Abstract
Vibrio cholerae O139, the second etiological serogroup of cholera, triggered the first outbreak of O139 cholera in China in 1993. To analyze the clone polymorphism of O139 isolates in China, 117 strains of V. cholerae O139, isolated from different areas in China between 1993 and 1999, were selected to characterize the phylogenetic relationships by molecular techniques. Analysis of restriction fragment length polymorphism in the conserved 16S rRNA gene revealed seven different ribotypes within the 117 strains. Among these strains, there were eight that lacked the cholera toxin gene (ctxAB), zot, and the repetitive sequence (RS); these eight strains belonged to three individual ribotypes. Our results suggested that V. cholerae O139 strains in China had clone diversity in phylogeny. The results of our hybridization patterns for CTX genetic elements (ctxAB, zot, and RS) showed that CTXΦ genomes in most V. cholerae O139 strains had two or more copies and had extensive restriction patterns even for the strains which belong to the same ribotype. For 22 (20.1%) strains, the copies of ctxAB were different from those of zot, suggesting that a ctxAB-negative CTXΦ genome may exist in O139 strains. This ctxAB-negative CTXΦ genome may coexist with the intact CTXΦ genome in a strain. In addition, the dendrogram for I-CeuI-generated pulsed-field gel electrophoresis patterns showed that V. cholerae serogroup O139 has a closer relationship with one strain of serogroup O22 than with the strains of serogroup O1. The results of this study showed the clonal diversity and the distribution of O139 strains in China, suggesting multiple origins of the O139 cholera epidemic or sporadic events.
Cholera is an infectious disease that causes severe diarrhea and is a major public health problem in developing countries. Vibrio cholerae serogroup O1 had been considered the only causative agent of epidemic cholera until the emergence of V. cholerae serogroup O139 Bengal in 1992 in southern India. O139 cholera rapidly spread and caused explosive epidemics throughout Bangladesh, India, and neighboring countries (16, 21, 22). V. cholerae O139 can disseminate widely, causing severe watery diarrhea that is clinically indistinguishable from that caused by V. cholerae O1 strains. Prior immunity to V. cholerae O1 does not provide protection against infection caused by V. cholerae O139 (4). In 1993, the first outbreak caused by serogroup O139 strains occurred in Xinjiang, China, where 200 cases were reported. In 1994, outbreaks of V. cholerae O139 were reported in six Chinese provinces. Although this newly recognized pathogen has not caused large-scale epidemics, as seen for El Tor, regions in China where sporadic cases are reported each year have been expanding: a total of 628 cases were reported up to 1999. Unlike the epidemics seen in Bangladesh and India, where explosive epidemics and reemergence following transient disappearance were observed (14, 15), O139 cholera in China appeared to have only rare outbreaks.
The rRNA gene restriction patterns (ribotypes) could be considered fairly stable markers for different clones. A standard ribotyping method has been developed (19), and it has been used to identify clonal diversity in the V. cholerae strains responsible for epidemics (6, 8, 20). Analysis of genomic DNA by pulsed-field gel electrophoresis (PFGE) has also been used to characterize clonal diversity and relationships among the V. cholerae isolates. This method allowed larger fragment distinctions, reflecting genome variation among epidemic strains. For the toxigenic strains of V. cholerae, there is a prophage known as CTXΦ integrated in the chromosome; this genetic element comprises a 4.5-kb central core region that contains the genes ctxAB, zot, ace, orfU, and cep, flanked by one or more copies of the repetitive sequence (RS) (7, 18, 24). The number and arrangement of the cholera toxin (CTX) genetic elements are known to vary in different toxigenic strains, which is a useful basis for the study of the diversity and characterization of strains (2, 5, 8, 9). The ribotyping and genotyping patterns are useful tools as molecular markers to monitor and analyze the spread of the pathogenic strains in disease surveillance. These molecular epidemiological approaches were used in the present study to analyze the clonal population by ribotyping and some genetic-structure characteristics of the CTX element of V. cholerae O139 strains isolated from 1993 to 1999 in China.
MATERIALS AND METHODS
V. cholerae strains
A total of 117 V. cholerae O139 strains isolated from cholera patients, healthy carriers, and environmental water between 1993 and 1999 in China were included in this study (Table 1). The strains were isolated in 12 provinces. Thirty-six of them were isolated from the first outbreak in Xinjiang in 1993. Others come from sporadic cases in different regions. The ten strains used in PFGE included six strains from serogroup O1 (of which three were classical and three were El Tor biovar), three from serogroup O139, and one from serogroup O22; all are preserved in our laboratory.
TABLE 1.
Strains and their patterns of ribotyping and hybridization with probes of CTX element (ctxAB, zot, and RS) among 117 strains of V. cholerae O139 between 1993 and 1999 in China
| Yr of isolation | Location (province) | Source | No. of isolates | Ribo- type (rb) | ctxAB genotype (ct) | RS genotype (R) | zot genotype (z) |
|---|---|---|---|---|---|---|---|
| 1993 | Xinjiang | Carrier | 14 | 3 | 1 | 1 | 1 |
| 1993 | Xinjiang | Carrier | 1 | 3 | 11 | 1 | 1 |
| 1993 | Xinjiang | Patient | 11 | 3 | 1 | 1 | 1 |
| 1993 | Xinjiang | Patient | 1 | 3 | 1 | 11 | 1 |
| 1993 | Xinjiang | Patient | 2 | 3 | 3 | 3 | 3 |
| 1993 | Xinjiang | Patient | 1 | 4 | −a | − | − |
| 1993 | Xinjiang | Patient | 1 | 3 | 9 | 3 | 9 |
| 1993 | Xinjiang | Patient | 1 | 6 | 1 | 1 | 1 |
| 1993 | Xinjiang | Patient | 1 | 3 | 11 | 1 | 1 |
| 1993 | Xinjiang | Patient | 1 | 3 | 1 | 1 | 1 |
| 1993 | Xinjiang | Patient | 1 | 1 | 1 | 1 | 1 |
| 1993 | Xinjiang | Water | 1 | 7 | − | − | − |
| 1994 | Beijing | Patient | 17 | 1 | 4 | 4 | 4 |
| 1996 | Zhejiang | Patient | 1 | 1 | 1 | 1 | 1 |
| 1996 | Zhejiang | Patient | 6 | 1 | 2 | 2 | 2 |
| 1996 | Zhejiang | Patient | 1 | 1 | 6 | 6 | 6 |
| 1996 | Zhejiang | Patient | 1 | 1 | 9 | 3 | 6 |
| 1996 | Zhejiang | Patient | 1 | 2 | 6 | 6 | 6 |
| 1997 | Zhejiang | Patient | 3 | 1 | 1 | 1 | 1 |
| 1997 | Zhejiang | Patient | 1 | 1 | 8 | 8 | 9 |
| 1998 | Zhejiang | Patient | 3 | 1 | 1 | 1 | 1 |
| 1998 | Zhejiang | Patient | 1 | 2 | 9 | 3 | 5 |
| 1993 | Guangdong | Patient | 1 | 1 | 1 | 1 | 1 |
| 1993 | Guangdong | Patient | 1 | 2 | 10 | 9 | 13 |
| 1994 | Guangdong | Patient | 1 | 1 | 1 | 1 | 12 |
| 1994 | Guangdong | Patient | 2 | 1 | 1 | 1 | 1 |
| 1995 | Guangdong | Patient | 1 | 4 | − | − | − |
| 1995 | Guangdong | Patient | 1 | 1 | 11 | 1 | 14 |
| 1995 | Guangdong | Patient | 1 | 2 | 5 | 5 | 5 |
| 1997 | Guangdong | Patient | 1 | 4 | − | − | − |
| 1997 | Guangdong | Fish | 1 | 4 | − | − | − |
| 1997 | Guangdong | Carrier | 1 | 1 | 7 | 4 | 7 |
| 1998 | Guangdong | Patient | 1 | 1 | 7 | 4 | 7 |
| 1998 | Guangdong | Patient | 1 | 1 | 4 | 4 | 6 |
| 1998 | Guangdong | Water | 1 | 1 | 10 | 9 | 10 |
| 1994 | Jiangxi | Patient | 1 | 1 | 4 | 9 | 10 |
| 1994 | Jiangxi | Patient | 1 | 1 | 1 | 9 | 10 |
| 1998 | Jiangxi | Patient | 5 | 2 | 1 | 12 | 9 |
| 1998 | Jiangxi | Patient | 3 | 5 | − | − | − |
| 1997 | Jiangsu | Patient | 1 | 1 | 1 | 1 | 1 |
| 1998 | Jiangsu | Patient | 1 | 1 | 1 | 1 | 1 |
| 1999 | Jiangsu | Patient | 1 | 1 | 1 | 1 | 1 |
| 1998 | Jiangsu | Patient | 1 | 2 | 5 | 10 | 9 |
| 1998 | Jiangsu | Patient | 2 | 1 | 7 | 7 | 9 |
| 1998 | Anhui | Patient | 8 | 3 | 1 | 1 | 1 |
| 1998 | Heilongjiang | Patient | 2 | 2 | 1 | 1 | 9 |
| 1998 | Heilongjiang | Patient | 1 | 6 | 1 | 12 | 9 |
| 1997 | Hunan | Patient | 1 | 3 | 5 | 4 | 7 |
| 1997 | Hunan | Patient | 1 | 1 | 7 | 4 | 7 |
| 1997 | Liaoning | Patient | 1 | 1 | 1 | 12 | 8 |
| 1996 | Hainan | Patient | 1 | 1 | 1 | 1 | 1 |
| 1996 | Ne Menggu | Patient | 1 | 1 | 10 | 12 | 11 |
−, negative.
Probes and Southern blot hybridization.
All DNA probes were products of PCR with the following primers, using the chromosomal DNA of V. cholerae strain O395 as a template: 16S rRNA gene probe (1.6 kb) with primers 5′-AGA GTT TGA TCA TGG CTC AG-3′ and 5′-AAG GAG GTG ATC CAA CCG CA-3′; ctxAB gene probe (1.1 kb) with primers 5′-AGT TCC ATG GGG CAG ATT CTA GAC-3′ and 5′-GAT CTA GAC GGT TGC TTC TCA TCA TCG-3′; zot gene probe (846 bp) with primers 5′-AAA CCT TGA ACG CAT AGC-3′ and 5′-GCC CAT AGA CCA CGA TAA-3′; and RS probe (900 bp) with primers 5′-CCG GTA CCA CTC ACC TTG TAT TCG-3′ and 5′-CGG GTA CCT CGA CAT CAA ATG GCA TG-3′. The probes used for hybridization were labeled with a random-primer DIG DNA Labeling and Detection kit (Roche Molecular Biochemicals, Indianapolis, Ind.).
The strains were cultured in Luria-Bertani medium, and 1 ml of culture was used to extract and purify the genomic DNA using the Wizard Genomic DNA Extraction kit (Promega, Madison, Wis.). Extracted aliquots of genomic DNA were digested with BglI for ribotyping and with PstI for CTX element restriction fragment length polymorphism analysis. The digested fragments were separated by agarose gel electrophoresis (0.7% gel) and were blotted on nitrocellulose membranes. For southern hybridization, the membranes were prehybridized at 42°C for 2 h in a solution containing 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 1% blocking reagent, 0.1% N-lauryl sarcosine, 0.02% sodium dodecyl sulfate (SDS), and 50% formamide. The membranes were then hybridized with the freshly denatured digoxigenin-labeled gene probes at 42°C for 12 h. The hybridized membranes were washed twice in 2× SSC- 0.1% SDS for 5 min at room temperature and then twice in 0.1× SSC- 0.1% SDS for 15 min at 68°C. Nonradioactive detection was based on digoxigenin- anti-digoxigenin enzyme-linked immunosorbent assay, according to the instructions for the DIG DNA Labeling and Detection kit.
PFGE.
The intact genomic DNA for PFGE analysis was prepared as described previously (13, 23). Briefly, overnight bacterial cultures grown to mid-logarithmic phase were washed with a washing buffer, resuspended with the same buffer, mixed with 2% low-melting-point agarose, and dispensed into an agarose plug mold. The agarose plug was incubated in lysozyme solution (1% sodium lauryl sarcosine, 1 mg of lysozyme/ml) at 37°C overnight and then deproteinated with a solution containing 1 mg of proteinase K (Roche) per ml at 50°C for 48 h. The agarose plug was washed twice for 2 h each time with Tris-EDTA (TE) buffer containing 1 mM phenylmethylsulfonyl fluoride, followed by two washes in TE buffer without phenylmethylsulfonyl fluoride. Then, the plugs were washed with 0.1× TE buffer for 1 h and equilibrated with enzyme buffer for 1 h. The DNA inside the plug was digested with 4 U of I-CeuI (New England Biolabs, Beverly, Mass.) in 100 μl of enzyme buffer at 37°C for 8 h.
Electrophoresis was carried out using the Pulsaphor Plus system with a hexagonal electrode (Pharmacia LKB, Uppsala, Sweden) at 3°C for 28 h at 10 V/cm. The following pulse parameters were used: 5 s (7 h), 10 s (7 h), 25 s (5 h), and 100 s (9 h).
RESULTS AND DISCUSSION
Ribotype analysis.
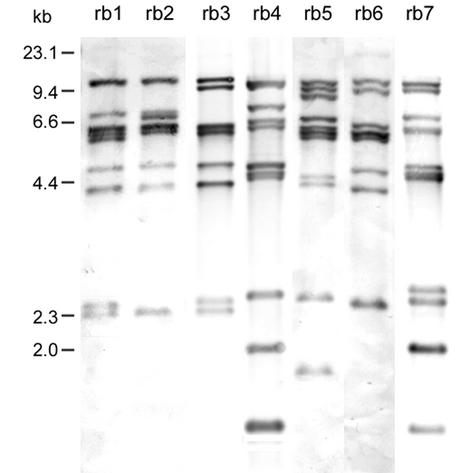
BglI was the most discriminatory restriction enzyme used for ribotyping (6, 8, 20). In the present study, 117 strains of V. cholerae O139 were differentiated into seven different ribotypes (Fig. 1, patterns rb1 to rb7) on the basis of rRNA gene restriction patterns using BglI digestion and 16S rRNA gene probe hybridization. The probe hybridization consisted of 7 to 10 bands between 12 and 1.5 kb in size. Forty-five strains (46.2% of the 117 tested strains) belonged to the pattern rb1, 41 (35.0%) strains belonged to rb3, and 12 (10.0%) strains belonged to rb2. Patterns rb4 through rb7 contained one to four strains each. Eight isolates that tested negative for ctxAB, zot, and RS produced three special restriction patterns (rb4, rb5, and rb7) that were clearly different from the other four restriction patterns of the toxigenic O139 strains. This result revealed an obvious divergence between the toxigenic and nontoxigenic O139 strains.
FIG. 1.
Southern hybridization analysis of rRNA genes in 117 V. cholerae O139 strains isolated from 1993 to 1999 in China. Genomic DNA was digested with BglI and hybridized with a digoxigenin-labeled 16S rRNA gene probe. The ribotyping patterns are rb1 to rb7. The numbers indicating the molecular sizes of the bands correspond to a λ DNA HindIII marker.
In the early emergence of the O139 strain in 1993 in China, the strains isolated from two regions, Xinjiang and Guangdong, showed four different patterns (rb1, rb3, rb4, and rb7) for the Xinjiang isolates and two patterns (rb1 and rb2) for the Guangdong isolates. Of these patterns, rb4 and rb7 were produced by nontoxigenic strains. With the exception of one strain belonging to rb1, all of the toxigenic strains from Xinjiang belonged to rb3. These results suggested that V. cholerae O139 was originally heterogeneous and raised the question of whether the origin of V. cholerae O139 was from the toxigenic non-O139 strains through acquisition of the serogroup-determinant genes or from the nontoxigenic O139 strains by horizontal transfer of the CTX elements.
The isolates from the small epidemic in Beijing in 1994 displayed only the rb1 pattern. Pattern rb1 includes strains isolated from eight different regions over the course of 6 years (1993 to 1998), suggesting that the later cases were not an extension of the first outbreak in Xinjiang. Overall, rb1 and rb3 are the predominant ribotypes among the analyzed strains and comprise most isolates that appeared in most regions.
The O139 strains isolated from sporadic cases in 13 different Chinese regions after 1994 belonged to the three patterns rb1, rb2, and rb3. The only exception was a strain isolated in Heilongjiang in 1998. This strain showed a special pattern, rb6. Our results show the clonal diversity among the isolates and the wide distribution of O139 strains in China, suggesting that V. cholerae O139 is undergoing genetic changes or some other unknown competitive mechanism to increase the ribotype diversity of the toxigenic and nontoxigenic strains. It has also been found that rrn recombination between loci may have generated variation in the seventh-pandemic V. cholerae clone (12).
Restriction patterns of CTX genetic elements.
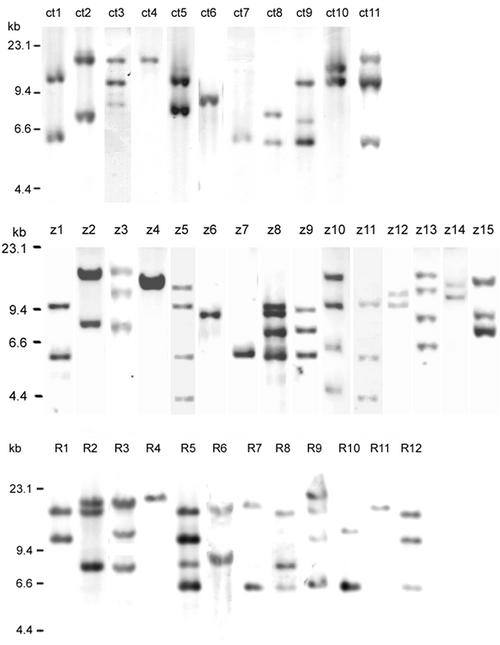
The toxin gene ctxAB is carried by the lysogenic phage CTXΦ, copies of whose genome may be amplified in tandem on the chromosome of V. cholerae (1). PstI is known to have only one internal site at orfU of the genome of CTXΦ. Southern blot hybridization of PstI-digested genomic DNAs of 109 strains isolated from 1993 to 1999 with the ctxAB gene probe showed 11 different patterns (Fig. 2 top, genotypes ct1 to ct11). These blots contained one to three bands 5.7 to 15 kb in size. Seventy-five isolates (68.8%) had two copies of ctxAB, 26 isolates (23.9%) had one copy, and 8 isolates (7.3%) had three copies or more. The genotypes ct1, ct2, ct5, ct8, and ct10 each contained two bands, and the genotypes ct3, ct9, and ct11 each contained three bands. However, the sizes of the fragments could not be completely identified. These results suggest that the strains carry two or more copies of the CTX genetic element located in different regions of the chromosome.
FIG. 2.
Southern hybridization analysis of the elements in the CTXΦ genome (ctxAB [top], zot [middle], and RS [bottom]) in V. cholerae O139 strains isolated from 1993 to 1999. Genomic DNA was digested with PstI and probed with digoxigenin-labeled ctxAB, zot, and RS, respectively. The numbers on the left indicate the molecular sizes of the bands and correspond to a λ DNA HindIII marker.
In our analysis, all of the toxigenic strains belong to four different ribotypes, while they have 11 different ctxAB genotypes with different numbers of hybridization bands of different sizes; for example, of the 32 toxigenic strains isolated in 1993 in Xinjiang, which belonged to ribotype rb3, 27 strains were genotype ct1, 2 strains were ct3, 1 strain was ct9, and 2 strains were ct11 (Table 1). They had different ctxAB probe hybridization bands when digested with PstI. These results show that O139 strains are undergoing rapid genetic variation, and in a single epidemic, some strains may take on local genetic variations resulting from mobile elements, such as the CTXφ genome.
The predominant genotype of the toxigenic strains was ct1, based on the numbers and the proportions of strains isolated in different regions. In our results, the clones causing the first outbreak in Xinjiang, and the strains isolated from sporadic cases in the other eight regions from 1993 to 1998, belonged to genotype ct1. The strains of type ct1 displaying two bands in Southern blot analysis (∼10 and 5.7 kb) accounted for 56.9% (62 of 109) of all analyzed ctxAB-positive strains. All strains isolated from Beijing belonged to a single genotype, ct4.
We noticed that eight of the studied O139 isolates, which showed no CTX elements, possessed three unique ribotype patterns (rb4, four strains; rb5, three strains; and rb7, one strain). ctxAB-positive strains were not included in these patterns. Because six of these ctxAB-negative strains were isolated from patients, an unknown pathogenic mechanism might exist. Their distinct ribotype patterns compared with those of the toxigenic strains may also indicate some tendency for clone differentiation.
In the same way as for the ctxAB probe, we performed zot gene probe hybridization (with PstI digestion) on 109 toxigenic strains. We were able to classify these strains into 15 different patterns (Fig. 2 middle, z1 to z15) containing one to four bands 4.3 to 15 kb in size. Thus, the pattern observed for the zot gene probe proved to be much more diverse than that of the CT genotype. In general, in the CTXΦ genome, there is one copy of zot and one copy of ctxAB (24); thus, in one strain containing multiple CTXΦ copies, the number of copies of ctxAB should be identical to that of zot in CTXΦ genomes. The patterns with the ctxAB and the zot probe hybridizations should be identical in a strain when the chromosomal DNA is digested with PstI, which has only one restriction site in orfU but not in zot or ctxAB.
Nevertheless, in the present study, the strains belonging to the same CT genotype patterns showed zot patterns that had different numbers and sizes of hybridization bands. A 4.2-kb fragment in the z5, z10, and z11 patterns was not present in any CT genotype patterns. In our study, the numbers of copies of ctxAB in 22 (20.1%) O139 strains were different from those of their own zot genes: 10 of 22 strains contained one more copy of zot than of ctxAB, 8 strains contained two more copies of zot than of ctxAB, and in four other strains, there were fewer copies of zot than of ctxAB. These results suggest that another CTXΦ phage family genome without a ctxAB gene probably exists in one O139 strain with a CTXΦ genome. Previously, it had been reported that ctxAB may not coexist with other genes of the CTX element (3, 10). This type of genome has been designated pre-CTXΦ (3) or nct-CTXΦ (10). We cloned the pre-CTXΦ-like genome from one such strain (our unpublished data and GenBank accession no. AF302794 and AF416590). However, it is still unclear how and why the genomes of CTXΦ and pre-CTXΦ integrate in the chromosome of one strain.
For hybridization with the RS probe, we found 12 different patterns consisting of one to four bands between 17 and 5.7 kb in size in 109 strains (Fig. 2 bottom, R1 to R12). It is possible that one or more copies of RS (including RS1 and RS2) are arranged in tandem in the genome of CTXΦ. As a result, the strains belonging to the same ctxAB genotype exhibit different and complicated RS patterns.
The strains belonging to the same genotypes of ctxAB, zot, and RS were differentiated in different ribotypes, suggesting that strains originating from different clones may possess identical CTX element characterizations. However, as an unstable element, it may result in amplification, rearrangement, or deletion in the same clonal strains under different selection pressures and inducible factors to generate the wide diversity of their arrangement on chromosomes.
rrn operons in V. cholerae O139 strains.
The endonuclease I-CeuI site is located only in the 23S rRNA gene of the rrn operon; therefore, the number of I-CeuI sites in the genome is taken as a measure of the number of rrn operons present in the genome. I-CeuI digestion followed by PFGE can be a useful tool for intra- and interspecies genome rearrangement analysis (13). Previous studies showed that V. cholerae strains belonging to serogroups O1 and O139 have nine rrn operons in their genomes and that V. cholerae strains belonging to serogroups non-O1 and non-O139 have 10 rrn operons (17).
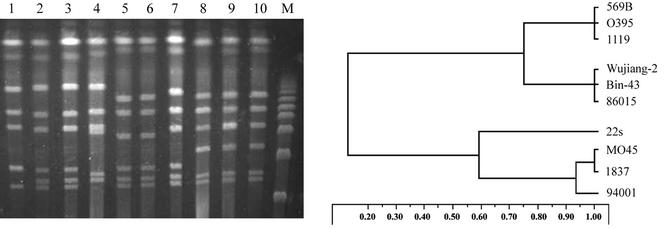
In our study, the PFGE profiles with I-CeuI digestion showed significant differences among strains of serogroups O1, O22, and O139 and between the classical and El Tor biotypes of serogroup O1 (Fig. 3). The positions of the operons and the fragment sizes in the chromosome are different, suggesting that genomic rearrangements may take place. This result agrees with some previous research (11, 17). The dendrogram for PFGE patterns generated by digestion with I-CeuI showed that V. cholerae O22 was genetically more closely related to V. cholerae O139 than to V. cholerae O1 (Fig. 3). In addition, it showed that the toxigenic V. cholerae O139 was genetically closer to nontoxigenic V. cholerae O139 than to V. cholerae O1 biotype El Tor. Based on these results, we hypothesize that the nontoxigenic O139 strains might be the potential reservoir of toxigenic O139 strains and that they might originate from the non-O1- non-O139 strains by serogroup-specific genetic changes and genome rearrangements. Once the induced CTXΦ is introduced among the nontoxigenic O139 strains in a natural habitat, they could obtain the virulence gene by the lysogenic conversion of CTXΦ and become toxigenic strains to trigger epidemics.
FIG. 3.
PFGE profiles (left) of the genomic DNAs of V. cholerae strains belonging to different serogroups and biotypes digested with I-CeuI and schematic diagram (right) of dendrogram analysis of the strains. Lane 1, 94001 (O139; cholera toxin negative; China); lane 2, 1837 (O139; ctxAB+; Bangladesh); lane 3, MO45 (O139; ctxAB+; India); lane 4, 22s (O22; cholera toxin negative; China); lane 5, 86015 (O1; El Tor biotype; cholera toxin negative; China); lane 6, Bin-43 (O1; El Tor biotype; ctxAB+; China); lane 7, Wujiang-2 (O1; El Tor biotype; ctxAB+; China); lane 8, 1119 (O1; classical biotype; ctxAB+; India); lane 9, O395 (O1; classical biotype; ctxAB+; Bangladesh); lane 10, 569B (O1; classical biotype; ctxAB+; Bangladesh); lane M, λ DNA ladder molecular size standard.
In summary, the clone diversity among the O139 strains demonstrated by ribotyping and restriction fragment length polymorphism of CTX element probe hybridization in this study suggests that O139 strains are undergoing rapid genetic changes resulting in the transient appearance of different clones. We hypothesize that the existence of different clones of toxigenic V. cholerae and the emergence of new clones are due to the interplay of genetic changes and natural selection caused by unidentified environmental factors, and also, the occasional contagion chance which introduces the bacteria into the human population should play a selective role, as the strains will become the dominant clones by spreading in the human population. The immune status of the host population is likely to influence the process as well. Moreover, using more than one typing techniques in epidemiological investigations helps to provide more information to reveal the genetics and variation of strains in evolution.
Acknowledgments
This research was supported by the National Basic Research Priorities Programme (grant G1999054102 to B.K.), Ministry of Science and Technology, People's Republic of China.
REFERENCES
- 1.Basu, A., A. K. Mukhopadhyay, C. Sharma, J. Jyot, N. Gupta, A. Ghosh, S. K. Bhattacharya, Y. Takeda, A. S. G. Farque, M. J. Albert, and G. B. Nair. 1998. Heterogeneity in the organization of the CTX genetic element in strains of Vibrio cholerae O139 Bengal isolated from Calcutta, India and Dhaka, Bangladesh and its possible link to the dissimilar incidence of O139 cholera in the two locales. Microb. Pathog. 24:175-183. [DOI] [PubMed] [Google Scholar]
- 2.Bhadra, R. K., S. Roychoudhury, R. K. Banerjee, S. Kar, R. Majumdar, S. Sengupta, S. Chatterjee, G. Khetawat, and J. Das. 1995. Cholera toxin (CTX) genetic element in Vibrio cholerae O139. Microbiology 411:1977-1983. [DOI] [PubMed] [Google Scholar]
- 3.Boyd, E. F., A. J. Heilpern, and M. K. Waldor. 2000. Molecular analyses of a putative CTXΦ precursor and evidence for independent acquisition of distinct CTXΦs by toxigenic Vibrio cholerae. J. Bacteriol. 182:5530-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cholera Working Group, International Center for Diarrhoeal Disease Research, Bangladesh. 1993. Large epidemic of cholera like disease in Bangladesh caused by Vibrio cholerae O139 synonym Bengal. Lancet 342:387-390. [PubMed] [Google Scholar]
- 5.Dalsgaard, A., H. F. Mortensen, K. Molbak, F. Dias, O. Serichantalergs, and P. Echeverria. 1996. Molecular characterization of Vibrio Cholerae O1 strains isolated during cholera outbreaks in Guinea-Bissau. J. Clin. Microbiol. 34:1189-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalsgaard, A., O. Serichantalergs, A. Forslund, C. Pitarangsi, and P. Echeverria. 1998. Phenotypic and molecular characterization of Vibrio cholerae O1 isolate in Samutsakorn, Thailand before, during and after the emergence of V. cholerae O139. Epidemiol. Infect. 121:259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faruque, S. M., Asadulghani, A. R. M. A. Alim, M. J. Albert, K. M. N. Islam, and J. J. Mekalanos. 1998. Induction of the lysogenic phage encoding cholera toxin in naturally occurring strains of toxigenic Vibrio cholerae O1 and O139. Infect. Immun. 66:3752-3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faruque, S. M., A. R. M. A. Alim, S. K. Roy, F. Khan, G. B. Nair, R. B. Sack, and M. J. Albert. 1994. Molecular analysis of rRNA and cholera toxin genes carried by the new epidemic strains of toxigenic Vibrio cholerae synonym Bengal. J. Clin. Microbiol. 32:1050-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faruque, S. M., M. J. Albert, and J. J. Mekalanos. 1998. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 62:1301-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kan, B., G. M. Qi, Y. Q. Liu, C. L. Liu, and S. Y. Gao. 1999. Genome of bacteriophage CTXΦ without the presence of ctxAB exists in ctxAB− strains of Vibrio cholerae. Chinese J. Microbiol. Immunol. 3:175-179. [Google Scholar]
- 11.Khetawat, G., R. K. Bhadra, S. Nandi, and J. Das. 1999. Resurgent Vibrio cholerae O139: rearrangement of cholera toxin genetic elements and amplification of rrn operon. Infect. Immun. 67:148-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lan, R., and P. R. Reeves. 1998. Recombination between rRNA operons created most of the ribotype variation observed in the seventh pandemic clone of Vibrio cholerae. Microbiology 144:1213-1221. [DOI] [PubMed] [Google Scholar]
- 13.Liu, S. L., A. Hessel, and K. E. Sanderson. 1993. Genomic mapping with I-CeuI, an intron-encoded endonuclease specific for genes for ribosomal RNA in Salmonella spp., Escherichia coli and other bacteria. Proc. Natl. Acad. Sci. USA 90:6874-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitra, R., A. Basu, D. Dutta, G. B. Nair, and Y. Takeda. 1996. Resurgence of Vibrio cholerae O139 Bengal with altered antibiogram in Calcutta, India. Lancet 348:1181-1186. [DOI] [PubMed] [Google Scholar]
- 15.Mukhopadhyay, A. K., A. Basu, P. Garg, P. K. Bag, A. Ghosh, S. K. Bhattacharya, Y. Takeda, and G. B. Nair. 1998. Molecular epidemiology of reemergent Vibrio cholerae O139 Bengal in India. J. Clin. Microbiol. 36:2149-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nair, G. B., T. Ramamurthy, S. K. Bhattcharya, A. K. Mukhopadhyay, S. Garg, M. K. Bhattacharya, T. Takeda, T. Shimada, Y. Takeda, and B. C. Deb. 1994. Spread of Vibrio cholerae O139 Bengal in India. J. Infect. Dis. 169:1029-1034. [DOI] [PubMed] [Google Scholar]
- 17.Nandi, S., G. Khetawat, S. Sengupta, R. Majumder, S. Kar, R. K. Bhadra, S. Roychoudhury, and J. Das. 1997. Rearrangement genomes of Vibrio cholerae strains belonging to serovars and biovars. Int. J. Syst. Bacteriol. 47:858-862. [DOI] [PubMed] [Google Scholar]
- 18.Pearson, G. D. N., A. Woods, S. L. Chiang, and J. J. Mekalanos. 1993. CTX genetic element encodes a site specific recombination system and international colonization. Proc. Natl. Acad. Sci. USA 90:3750-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popvic, T., C. Bopp, O. Olsvic, and K. Wachsmuth. 1993. Epidemiological application of a standardized ribotype scheme for Vibrio cholerae O1. J. Clin. Microbiol. 31:2472-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popvic, T., O. Olsvik, J. G. Wells, G. M. Evins, D. N. Cameron, J. J. Farmer III, C. A. Bopp, K. Wachsmuth, G. B. Nair, R. B. Sack, M. J. Albert, T. Shimada, and J. C. Feeley. 1995. Molecular subtyping of toxigenic Vibrio cholerae O139 causing epidemic cholera in India and Bangladesh 1992-1993. J. Clin. Dis. 171:122-127. [DOI] [PubMed] [Google Scholar]
- 21.Ramamurthy, T., S. Garg, R. Sharma, S. K. Bhattacharya, G. B. Nair, T. Shimada, T. Takeda, T. Karasawa, H. Kurazano, A. Pal, and Y. Takeda. 1993. Emergence of a novel strain of Vibrio cholerae with epidemic potential in Southern and Eastern India. Lancet 341:703-704. [DOI] [PubMed] [Google Scholar]
- 22.Rivas, M., C. Toma, E. Miliwebsky, M. I. Caffer, M. Galas, P. Varela, M. Tous, A. M. Bru, and N. Binsztein. 1993. Cholera isolates in relation to the ‘eighth pandemic’. Lancet 342:926-927. [PubMed] [Google Scholar]
- 23.Roychoudhury, S., R. K. Bhadra, and J. Das. 1994. Genome size and restriction fragment length polymorphism analysis of Vibrio cholerae strains belonging to different serovars and biotypes. FEMS Microbiol. Lett. 115:329-334. [DOI] [PubMed] [Google Scholar]
- 24.Waldor, M. K., and J. J. Mekalanos. 1996. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272:1910-1914. [DOI] [PubMed] [Google Scholar]