caveolae along with L-type Ca2+ channels.
Caveolae-Localized L-Type Ca2+ Channels and Integrated Caveolar Ca2+ Signaling.
This study adds the L-type Ca2+ channel to a growing collection of proteins involved in cellular Ca2+ cycling that have been localized to caveolae. The IP3 receptor, Na+/Ca2+ exchanger, plasma membrane Ca2+-ATPase, and members of the transient receptor potential family have been localized to caveolae in various cell types (17, 18, 31, 32). Previous studies of cardiomyocytes have suggested that the Na+/Ca2+ exchanger protein and the plasma membrane Ca2+-ATPase are present in caveolae (15, 33). Therefore, the caveolar submembrane domain employs multiple proteins to regulate Ca2+ levels, which in turn regulate a variety of caveolar proteins such as Ca2+-sensitive isoforms of adenylyl cyclase (AC5, AC6, and AC8) and endothelial NO synthase (9, 10, 34, 35).
The role of caveolar L-type Ca2+ channels in excitation-contraction coupling in the heart is not well defined. One previous study demonstrated that disruption of caveolae in neonatal rat ventricular myocytes with MβCD reduced the frequency, amplitude, and width of Ca2+ sparks (36). The authors suggested that Ca2+ sparks form specifically in caveolar microdomains. However, the ultrastructure of adult cardiomyocytes is different, with the formation of the T-tubule system and highly structured junctional domains. The junctional domains between sarcolemma-containing L-type Ca2+ channels and junctional sarcoplasmic reticulum likely provide the major site for excitation-contraction coupling and Ca2+ spark generation, and thus the caveolar Ca2+ channels may be more peripherally involved in excitation-contraction coupling in adult myocytes. Even without providing the triggering Ca2+ for sarcoplasmic reticulum release, they may provide an important source for sarcoplasmic reticulum Ca2+ loading.
Conclusions and Limitations
These findings demonstrate that a subpopulation of L-type Ca2+ channels in mouse ventricular myocytes are localized to caveolae, where they are part of a macromolecular signaling complex necessary for β2-AR, but not β1-AR, regulation of Cav1.2 channels. Many questions remain to understand the functional role and molecular identity of the caveolar-localized Ca2+ channels. What mechanisms target the channels to caveolae? What is the molecular composition of the caveolar channels regarding auxiliary subunit composition as well as alternatively spliced forms of Cav1.2? Do alterations in caveolae and caveolar signaling processes play a role in pathophysiology of the heart such as cardiac hypertrophy and heart failure? Our studies provide the basis to address these critical questions in the future.
Materials and Methods
Isolation of Ventricular Myocytes and Purification of Caveolae-Rich Fractions.
Neonatal or adult mouse as well as adult canine ventricular myocytes were enzymatically isolated as previously described (37, 38). Caveolin-rich fractions from neonatal mouse ventricular myocytes were prepared by using a previously described detergent-free method (39) with some modifications (see Supporting Methods, which is published as supporting information on the PNAS web site). From each gradient, 1-ml gradient fractions were collected to yield a total of 12 fractions.
Immunoblotting.
Gradient fractions were analyzed by SDS/PAGE and Western blot analysis (see Supporting Methods). Immunoblots were probed with antibodies to Cav1.2 (rabbit polyclonal) (40), Cav-3, PKARIIα, PP2A, and Gαi (mouse monoclonal antibodies; BD Transduction Laboratories), AC V/VI, β1-AR, β2-AR (rabbit polyclonal antibodies; Santa Cruz Biotechnology), Gαs (rabbit polyclonal; Chemicon International, Temecula, CA), and anti-KCNH2 (rabbit polyclonal) (41).
Immunofluorescence.
Immunolabeling was performed on isolated mouse neonatal and adult canine ventricular myocytes by using a rabbit polyclonal anti-Cav1.2 subunit and monoclonal anti-Cav-3 antibodies as described elsewhere (1). Cells were randomly selected and used for imaging and analysis, and immunolabeling experiments were repeated at least five times.
Electron Microscopy.
Immunogold labeling and colocalization of Cav-3 and Cav1.2 were performed on isolated neonatal mouse myocytes by using a silver enhancement method (42) (for details see Supporting Methods).
Immunoprecipitation.
For immunoprecipitations, adult or neonatal mouse myocyte lysates (≈2 mg of protein) were used, and immunoprecipitations were carried out by using anti-Cav-3 (5 μg) or anti-Cav1.2 (10 μg) antibodies, or control Igs were used at the same concentrations as the specific antibodies (see Supporting Methods). Immune complexes were analyzed by SDS/PAGE and Western blot by probing with antibodies to Cav1.2, Cav-3, PKARIIα, PP2A, AC V/VI, β1-AR, β2-AR, Gαs, Gαi, and KCNH2.
siRNA-Mediated Cav-3 Knockdown.
Three predesigned Cav-3-specific siRNA sequences (Ambion Silencer Predesigned siRNA, HPLC-purified, annealed, catalog nos. 160017, 160018, and 160019 for mouse Cav-3, GenBank accession no. NM_007617) and control nonspecific siRNA oligos for mouse (Ambion, Silencer Negative Control #1 siRNA) were used for Cav-3 knockdown as described in Supporting Methods.
Electrophysiology.
Whole-cell perforated patch clamp was performed on isolated mouse neonatal cardiomyocytes as described in Supporting Methods.
Supplementary Material
Acknowledgments
We are grateful for technical assistance with electron microscopy studies from Randall Massey, Benjamin August (both of the University of Wisconsin School of Medicine Electron Microscope Facility), and Dr. Terry Oberley (Department of Pathology and Laboratory Medicine, University of Wisconsin School of Medicine). We are grateful to Thankful Sanftleben for assistance with manuscript preparation. This work was supported by National Institutes of Health Grants P01 HL47053 (to T.J.K. and J.W.H.), R01 NS035563 (to J.W.H.), T32-HL 07936 (to J.D.F.), and T32 HL07121 (to D.D.H.).
Abbreviations
- MβCD
methyl β-cyclodextrin
- siRNA
small interfering RNA
- AR
adrenergic receptor
- Cav-3
caveolin-3
- PKA
protein kinase A
- AC
adenylyl cyclase
- Salb
salbutamol
- Aten
atenolol
- PTX
pertussis toxin
- PP2A
protein phosphatase 2A
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Balijepalli R. C., Lokuta A. J., Maertz N. A., Buck J. M., Haworth R. A., Valdivia H. H., Kamp T. J. Cardiovasc. Res. 2003;59:67–77. doi: 10.1016/s0008-6363(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 2.Carl S. L., Felix K., Caswell A. H., Brandt N. R., Ball W. J., Jr., Vaghy P. L., Meissner G., Ferguson D. G. J. Cell Biol. 1995;129:673–682. doi: 10.1083/jcb.129.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun X. H., Protasi F., Takahashi M., Takeshima H., Ferguson D. G., Franzini-Armstrong C. J. Cell Biol. 1995;129:659–671. doi: 10.1083/jcb.129.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franzini-Armstrong C., Protasi F., Ramesh V. Ann. N.Y. Acad. Sci. 1998;853:20–30. doi: 10.1111/j.1749-6632.1998.tb08253.x. [DOI] [PubMed] [Google Scholar]
- 5.Tang Z., Scherer P. E., Okamoto T., Song K., Chu C., Kohtz D. S., Nishimoto I., Lodish H. F., Lisanti M. P. J. Biol. Chem. 1996;271:2255–2261. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- 6.Doyle D. D., Ambler S. K., Upshaw-Earley J., Bastawrous A., Goings G. E., Page E. Circ. Res. 1997;81:86–91. doi: 10.1161/01.res.81.1.86. [DOI] [PubMed] [Google Scholar]
- 7.Feldman A. M., Cates A. E., Veazey W. B., Hershberger R. E., Bristow M. R., Baughman K. L., Baumgartner W. A., Van Dop C. J. Clin. Invest. 1988;82:189–197. doi: 10.1172/JCI113569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lasley R. D., Narayan P., Uittenbogaard A., Smart E. J. J. Biol. Chem. 2000;275:4417–4421. doi: 10.1074/jbc.275.6.4417. [DOI] [PubMed] [Google Scholar]
- 9.Rybin V. O., Xu X., Lisanti M. P., Steinberg S. F. J. Biol. Chem. 2000;275:41447–41457. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- 10.Ostrom R. S., Violin J. D., Coleman S., Insel P. A. Mol. Pharmacol. 2000;57:1075–1079. [PubMed] [Google Scholar]
- 11.Xiang Y., Rybin V. O., Steinberg S. F., Kobilka B. J. Biol. Chem. 2002;277:34280–34286. doi: 10.1074/jbc.M201644200. [DOI] [PubMed] [Google Scholar]
- 12.Rybin V. O., Xu X., Steinberg S. F. Circ. Res. 1999;84:980–988. doi: 10.1161/01.res.84.9.980. [DOI] [PubMed] [Google Scholar]
- 13.Yarbrough T. L., Lu T., Lee H. C., Shibata E. F. Circ. Res. 2002;90:443–449. doi: 10.1161/hh0402.105177. [DOI] [PubMed] [Google Scholar]
- 14.Martens J. R., Sakamoto N., Sullivan S. A., Grobaski T. D., Tamkun M. M. J. Biol. Chem. 2001;276:8409–8414. doi: 10.1074/jbc.M009948200. [DOI] [PubMed] [Google Scholar]
- 15.Bossuyt J., Taylor B. E., James-Kracke M., Hale C. C. Ann. N.Y. Acad. Sci. 2002;976:197–204. doi: 10.1111/j.1749-6632.2002.tb04741.x. [DOI] [PubMed] [Google Scholar]
- 16.Barbuti A., Gravante B., Riolfo M., Milanesi R., Terragni B., DiFrancesco D. Circ. Res. 2004;94:1325–1331. doi: 10.1161/01.RES.0000127621.54132.AE. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto T., Nakade S., Miyawaki A., Mikoshiba K., Ogawa K. J. Cell Biol. 1992;119:1507–1513. doi: 10.1083/jcb.119.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujimoto T. J. Cell Biol. 1993;120:1147–1157. doi: 10.1083/jcb.120.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rybin V. O., Pak E., Alcott S., Steinberg S. F. Mol. Pharmacol. 2003;63:1338–1348. doi: 10.1124/mol.63.6.1338. [DOI] [PubMed] [Google Scholar]
- 20.Xiao R. P., Avdonin P., Zhou Y. Y., Cheng H., Akhter S. A., Eschenhagen T., Lefkowitz R. J., Koch W. J., Lakatta E. G. Circ. Res. 1999;84:43–52. doi: 10.1161/01.res.84.1.43. [DOI] [PubMed] [Google Scholar]
- 21.Heubach J. F., Graf E. M., Molenaar P., Jager A., Schroder F., Herzig S., Harding S. E., Ravens U. Br. J. Pharmacol. 2001;133:73–82. doi: 10.1038/sj.bjp.0704045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galbiati F., Engelman J. A., Volonte D., Zhang X. L., Minetti C., Li M., Hou H., Jr., Kneitz B., Edelmann W., Lisanti M. P. J. Biol. Chem. 2001;276:21425–21433. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- 23.Hulme J. T., Lin T. W., Westenbroek R. E., Scheuer T., Catterall W. A. Proc. Natl. Acad. Sci. USA. 2003;100:13093–13098. doi: 10.1073/pnas.2135335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L., Liu G., Zakharov S. I., Morrow J. P., Rybin V. O., Steinberg S. F., Marx S. O. J. Biol. Chem. 2005;280:207–214. doi: 10.1074/jbc.M410509200. [DOI] [PubMed] [Google Scholar]
- 25.Davare M. A., Avdonin V., Hall D. D., Peden E. M., Burette A., Weinberg R. J., Horne M. C., Hoshi T., Hell J. W. Science. 2001;293:98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- 26.Hering H., Lin C. C., Sheng M. J. Neurosci. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y.-Y., Cheng H., Bogdanov K. Y., Hohl C., Altschuld R., Lakatta E. G., Xiao R.-P. Am. J. Physiol. 1997;273:H1611–H1618. doi: 10.1152/ajpheart.1997.273.3.H1611. [DOI] [PubMed] [Google Scholar]
- 28.Jurevicius J., Fischmeister R. Proc. Natl. Acad. Sci. USA. 1996;93:295–299. doi: 10.1073/pnas.93.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen-Izu Y., Xiao R. P., Izu L. T., Cheng H., Kuschel M., Spurgeon H., Lakatta E. G. Biophys. J. 2000;79:2547–2556. doi: 10.1016/S0006-3495(00)76495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroder F., Herzig S. Am. J. Physiol. 1999;276:H834–H843. doi: 10.1152/ajpheart.1999.276.3.H834. [DOI] [PubMed] [Google Scholar]
- 31.Lockwich T. P., Liu X., Singh B. B., Jadlowiec J., Weiland S., Ambudkar I. S. J. Biol. Chem. 2000;275:11934–11942. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- 32.Teubl M., Groschner K., Kohlwein S. D., Mayer B., Schmidt K. J. Biol. Chem. 1999;274:29529–29535. doi: 10.1074/jbc.274.41.29529. [DOI] [PubMed] [Google Scholar]
- 33.Hammes A., Oberdorf-Maass S., Rother T., Nething K., Gollnick F., Linz K. W., Meyer R., Hu K., Han H., Gaudron P., et al. Circ. Res. 1998;83:877–888. doi: 10.1161/01.res.83.9.877. [DOI] [PubMed] [Google Scholar]
- 34.Barouch L. A., Harrison R. W., Skaf M. W., Rosas G. O., Cappola T. P., Kobeissi Z. A., Hobai I. A., Lemmon C. A., Burnett A. L., O’Rourke B., et al. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 35.Crossthwaite A. J., Seebacher T., Masada N., Ciruela A., Dufraux K., Schultz J. E., Cooper D. M. J. Biol. Chem. 2005;280:6380–6391. doi: 10.1074/jbc.M411987200. [DOI] [PubMed] [Google Scholar]
- 36.Lohn M., Furstenau M., Sagach V., Elger M., Schulze W., Luft F. C., Haller H., Gollasch M. Circ. Res. 2000;87:1034–1039. doi: 10.1161/01.res.87.11.1034. [DOI] [PubMed] [Google Scholar]
- 37.He J., Conklin M. W., Foell J. D., Wolff M. R., Haworth R. A., Coronado R., Kamp T. J. Cardiovasc. Res. 2001;49:298–307. doi: 10.1016/s0008-6363(00)00256-x. [DOI] [PubMed] [Google Scholar]
- 38.Wagoner L. E., Zhao L., Bishop D. K., Chan S., Xu S., Barry W. H. Circulation. 1996;93:111–119. doi: 10.1161/01.cir.93.1.111. [DOI] [PubMed] [Google Scholar]
- 39.Song W.-J., Surmeier D. J. J. Neurophysiol. 1996;76:2290–2306. doi: 10.1152/jn.1996.76.4.2290. [DOI] [PubMed] [Google Scholar]
- 40.Hell J. W., Westenbroek R. E., Warner C., Ahlijanian M. K., Prystay W., Gilbert M. M., Snutch T. P., Catterall W. A. J. Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Z., January C. T. Biophys. J. 1998;74:1830–1839. doi: 10.1016/S0006-3495(98)77893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yi H., Leunissen J., Shi G., Gutekunst C., Hersch S. J. Histochem. Cytochem. 2001;49:279–284. doi: 10.1177/002215540104900301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.