Abstract
We characterized 32 levofloxacin-nonsusceptible Streptococcus pneumoniae (LNSP) isolates obtained from a broad geographic region of North America over a 5-year period by using capsular serotypes, antimicrobial susceptibility profiles, BOX-PCR, multilocus sequence typing (MLST), and pulsed-field gel electrophoresis (PFGE). Sixteen international clones identified by the Pneumococcal Molecular Epidemiology Network also were included for comparison. Fifteen serotypes were represented, with serogroups 6, 9, 14, 19, and 23 accounting for 63% of isolates. Among isolates whose quinolone resistance-determining regions were sequenced, all contained gyrA and parC point mutations. Sixty-three percent were penicillin susceptible, and 84% were erythromycin susceptible. BOX-PCR analysis identified 39 different band patterns among 32 LNSP and 16 international clones and grouped 16 isolates, including 2 international clones, into seven unrelated groups of 2 to 4 isolates each. PFGE analysis identified 35 different band patterns among 32 LNSP and 16 international clones and grouped 21 isolates, including 3 international clones, into eight unrelated groups of 2 to 6 isolates each. MLST performed on 10 isolates identified five allelic profiles and separated 9 isolates into four groups of 2 to 3 isolates each. Overall, each typing method indicated that the LNSP were heterogeneous and that resistance to fluoroquinolones was not closely associated with a particular serotype or with coresistance to other antimicrobial classes and suggests that LNSP have likely arisen through independent mutational events as a result of selective pressure. However, seven LNSP were found to be related to three international clones by PFGE.
Streptococcus pneumoniae is a major causative agent of community-acquired pneumonia, otitis media, meningitis, and bacteremia, and it remains an important cause of morbidity and mortality among all age groups worldwide. Pneumococcal infections are often treated with β-lactams, macrolides, and, more recently, fluoroquinolones.
Fluoroquinolones act by forming irreversible tertiary complexes with actively replicating DNA and type II DNA topoisomerases (16, 30). Resistance to fluoroquinolones is mediated primarily by point mutations in the quinolone resistance-determining regions (QRDRs) of the genes encoding DNA gyrase (gyrA and gyrB) and topoisomerase IV (parC and parE) (22, 37, 38). A fluoroquinolone resistance efflux mechanism has also been reported for S. pneumoniae that usually results in low-level resistance (3, 18).
Fluoroquinolone resistance in S. pneumoniae has been well documented (5, 7, 10, 11, 19) but is still less than 2% overall for ciprofloxacin in the United States according to recent surveys, despite a dramatic increase in fluoroquinolone use to treat community-acquired respiratory tract infections since the late 1990s (4, 5, 10, 11, 19, 23, 29). However, rapidly emerging resistance in the well-characterized Spain23F-1 clone was apparently responsible for a fluoroquinolone resistance rate of 13.3% reported in Hong Kong during 2000 (20). This Spain23F-1 clone is already responsible for an estimated 38.7% of penicillin-resistant S. pneumoniae respiratory tract isolates in the United States (6). While there have been many studies that address the epidemiology of S. pneumoniae with reduced susceptibility to macrolides and β-lactams (2, 6, 8, 12, 15, 25, 28, 31), much remains unknown about the epidemiology of fluoroquinolone resistance in S. pneumoniae, since it has been documented only within the past few years and relatively small numbers of clinical isolates are available for study. It is important to assess the characteristics of these organisms to gain a better understanding of their clonal relationships and their potential for clonal dissemination and to aid in the selection of appropriate antimicrobials for empirical therapy.
In order to improve our understanding of the development of fluoroquinolone resistance in S. pneumoniae, we used capsular serotyping, antimicrobial susceptibility profiles, QRDR point mutations, BOX-PCR typing, multilocus sequence typing (MLST), and pulsed-field gel electrophoresis (PFGE) to study 32 clinical isolates with reduced susceptibility to levofloxacin and 16 Pneumococcal Molecular Epidemiology Network-recognized international clones.
(This work was presented at the 3rd International Symposium on Pneumococci and Pneumococcal Diseases, Anchorage, Alaska, May 2002.)
MATERIALS AND METHODS
Microorganisms.
Thirty-two levofloxacin-nonsusceptible S. pneumoniae (LNSP) isolates (levofloxacin MICs of >4 μg/ml) originating from blood, sputum, and pleural fluid specimens were obtained through active bacterial surveillance studies conducted by the Centers for Disease Control and Prevention as part of the Emerging Infections Program Network and from culture collections from local laboratories. Partial characterization of some of these isolates has been previously reported (43). Eleven isolates originated from Connecticut, four from Georgia, three each from Maryland, Minnesota, Texas, and Ontario, Canada, two from Alabama, and one each from California, New York, and Oregon between 1995 and 1999. Isolates were shipped to the University of Alabama at Birmingham frozen and were stored at −70°C until tested. Upon receipt they were grown overnight at 35°C on trypticase soy agar with 5% defibrinated sheep blood under 5% CO2, streaked for isolation, subcultured from a single colony, reidentified by hemolytic pattern and optochin susceptibility, and stored at −70°C in Todd Hewitt broth with Bacto-yeast extract plus 23% glycerol. Sixteen international clones were obtained from existing laboratory collections or directly from the American Type Culture Collection (Manassas, Va.) as shown in Table 1.
TABLE 1.
Characteristics of 32 LNSP and 16 international clonesa
| Strain designation | ATCC No. | Type | Originb (yr) | Typing method
|
||
|---|---|---|---|---|---|---|
| PFGE | BOX-PCR | MLST allelic profilec | ||||
| SP2072 | 6B | TX (1999) | 1 | A | ||
| MN04618 | 6B | MN (1998) | 1 | B | ||
| CT00815 | 6B | CT (1995) | 2 | B | ||
| South Africa19A-7 | 700674 | 19A | SAF | 3 | C | 2-13-8-25-25-6-8 |
| CT70490 | 23F | CT (1997) | 4 | D | 4-4-2-4-4-1-1 | |
| CT00090 | 23F | CT (1995) | 4 | E | ||
| CT90233 | 19F | CT (1999) | 4 | E | ||
| SF10029 | 23F | CA (1997) | 4 | E | 4-4-2-4-4-1-1 | |
| GA06221 | 23F | GA (1996) | 4 | E | 4-4-2-4-4-1-1 | |
| Spain23F-1 | 700669 | 23F | SPN | 4 | F | 4-4-2-4-4-1-1 |
| CSR14-10 | 700677 | 14 | CSR | 5 | G | 1-5-4-1-5-3-3 |
| CT71119 | 13 | CT (1997) | 6 | H | ||
| GA04388 | 20 | GA (1995) | 6 | I | ||
| Taiwan23F-15 | 700906 | 23F | TAI | 7 | J | 15-29-4-21-30-1-14 |
| MN00418 | 31 | MN (1998) | 8 | K | ||
| MN01871 | 6A | MN (1997) | 9 | F | 10-13-1-1-5d-1-20 | |
| SP1099 | 6A | TX (1999) | 9 | L | 10-13-1-1-5-1-20 | |
| Poland23F-16 | BAA-343 | 23F | POL | 10 | M | 7-13-8-1-10-6-36 |
| CT90238 | 16 | CT (1999) | 1 | N | ||
| CT81035 | 18C | CT (1998) | 12 | O | ||
| England14-9 | 700676 | 14 | ENG | 13 | P | 1-5-4-5-5-1-8 |
| MD09289 | 19F | MD (1995) | 14 | Q | ||
| Taiwan19F-14 | 700905 | 19F | TAI | 15 | R | 15-16-19-15-6-20-26 |
| TO03022 | 28 | ON (1995) | 16 | S | ||
| GA06781 | 23F | GA (1997) | 17 | T | ||
| RP1 | 6A | GA (ND) | 18 | U | ||
| Spain14-5 | 700902 | 14 | SPN | 19 | V | 1-5-4-11-9-3-16 |
| Spain9V-3 | 700671 | 9V | SPN | 20 | W | 7-11-10-1-6-8-1 |
| T703626 | 9V | TX (1997) | 20 | X | ||
| Hungary19A-6 | 700673 | 19A | HUN | 21 | Y | 7-13-42-6-10-6-56 |
| CT90403 | 19F | CT (1999) | 22 | U | ||
| CT80314 | 6A | CT (1999) | 23 | Z | 1-25-1-8-15-20-14 | |
| NY00779 | 6B | NY (1999) | 24 | AA | ||
| CT01147 | 18C | CT (1996) | 25 | BB | ||
| Finland6B-12 | 700903 | 6B | FIN | 26 | CC | 5-6-43-2-6-1-28 |
| MD71350 | 35 | MD (1999) | 27 | DD | 18-12-4-44-14-77-97 | |
| O804923 | 34 | OR (1998) | 27 | EE | 18-12-4-44-14-77d-97 | |
| TO3607 | 34 | ON (ND) | 28 | FF | ||
| South Africa6B-8 | 700675 | 6B | SAF | 29 | GG | 7-22-1-2-5-1-14 |
| Tennessee23F-4 | 51916 | 23F | TN | 30 | HH | 1-8-6-2-6-4-6 |
| CT90253 | 22F | CT (1999) | 31 | II | 1-1-4-1-18-58-17 | |
| MD70023 | 22F | MD (1997) | 31 | II | 1-1-4-1-18-58-17 | |
| UAB169 | 6A | AL (1996) | 31 | JJ | ||
| Spain6B-2 | 700670 | 6B | SPN | 32 | JJ | 5-6-1-2-6-3-4 |
| UAB93 | 6B | AL (1995) | 32 | KK | ||
| TO3520 | 14 | ON (ND) | 33 | Z | ||
| South Africa19A-13 | 700904 | 19A | SAF | 34 | LL | 1-8-9-5-11-1-12 |
| CSR19A-11 | 700678 | 19A | CSR | 35 | MM | 7-5-4-26-10-3-55 |
International clones are identified in bold, and italics signifies the groups of microorganisms that were shown to be related to one another as described in the text.
Abbreviations: AL, Alabama; CA, California; CT, Connecticut; GA, Georgia, MD, Maryland; MN, Minnesota; NY, New York; OR, Oregon; TX, Texas; ON, Ontario; CSR, Czech Republic; FIN, Finland; HUN, Hungary; POL, Poland; SAF, South Africa; SPN, Spain; TAI, Taiwan.
Data from McGee et al. (34).
MLST alleles indicated represent closest matches based on query results obtained from http://www.mlst.net.
Previously determined organism characteristics.
Capsular serotypes, QRDR point mutations, and antimicrobial susceptibility profiles reported previously (43) were used to determine clonal relatedness. Capsular serotyping was accomplished by using anticapsular antibodies or fluorescence-activated cell sorting.
BOX-PCR typing.
BOX-PCR was performed on 32 LNSP and 16 international clones. DNA from all isolates was purified for BOX-PCR by using an ethanol precipitation method as follows. Organisms were grown to confluency, and approximately 2.5 cm was scraped and suspended in 500 μl of Tris-EDTA (TE) with 1% sodium dodecyl sulfate (SDS). This was incubated at 37°C for 15 min and then was transferred to 65°C for 15 min. A volume of 100 μl of 5 M potassium acetate was added, inverted to mix, and incubated at 65°C for 30 min. The mixture was then incubated on ice for 1 h. The mixture was then centrifuged for 10 min at 16,060 × g, and 500 μl of supernatant was removed and added to 1 ml of cold ethanol and was inverted to mix. The mixture was centrifuged for 10 min at 13,000 rpm, all ethanol was removed, and the DNA pellet was allowed to dry. The DNA pellet was then eluted in 50 to 500 μl of Millipore water and was quantitated on a Gene Quant II spectrophotometer (Amersham Pharmacia Biotech, Piscataway, N.J.).
BOX-PCR was performed by using Ready-to-Go PCR beads (Amersham Pharmacia Biotech, Piscataway, N.J.) in a 25-μl reaction mixture containing 2 μl of 100- to 200-μg/ml genomic DNA, 10 μl of 5-pmol/μl BOXA1R primer (5′-CTACGGCAAGGCGACGCTGACG-3′) (27), 9.25 μl of Millipore water, and 3.75 μl of 10 mM MgCl2. Cycling conditions were as follows: 94°C for 5 min, 35 cycles of denaturation (95°C) for 1 min, annealing (58°C) for 2 min, and extension (72°C) for 2 min, followed by holding at 4°C. BOX-PCR was carried out on a Perkin Elmer 9600 thermal cycler. Amplicons were analyzed for band patterns by agarose gel electrophoresis. Four groups of 10 isolates initially detected by BOX-PCR analysis were selected for further analysis by MLST. Analysis of BOX-PCR bands and generation of dendrograms was carried out with GelCompar II analysis software (Applied Maths, Kortrijk, Belgium) with Pierson and unweighted pair group method with arithmetic mean parameters with 1% optimization.
MLST.
Ten isolates chosen on the basis of BOX-PCR similarities underwent MLST analysis as described by Enright and Spratt (14). Fragments of 405 to 486 bp were amplified from seven housekeeping genes by using Ready-to-Go PCR beads (Amersham Pharmacia Biotech) with the following cycling conditions: an initial denaturation at 95°C, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 57°C for 30 s, and extension at 72°C for 60 s, and a final hold at 4°C on a Perkin Elmer 9600 thermal cycler. PCR products were purified by using the Qiagen PCR Purification kit (Valencia, Calif.), and then both strands were sequenced by using the Amersham DyeET dye terminator kit (Amersham Pharmacia Biotech) with the following cycling conditions: 25 cycles of denaturation at 95°C for 20 s, annealing at 50°C for 15 s, and extension at 60°C for 60 s. Sequencing reactions were separated from the excess dye terminator by using Performa gel filtration cartridges (Edge BioSystems, Gaithersburg, Md.). Sequencing reactions were run on an ABI 377 automated DNA sequencer. Results were analyzed by using the ABI Prism Sequencing Analysis program (Perkin-Elmer Applied Biosystems). Sequences were assembled and edited by using the Sequencher program (Gene Codes Corporation, Ann Arbor, Mich.). Edited sequences were then submitted to the MLST website (http://www.mlst.net) for final assignment of allele and sequence types as well as alignment and comparison with published MLST allele type sequences (34).
PFGE.
Thirty-two LNSP isolates, 16 international clones, and S. pneumoniae reference strain R6 were subjected to PFGE as described by Louie et al. (28) with some modifications. To make PFGE plugs, organisms were grown to confluency from single colonies and were suspended in 4 ml of saline to an optical density of 0.7 to 1.1 at 450 nm. One hundred-fifty microliters was removed, centrifuged, resuspended in TE, and added to 150 μl of 1.8% low-melting-point agarose (Bio-Rad Laboratories, Hercules, Calif.). Solidified plugs were lysed on a rotator overnight at 37°C in fresh lysis buffer (6 mM Tris-HCl [pH 8.0], 1 M NaCl, 100 mM EDTA [pH 8.0], 0.5% Brij 58, 0.2% deoxycholate, 0.5% N-lauroylsarcosine, 20 μg of RNase/ml, 1 mg of lysozyme/ml) and incubated in ESP buffer (0.5 M EDTA [pH 9 to 9.5], 1% N-lauroylsarcosine, 50 μg of proteinase K/ml) at 55°C overnight. Plugs were washed in TE and were stored at 4°C. Half of each plug was digested in 4 μl of SmaI solution overnight at 25°C. Electrophoresis on a 1% Pulsed Field Certified Agarose gel (Bio-Rad Laboratories) was performed for 22 h by using linearly ramped pulse times beginning with 1 s and ending with 40 s at 6 V/cm at 14°C. The gel was stained with ethidium bromide for 1 h and was destained for 1 h and then photographed by using Gel-Doc 2000 (Bio-Rad Laboratories). Isolates were initially aligned on the basis of band similarities and dendrograms generated by using GelCompar II analysis software (Applied Maths) with Pierson and unweighted pair group method with arithmetic mean parameters. Organisms were ultimately designated as indistinguishable (having identical band patterns), closely related (having three or fewer band differences), or unrelated (having four or more band differences) by using a method similar to that described by Tenover et al. (40).
RESULTS
Capsular serotyping.
Fifteen serotypes were represented among the 32 LNSP isolates (Table 1). Five serotypes commonly associated with antimicrobial resistance (serotypes 6, 9, 14, 19, and 23) accounted for 20 (63%) of the 32 isolates.
DNA sequencing.
Among the 30 isolates for which QRDR sequence data were available, 27 had a Ser81→Phe mutation and 3 had a Glu85→Lys mutation in GyrA. Among 29 isolates for which ParC data were available, 27 had a Ser79→Phe mutation, 1 had a Ser79→Tyr mutation, and 1 had a Ser80→Pro mutation and an Asp83→Tyr mutation. These QRDR sequence data have been published previously (43).
Antimicrobial susceptibility testing.
Levofloxacin MICs for two isolates were intermediate (4 μg/ml), and 30 isolates were fully resistant to levofloxacin (MIC ≥ 8 μg/ml). All 32 isolates were susceptible or intermediately resistant to moxifloxacin. The majority of isolates were susceptible to the other drugs tested, including penicillin, clindamycin, and erythromycin. Among the five erythromycin-resistant isolates, two contained mefA and three contained ermB, as determined by PCR methods described previously (21). Five isolates had reduced susceptibility to three or more drug classes.
BOX-PCR analysis.
A dendrogram illustrating the relationships among 32 LNSP and 16 international S. pneumoniae clones was generated (data not shown). Thirty-nine different BOX types were identified among the 32 LNSP isolates and 16 international clones by using a limit of 80% similarity. Among 23 BOX types detected in the 32 LNSP isolates, five unrelated groups, designated B, E, U, Z, and II, were identified, each consisting of two to four isolates (each is italicized in Table 1). The BOX-PCR pattern for MN01871 also appeared to match the isolate from the international clone Spain23F-1 (group F), a relationship which was not confirmed by MLST or PFGE. The BOX-PCR pattern for UAB169 matched that of Spain6B-2 (group JJ) but was not confirmed by PFGE.
MLST.
MLST was used to characterize 10 isolates (CT70490,GA06221, SF10029, MN01871, SP1099, O804923, MD71350, CT90253, MD70023, and CT80314) initially specified by BOX-PCR as representing patterns held by more than one isolate. Allelic profiles for these isolates as well as for the 16 international clones are shown in Table 1. Overall, MLST identified four groups and 1 distinct isolate among the 10 isolates examined.
Somewhat surprisingly, isolates CT70490, GA06221, and SF10029 were found to have MLST allelic profiles identical to that of the Spain23F-1 clone despite having BOX-PCR patterns that were distinct from that of this clone. Two LNSP alleles failed to match allelic sequences already present in the MLST database. Isolates MN01871 and SP1099 were identical to each other at six out of seven loci (single-locus variants). Isolates O804923 and MD71350 were identical to each other at all seven loci.
PFGE.
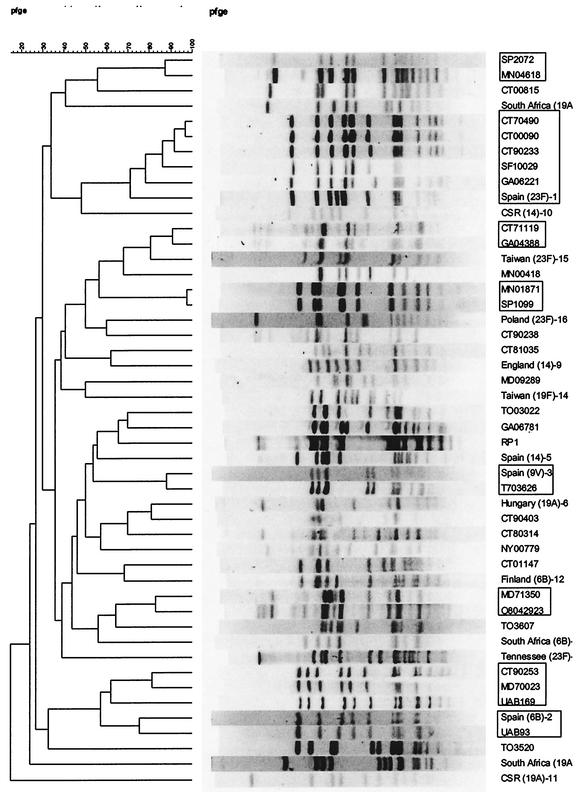
A dendrogram illustrating the relationships of the 32 LNSP and 16 international clones was generated (Fig. 1). Twenty different PFGE types were identified among the 32 LNSP isolates. Among these, there were six groups consisting of two to five isolates each (groups 1, 4, 6, 9, 27, and 31) shown in italics in Table 1. Band patterns for isolates in groups 1, 6, 27, and 31 were closely related to each other within those groups, and band patterns for isolates in group 9 were indistinguishable from each other. In group 2, band patterns for SF10029, GA06221, and Spain23F-1 were all closely related to each other and to the three isolates CT70490, CT00090, and CT90233 which were indistinguishable from each other. Overall, PFGE identified five isolates which were closely related to Spain23F-1, one of which was indistinguishable from Spain9V-3 and one of which was closely related to Spain6B-2.
FIG. 1.
Dendrogram illustrating clonal relationships among 32 LNSP and 16 international clones. Related groups are indicated by boxes.
There was 100% correlation between PFGE and MLST methods for identifying genetic relatedness. Results of PFGE and MLST did not correlate well with results of BOX-PCR. Correlation data for the three typing methods are summarized in Table 2.
TABLE 2.
Congruence between molecular typing methods
| Method | No. of distinct patterns | No. of groups with >1 isolate | Correlation results (%)
|
||
|---|---|---|---|---|---|
| PFGE | BOX-PCR | MLST | |||
| PFGE | 23 | 6 | 100 | ||
| BOX-PCR | 23 | 5 | 40 | 100 | |
| MLSTa | 5 | 4 | 100 | 30 | 100 |
MLST was performed on only 10 isolates.
DISCUSSION
Very few studies have addressed the epidemiology of fluoroquinolone-nonsusceptible S. pneumoniae in North America or elsewhere. The recent emergence of S. pneumoniae with reduced susceptibilities to fluoroquinolones and well-documented treatment failures with the emergence of resistance during levofloxacin therapy for pneumococcal pneumonia (7, 24) underscore the need for such evaluations.
In a molecular epidemiology study of penicillin-resistant S. pneumoniae published in 1998, Corso et al. (6) detected 20 isolates with reduced susceptibility to levofloxacin in a national survey of 9,160 isolates. Sixteen of these 20 isolates were highly resistant to levofloxacin (MIC = 32 μg/ml). The isolates were predominantly heterogeneous based on PFGE. However, they discovered four strains that were related to the Spain23F-1 clone and one strain that was related to the French serogroup 9/14 clone. This was the first documentation of fluoroquinolone resistance in these international clones in the United States. During the past 5 years, fluoroquinolone resistance in other international clones, including Spain6B-2, Spain9V-3, and Spain14-5, has been reported from various countries (1, 20, 33, 39). Ours is the first evaluation to employ serotyping, antimicrobial susceptibility profiles, QRDR point mutations, BOX-PCR, MLST, and PFGE to characterize the epidemiology of pneumococcal fluoroquinolone resistance in North America.
In our study, reduced susceptibility to levofloxacin was not closely associated with resistance to other classes of antimicrobial agents. Only 5 of 32 isolates had reduced susceptibilities to three or more drug classes, and the majority remained susceptible to penicillin (63%) and erythromycin (84%). These percentages are reflective of the average susceptibility rates for penicillin and erythromycin in the United States and Canada (9, 10, 23). Weigel et al. reported a similar finding (41). In contrast, Corso et al. found that 65% of 20 fluoroquinolone-resistant isolates had reduced susceptibility to penicillin (6). Ho et al. (19, 20) found that all fluoroquinolone-resistant organisms in their study from Hong Kong had reduced susceptibilities to penicillin, cefotaxime, and erythromycin, which is not surprising, because they were all a single clone related to Spain23F-1. In addition, Brueggemann et al. (4) found that 42.9% of 49 fluoroquinolone-resistant isolates were concomitantly resistant to two to five classes of nonfluoroquinolone agents.
The capsular serotype distribution for isolates in our study was more diverse than that typically seen in other studies of β-lactam- and macrolide-resistant S. pneumoniae in the United States and Canada. Typically, serogroups 6, 9, 14, 19, and 23 account for 90% or more of isolates in other studies of antimicrobial-resistant S. pneumoniae (8, 17, 26, 32, 35, 39). In contrast, we found 15 different serotypes in our study, and serogroups 6, 9, 14, 19, and 23 represented only 63% of our isolates. Serotype diversity among pneumococcal isolates with reduced susceptibility to ciprofloxacin has also been observed by others (1, 42). These findings are more consistent with the independence of QRDR mutational events than with clonal spread.
We evaluated point mutations in QRDRs of 29 isolates and found no relationship between the position of QRDR mutations and genetic relatedness of the isolates, with the majority of isolates having the same two common mutations (43). Thus, characterization of QRDRs appears not to be helpful in understanding potential differences that may be present among LNSP.
In comparing the relatedness of LNSP isolates, there was a clear advantage to using MLST or PFGE. Overall, PFGE and MLST results were in agreement, whereas BOX-PCR analysis differentiated isolates shown to be related by PFGE and MLST, and it grouped isolates shown to be unrelated by PFGE and MLST. It is possible that since BOX elements do not code for proteins but rather for intergenic regulatory sequences, they may be more susceptible to genomic changes over time than are regions of SmaI restriction sites and housekeeping genes. Some advantages of MLST are the ability to instantly compare results between labs and the ability to compare results to those for international clones and other strains without the need to ever acquire the organisms in hand. BOX-PCR has some value for the purpose of initial assignment of clonal relatedness among isolates gathered in limited time and space; however, without software for rigorous analysis of band sizes or the inclusion of more commonly accepted methods (e.g., PFGE and MLST), its value is very limited. The discrepancies we report here support others' suggestions that the use of more than one typing method is necessary (8, 13).
Alou et al. (1), Richter et al. (39), Weigel et al. (41), Nichol et al. (36), and Zhanel et al. (42) all report high levels of genetic diversity among fluoroquinolone-resistant S. pneumoniae originating from Spain, the United States, and Canada. However, McGee et al. (33) described six fluoroquinolone-resistant pneumococci that appeared in Ireland in 1997, all of which were genetically related to the Spain9V-3 clone based on BOX-PCR and PFGE. Ho et al. (19, 20) reported 24 fluoroquinolone-resistant isolates that appeared in Hong Kong in 2000, all of which were genetically related to the Spain23F-1 clone based on PFGE and MLST. Thus, fluoroquinolone resistance occurs in organisms with diverse genetic backgrounds, probably arising de novo as a result of antimicrobial pressure, but can occur in international clones that have disseminated locally and globally, as we have also shown in the present study.
There are several limitations to our study. First, the rate of fluoroquinolone resistance is presently too low to draw significant epidemiological conclusions. This is reflected by the small number of isolates available for evaluation, although their geographic origins are diverse. Second, BOX-PCR was used to select isolates that were clonally related so that these clones could undergo more stringent analysis by MLST. As a result, MLST, a very technically demanding, time-consuming, and expensive procedure, was not performed on all 32 isolates but was restricted to those 10 isolates initially selected because of clonal relatedness on the basis of visual inspection of BOX-PCR band patterns. The use of PFGE analysis to determine clonal relationships and the use of GelCompar II software were therefore highly valuable for this study.
Mere documentation of fluoroquinolone resistance in widely disseminated international clones does not infer that these clones are now the vehicle for spreading these organisms throughout North America. However, the existence of LNSP related to international clones underscores the potential for fluoroquinolone resistance to occur and disseminate in a mode that is distinct from independent mutational events, provided that organisms containing these resistance genes have the same capacity for colonization and clonal spread. The appearance of fluoroquinolone resistance in international clones stresses the need for continued epidemiological studies. Finally, no clinical information is available for the isolates that were obtained mainly from surveillance studies, so it is not possible to assess directly the clinical significance of these bacterial isolates in the persons from which they were originally derived.
Acknowledgments
This study was supported by a grant from Bayer Corporation, Pharmaceutical Division, West Haven, Conn.
We thank the Centers for Disease Control and Prevention Active Bacterial Core Surveillance component of the Emerging Infections Program Network, Robert Jerris, and the Microbiology Laboratory of the University of Alabama Hospital for providing the LNSP isolates. We also thank Ashley Robinson for assistance in the development of BOX-PCR and MLST methods, David Briles for advice concerning methodology and evaluation of results, and Sarah Armstrong and Anita Smith for technical support.
REFERENCES
- 1.Alou, L., M. Ramirez, C. Garcia-Rey, J. Prieto, and H. de Lencastre. 2001. Streptococcus pneumoniae isolates with reduced susceptibility to ciprofloxacin in Spain: clonal diversity and appearance of ciprofloxacin-resistant epidemic clones. Antimicrob. Agents Chemother. 45:2955-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelbaum, P. C. 1992. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin. Infect. Dis 15:77-83. [DOI] [PubMed] [Google Scholar]
- 3.Bast, D. J., D. E. Low, C. L. Duncan, L. Kilburn, L. A. Mandell, R. J. Davidson, and J. C. de Azavedo. 2000. Fluoroquinolone resistance in clinical isolates of Streptococcus pneumoniae: contributions of type II topoisomerase mutations and efflux to levels of resistance. Antimicrob. Agents Chemother. 44:3049-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brueggemann, A. B., S. L. Coffman, P. Rhomberg, H. Huynh, L. Almer, A. Nilius, R. Flamm, and G. V. Doern. 2002. Fluoroquinolone resistance in Streptococcus pneumoniae in the United States since 1994-1995. Antimicrob. Agents Chemother. 46:680-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, D. K., A. McGeer, J. C. de Azavedo, and D. E. Low. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. Canadian Bacterial Surveillance Network. N. Engl. J. Med. 341:233-239. [DOI] [PubMed] [Google Scholar]
- 6.Corso, A., E. P. Severina, V. F. Petruk, Y. R. Mauriz, and A. Tomasz. 1998. Molecular characterization of penicillin-resistant Streptococcus pneumoniae isolates causing respiratory disease in the United States. Microb. Drug Resist. 4:325-337. [DOI] [PubMed] [Google Scholar]
- 7.Davidson, R., R. Cavalcanti, J. L. Brunton, D. J. Bast, J. C. de Azavedo, P. Kibsey, C. Fleming, and D. E. Low. 2002. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N. Engl. J. Med. 346:747-750. [DOI] [PubMed] [Google Scholar]
- 8.Doern, G. V., A. B. Brueggemann, M. Blocker, M. Dunne, H. P. Holley, Jr., K. S. Kehl, J. Duval, K. Kugler, S. Putnam, A. Rauch, and M. A. Pfaller. 1998. Clonal relationships among high-level penicillin-resistant Streptococcus pneumoniae in the United States. Clin. Infect. Dis. 27:757-761. [DOI] [PubMed] [Google Scholar]
- 9.Doern, G. V., A. B. Brueggemann, H. Huynh, and E. Wingert. 1999. Antimicrobial resistance with Streptococcus pneumoniae in the United States, 1997-98. Emerg. Infect. Dis. 5:757-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doern, G. V., K. P. Heilmann, H. K. Huynh, P. R. Rhomberg, S. L. Coffman, and A. B. Brueggemann. 2001. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999-2000, including a comparison of resistance rates since 1994-1995. Antimicrob. Agents Chemother. 45:1721-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doern, G. V., M. A. Pfaller, M. E. Erwin, A. B. Brueggemann, and R. N. Jones. 1998. The prevalence of fluoroquinolone resistance among clinically significant respiratory tract isolates of Streptococcus pneumoniae in the United States and Canada-1997 results from the SENTRY Antimicrobial Surveillance Program. Diagn Microbiol. Infect. Dis. 32:313-316. [DOI] [PubMed] [Google Scholar]
- 12.Doit, C., B. Picard, C. Loukil, P. Geslin, and E. Bingen. 2000. Molecular epidemiology survey of penicillin-susceptible and -resistant Streptococcus pneumoniae recovered from patients with meningitis in France. J. Infect. Dis. 181:1971-1978. [DOI] [PubMed] [Google Scholar]
- 13.Dunne, W. M., Jr., K. S. Kehl, C. A. Holland-Staley, A. B. Brueggemann, M. A. Pfaller, and G. V. Doern. 2001. Comparison of results generated by serotyping, pulsed-field restriction analysis, ribotyping, and repetitive-sequence PCR used to characterize penicillin-resistant pneumococci from the United States. J. Clin. Microbiol. 39:1791-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049-3060. [DOI] [PubMed] [Google Scholar]
- 15.Fenoll, A., I. Jado, D. Vicioso, A. Perez, and J. Casal. 1998. Evolution of Streptococcus pneumoniae serotypes and antibiotic resistance in Spain: update (1990 to 1996). J. Clin. Microbiol. 36:3447-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuda, H., and K. Hiramatsu. 1999. Primary targets of fluoroquinolones in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:410-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gherardi, G., J. S. Inostrozo, M. O'Ryan, V. Prado, S. Prieto, C. Arellano, R. R. Facklam, and B. Beall. 1999. Genotypic survey of recent beta-lactam-resistant pneumococcal nasopharyngeal isolates from asymptomatic children in Chile. J. Clin. Microbiol. 37:3725-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill, M. J., N. P. Brenwald, and R. Wise. 1999. Identification of an efflux pump gene, pmrA, associated with fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:187-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho, P. L., T. L. Que, D. N. Tsang, T. K. Ng, K. H. Chow, and W. H. Seto. 1999. Emergence of fluoroquinolone resistance among multiply resistant strains of Streptococcus pneumoniae in Hong Kong. Antimicrob. Agents Chemother. 43:1310-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho, P. L., R. W. Yung, D. N. Tsang, T. L. Que, M. Ho, W. H. Seto, T. K. Ng, W. C. Yam, and W. W. Ng. 2001. Increasing resistance of Streptococcus pneumoniae to fluoroquinolones: results of a Hong Kong multicentre study in 2000. J. Antimicrob. Chemother. 48:659-665. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, C. N., W. H. Benjamin, Jr., B. M. Gray, M. C. Crain, K. M. Edwards, and K. B. Waites. 2001. In vitro activity of ABT-773, telithromycin and eight other antimicrobials against erythromycin-resistant Streptococcus pneumoniae respiratory isolates of children. Int. J. Antimicrob. Agents 18:531-535. [DOI] [PubMed] [Google Scholar]
- 22.Jones, M. E., D. F. Sahm, N. Martin, S. Scheuring, P. Heisig, C. Thornsberry, K. Kohrer, and F. J. Schmitz. 2000. Prevalence of gyrA, gyrB, parC, and parE mutations in clinical isolates of Streptococcus pneumoniae with decreased susceptibilities to different fluoroquinolones and originating from Worldwide Surveillance Studies during the 1997-1998 respiratory season. Antimicrob. Agents Chemother. 44:462-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones, R. N., and M. A. Pfaller. 2000. Macrolide and fluoroquinolone (levofloxacin) resistances among Streptococcus pneumoniae strains: significant trends from the SENTRY Antimicrobial Surveillance Program (North America, 1997-1999). J. Clin. Microbiol. 38:4298-4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kays, M. B., and G. A. Denys. 2001. Fluoroquinolone susceptibility, resistance, and pharmacodynamics versus clinical isolates of Streptococcus pneumoniae from Indiana. Diagn. Microbiol. Infect. Dis. 40:193-198. [DOI] [PubMed] [Google Scholar]
- 25.Klugman, K. P. 1990. Pneumococcal resistance to antibiotics. Clin. Microbiol. Rev. 3:171-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klugman, K. P., and H. J. Koornhof. 1988. Drug resistance patterns and serogroups or serotypes of pneumococcal isolates from cerebrospinal fluid or blood, 1979-1986. J. Infect. Dis. 158:956-964. [DOI] [PubMed] [Google Scholar]
- 27.Koeuth, T., J. Versalovic, and J. R. Lupski. 1995. Differential subsequence conservation of interspersed repetitive Streptococcus pneumoniae BOX elements in diverse bacteria. Genome Res. 5:408-418. [DOI] [PubMed] [Google Scholar]
- 28.Louie, M., L. Louie, G. Papia, J. Talbot, M. Lovgren, and A. E. Simor. 1999. Molecular analysis of the genetic variation among penicillin-susceptible and penicillin-resistant Streptococcus pneumoniae serotypes in Canada. J. Infect. Dis. 179:892-900. [DOI] [PubMed] [Google Scholar]
- 29.Luna, V. A., and M. C. Roberts. 1999. In-vitro activities of 11 antibiotics against 75 strains of Streptococcus pneumoniae with reduced susceptibilities to penicillin isolated from patients in Washington state. J. Antimicrob. Chemother. 44:578-580. [DOI] [PubMed] [Google Scholar]
- 30.Marians, K. J., and H. Hiasa. 1997. Mechanism of quinolone action. A drug-induced structural perturbation of the DNA precedes strand cleavage by topoisomerase IV. J. Biol. Chem. 272:9401-9409. [DOI] [PubMed] [Google Scholar]
- 31.McDougal, L. K., J. K. Rasheed, J. W. Biddle, and F. C. Tenover. 1995. Identification of multiple clones of extended-spectrum cephalosporin-resistant Streptococcus pneumoniae isolates in the United States. Antimicrob. Agents Chemother. 39:2282-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McEllistrem, M. C., M. Pass, J. A. Elliott, C. G. Whitney, and L. H. Harrison. 2000. Clonal groups of penicillin-nonsusceptible Streptococcus pneumoniae in Baltimore, Maryland: a population-based, molecular epidemiologic study. J. Clin. Microbiol. 38:4367-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGee, L., C. E. Goldsmith, and K. P. Klugman. 2002. Fluoroquinolone resistance among clinical isolates of Streptococcus pneumoniae belonging to international multiresistant clones. J. Antimicrob. Chemother. 49:173-176. [DOI] [PubMed] [Google Scholar]
- 34.McGee, L., L. McDougal, J. Zhou, B. G. Spratt, F. C. Tenover, R. George, R. Hakenbeck, W. Hryniewicz, J. C. Lefevre, A. Tomasz, and K. P. Klugman. 2001. Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the pneumococcal molecular epidemiology network. J. Clin. Microbiol. 39:2565-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGee, L., H. Wang, A. Wasas, R. Huebner, M. Chen, and K. P. Klugman. 2001. Prevalence of serotypes and molecular epidemiology of Streptococcus pneumoniae strains isolated from children in Beijing, China: identification of two novel multiply-resistant clones. Microb. Drug Resist. 7:55-63. [DOI] [PubMed] [Google Scholar]
- 36.Nichol, K. A., G. G. Zhanel, and D. J. Hoban. 2003. Molecular epidemiology of penicillin-resistant and ciprofloxacin-resistant Streptococcus pneumoniae in Canada. Antimicrob. Agents Chemother. 47:804-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan, X. S., J. Ambler, S. Mehtar, and L. M. Fisher. 1996. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 40:2321-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan, X. S., and L. M. Fisher. 1996. Cloning and characterization of the parC and parE genes of Streptococcus pneumoniae encoding DNA topoisomerase IV: role in fluoroquinolone resistance. J. Bacteriol. 178:4060-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richter, S. S., K. P. Heilmann, S. L. Coffman, H. K. Huynh, A. B. Brueggemann, M. A. Pfaller, and G. V. Doern. 2002. The molecular epidemiology of penicillin-resistant Streptococcus pneumoniae in the United States, 1994-2000. Clin. Infect. Dis. 34:330-339. [DOI] [PubMed] [Google Scholar]
- 40.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weigel, L. M., G. J. Anderson, R. R. Facklam, and F. C. Tenover. 2001. Genetic analyses of mutations contributing to fluoroquinolone resistance in clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:3517-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhanel, G. G., A. Walkty, K. Nichol, H. Smith, A. Noreddin, and D. J. Hoban. 2003. Molecular characterization of fluoroquinolone resistant Streptococcus pneumoniae clinical isolates obtained from across Canada. Diagn. Microbiol. Infect. Dis. 45:63-67. [DOI] [PubMed] [Google Scholar]
- 43.Zheng, X., C. Johnson, Y. Lu, R. Yanagihara, S. Hollingshead, M. Crain, W. Benjamin, Jr., and K. B. Waites. 2001. Clinical isolates of Streptococcus pneumoniae resistant to levofloxacin contain mutations in both gyrA and parC genes. Int. J. Antimicrob. Agents 18:373-378. [DOI] [PubMed] [Google Scholar]