Abstract
Chlamydophila felis and feline herpesvirus (FHV) are pathogens commonly associated with feline respiratory and ocular disease. A real-time multiplex PCR assay was developed to allow detection of these organisms, together with feline 28S ribosomal DNA, in a single tube. Of 538 ocular swab samples tested, 123 were positive for FHV, 97 were positive for C. felis, and 16 were positive for both pathogens.
Feline herpesvirus (FHV) and Chlamydophila felis are two pathogens commonly isolated from domestic cats with ocular disease (4, 9). Together these pathogens comprise the most common infectious causes of conjunctivitis in cats (5, 12, 13). FHV also causes cat flu and tends to cause more severe clinical disease than feline calicivirus, the other major cause of cat flu (2). C. felis can also cause fever, lethargy, lameness, and reduction in weight gain in infected kittens (11). Isolation has traditionally been used to identify these pathogens. Recently, PCR tests which are more sensitive than isolation have been developed for the detection of FHV and C. felis (1, 6-8, 10).
We have previously reported the detection of C. felis by real-time PCR using a molecular beacon (3). This type of assay has considerable advantages over conventional nested PCR for the detection of pathogens. Importantly, the reaction is performed in a sealed tube, which dramatically reduces the risk of amplicon carryover and, hence, the chance of false-positive results. Also, the starting copy number can easily and accurately be quantified, allowing the efficacy of treatment regimens to be determined.
In this paper, we report the use of three-way real-time multiplex PCR to simultaneously detect C. felis, FHV, and feline 28S ribosomal DNA (rDNA). By detecting both pathogens in the same tube at the same time, the assay is fast and efficient and allows a rapid throughput of samples.
Sequence data for the feline 28S rDNA gene were obtained using conserved primers from human, rat, and mouse 28S rDNA gene sequences and deposited in the GenBank database under the accession number AF353617. This sequence was used to design real-time PCR primers and probe.
Real-time PCR primers and probes were designed using MacVector 6.5 (Oxford Molecular, Oxford, United Kingdom) (Table 1). Molecular beacons and fluorescently quenched (FQ) probes were designed to have annealing temperatures 10°C higher than those of their primer pairs. The mfold program (http://bioinfo.math.rpi.edu/∼mfold/dna/) was used to ensure that minimal secondary structure formed in the region of the primers or probe at the annealing temperature. This program was also used to calculate the melting temperature of the stem-loop in the molecular beacon and to ensure that the fluorophore and quencher were adjacent in the folded beacon.
TABLE 1.
PCR primers and probes used in the multiplex real-time PCR assay
| Primer or probea | Primer or probe sequence (5′→3′) | Region of gene (nt)b |
|---|---|---|
| 28S rDNA | ||
| Forward primer | CGCTAATAGGGAATGTGAGCTAGG | 663-686 |
| Reverse primer | TGTCTGAACCTCCAGTTTCTCTGG | 783-760 |
| Molecular beacon | Texas red-CGCGCACCCTACTGATGATGTGTTGTTGCCGCGCG-DABCYL | 716-740 |
| C. felis OmpA | ||
| Forward primer | GAACTGCAAGCAACACCACTG | 281-301 |
| Reverse primer | CCATTCGGCATCTTGAAGATG | 357-337 |
| FQ probe | 6-FAM-CGCTGCCGACAGATCAAATTTTGCC-BHQ1 | 303-327 |
| FHV TKc | ||
| Forward primer | GGACAGCATAAAAGCGATTG | 173-192 |
| Reverse primer | AACGTGAACAACGACGCAG | 247-229 |
| FQ probe | Cy5-AATTCCAGCCCGGAGCCTCAAT-BHQ2 | 201-222 |
All primers and probes were synthesized by Cruachem Ltd., Glasgow, Scotland. The 28S rDNA molecular beacon was labeled with Texas red at the 5′ end and a 4-(4′-dimethylaminophenylazo)benzoic acid (DABCYL) quencher at the 3′ end. The regions of stem-loop formation are underlined. FQ probes were labeled with either 6-carboxyfluorescein (6-FAM) or cyanine 5 (Cy5) at the 5′ end and the appropriate black hole quencher (BHQ) at the 3′ end.
The following gene sequences were used: feline 28S rDNA (GenBank accession number AF353617), C. felis major outer membrane protein gene (GenBank accession number AF269258) and FHV thymidine kinase gene (GenBank accession number M26660). nt, nucleotides.
TK, thymidine kinase.
A mixture of the two pathogens was prepared by combining 1 ml of FHV-infected feline kidney cells and 1 ml of a lysate from C. felis-infected feline cells. Both pathogens were field isolates from cats with clinical signs of infection. Template genomic DNA was extracted from 200 μl of the above mixture using the DNeasy tissue kit (Qiagen) following the manufacturer's instructions for animal cells. Plain cotton swabs were used to collect ocular cells from cats. Genomic DNA was isolated by placing the swab in a solution of 200 μl of phosphate-buffered saline, 200 μl of buffer AL (Qiagen), and 20 μl of proteinase K (Qiagen) and incubating the swab and solution at 70°C for 10 min; subsequent procedures were performed as outlined by the manufacturer.
Real-time PCR was performed using an iCycler (Bio-Rad Laboratories Ltd., Hemel Hempstead, United Kingdom) with optical upgrade system. The PCR mixture consisted of 12.5 μl of Platinum Q PCR SuperMix-UDG (Life Technologies Ltd.), 150 nM each of the 28S rDNA forward and reverse primers (see Table 1 for primer and probe sequences), 100 nM each of the FHV forward and reverse primers, 100 nM each of the C. felis forward and reverse primers, 200 nM 28S rDNA molecular beacon, 100 nM herpesvirus FQ probe, 100 nM C. felis FQ probe, 6 mM MgCl2, 1.25 U of Platinum Taq DNA polymerase (Life Technologies Ltd.) (to give 2 U of Taq per 25-μl reaction mixture), 5 μl of genomic DNA, and water to 25 μl. After an initial incubation at 50°C for 3 min to allow uracil DNA glycosylase (UDG) to digest any amplicon carryover and a subsequent incubation at 95°C for 2 min to inactivate the UDG and activate the Platinum Taq, 45 cycles of PCR, with 1 cycle consisting of 10 s at 95°C and 30 s at 60°C, were performed. Fluorescence was detected at 530-, 575-, and 620-nm wavelengths at each annealing step (60°C). All reactions were run in triplicate.
Reproducible results were obtained only when the concentration of Taq DNA polymerase was increased from 0.75 to 2 U and the MgCl2 concentration was increased from 3 to 6 mM per multiplex reaction mixture. It was also found that titration of the primer concentrations was essential to limit PCR product formation from the FHV assay, which was very efficient.
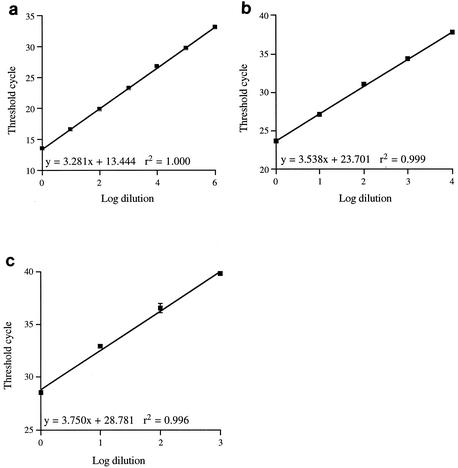
Figure 1 shows the linearity and reproducibility of the three-way multiplex real-time PCR assay. It can be seen that the linearity ranges from 103 for the 28S rDNA PCR to 106 for the herpesvirus PCR. This difference is due to the starting copy number, since the threshold cycle (Ct) values for the undiluted sample were 13.4 for the herpesvirus PCR and 28.8 for the 28S rDNA PCR, indicating that there was considerably more FHV template than feline genomic DNA in the initial sample. The correlation coefficient ranges from 0.996 to 1.000, indicating that the assays are highly reproducible. The slope of each line indicates the efficiency of the reaction, giving efficiencies of 102% for FHV, 92% for C. felis, and 85% for 28S rDNA. These efficiencies are acceptable for quantifying differences of the order of magnitude. The formation of primer dimers or the secondary structures of the PCR products, which were not predicted by the mfold program, are possible factors that could affect efficiency.
FIG. 1.
Dilution curves for each component of the three-way PCR. Genomic DNA was isolated from a mixture of FHV-infected feline cells and C. felis and was serially diluted in 10-fold steps. After multiplex PCR, the threshold cycle was plotted against the log10 of the dilution. Dilution curves for FHV (a), C. felis (b), and feline 28S rDNA (c) are shown. Each point represents the average ± standard deviation for three PCRs.
We have previously reported a singleplex real-time PCR for the detection of C. felis (3). The three-way PCR described in this paper also detects C. felis, but it uses newly designed primers and probe because the original ones would not work in multiplex PCR. We have seen no difference in the sensitivity and specificity of the two assays, even though one uses a molecular beacon and the other uses an FQ probe.
Of 538 ocular swab samples submitted over a 4-month period to the Diagnostic Service, University of Bristol, Langford, Bristol, United Kingdom, 123 were positive for FHV, 97 were positive for C. felis, and 16 were positive for both FHV and C. felis using the real-time PCR assay described in this paper. In a parallel study comparing FHV isolation and real-time PCR, 11 samples were found to be positive by virus isolation, while 58 were positive by real-time PCR, including the 11 positive by virus isolation. All samples negative by real-time PCR were negative by virus isolation. Overall, the Ct values of the samples where FHV was isolated were significantly lower (P < 0.0001) than the Ct values of samples where virus could not be isolated, indicating that the real-time PCR assay is more sensitive than virus isolation. No such comparison could be done for C. felis, since isolation was never undertaken on the samples used for real-time PCR. We have, however, tested six C. felis field isolates grown in tissue culture using the multiplex PCR, and all were positive.
Of 538 samples, 9 were negative for 28S rDNA by the real-time PCR assay. Of these nine samples, four showed a signal for either FHV or C. felis, although the Ct value was always greater than 35. One possible explanation is that some samples contained very low numbers of feline cells and therefore little 28S rDNA but also contained measurable numbers of pathogen. This could arise from the swabbing technique used to take the sample. Additionally, the less-than-optimal efficiency of the 28S rDNA assay may reduce its sensitivity. Overall, only 1.67% of samples in this study showed no 28S rDNA signal. It is suggested that in such cases, new samples should be taken from the cats for diagnostic testing to reduce the number of false-negative results for the pathogens being tested.
The inclusion of 28S rDNA as an internal control in the multiplex real-time PCR assay enables samples containing different amounts of DNA to be normalized to allow quantification. This allows direct comparison between pre- and posttreatment samples to allow measurement of treatment efficacy.
Other multiplex PCR assays have been reported for FHV, C. felis, and feline calicivirus, but these methods rely on conventional PCR (8), which does not enable accurate quantification of starting template and is time-consuming because of the need to run PCR products on gels. The use of hybridization probes increases specificity, since they will not bind to nonspecific PCR product. The inclusion of the 28S rDNA positive control ensures that samples negative for FHV or C. felis are truly negative by confirming that amplifiable DNA was present on the swab sample. The assay is fast and efficient, allowing 96 samples to undergo three-way real-time PCR within 90 min. This type of assay is especially suited to epidemiological studies, as it allows large numbers of samples to be assayed quickly at a relatively low cost and with minimum hands-on time.
Acknowledgments
We are grateful to Intervet International BV, Boxmeer, The Netherlands, for their support.
REFERENCES
- 1.Burgesser, K. M., S. Hotaling, A. Schiebel, S. E. Ashbaugh, S. M. Roberts, and J. K. Collins. 1999. Comparison of PCR, virus isolation, and indirect fluorescent antibody staining in the detection of naturally occurring feline herpesvirus infections. J. Vet. Diagn. Investig. 11:122-126. [DOI] [PubMed] [Google Scholar]
- 2.Gaskell, R., and S. Dawson. 1998. Feline respiratory disease, p. 97-102. In C. E. Greene (ed.), Infectious diseases of the dog and cat, 2nd ed. W. B. Saunders, Philadelphia, Pa.
- 3.Helps, C., N. Reeves, S. Tasker, and D. Harbour. 2001. Use of real-time quantitative PCR to detect Chlamydophila felis infection. J. Clin. Microbiol. 39:2675-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoskins, J. D. 1999. Feline respiratory diseases. Vet. Clin. N. Am. Small Anim. Pract. 29:945-958. [DOI] [PubMed] [Google Scholar]
- 5.Johnson, F. W. 1984. Isolation of Chlamydia psittaci from nasal and conjunctival exudate of a domestic cat. Vet. Rec. 114:342-344. [DOI] [PubMed] [Google Scholar]
- 6.McDonald, M., B. J. Willett, O. Jarrett, and D. D. Addie. 1998. A comparison of DNA amplification, isolation and serology for the detection of Chlamydia psittaci infection in cats. Vet. Rec. 143:97-101. [DOI] [PubMed] [Google Scholar]
- 7.Stiles, J., M. McDermott, M. Willis, W. Roberts, and C. Greene. 1997. Comparison of nested polymerase chain reaction, virus isolation, and fluorescent antibody testing for identifying feline herpesvirus in cats with conjunctivitis. Am. J. Vet. Res. 58:804-807. [PubMed] [Google Scholar]
- 8.Sykes, J. E., J. L. Allen, V. P. Studdert, and G. F. Browning. 2001. Detection of feline calicivirus, feline herpesvirus 1 and Chlamydia psittaci mucosal swabs by multiplex RT-PCR/PCR. Vet. Microbiol. 81:95-108. [DOI] [PubMed] [Google Scholar]
- 9.Sykes, J. E., G. A. Anderson, V. P. Studdert, and G. F. Browning. 1999. Prevalence of feline Chlamydia psittaci and feline herpesvirus 1 in cats with upper respiratory tract disease. J. Vet. Intern. Med. 13:153-162. [DOI] [PubMed] [Google Scholar]
- 10.Sykes, J. E., V. P. Studdert, and G. F. Browning. 1999. Comparison of the polymerase chain reaction and culture for the detection of feline Chlamydia psittaci in untreated and doxycycline-treated experimentally infected cats. J. Vet. Intern. Med. 13:146-152. [DOI] [PubMed] [Google Scholar]
- 11.TerWee, J., M. Sabara, K. Kokjohn, J. Sandbulte, P. Frenchick, and K. J. Dreier. 1998. Characterization of the systemic disease and ocular signs induced by experimental infection with Chlamydia psittaci in cats. Vet. Microbiol. 59:259-281. [DOI] [PubMed] [Google Scholar]
- 12.Wills, J., T. J. Gruffydd-Jones, S. Richmond, and I. D. Paul. 1984. Isolation of Chlamydia psittaci from cases of conjunctivitis in a colony of cats. Vet. Rec. 114:344-346. [DOI] [PubMed] [Google Scholar]
- 13.Wills, J. M., P. Howard, T. J. Gruffydd-Jones, and C. M. Wathes. 1988. Prevalence of Chlamydia psittaci in different cat populations in Britain. J. Small Anim. Pract. 29:327-339. [Google Scholar]