Abstract
Asymptomatic genital shedding of herpes simplex virus type 2 (HSV-2) DNA was evidenced by real-time PCR in 25 (13.2%) of 188 cervicovaginal lavage samples and in only 13 (6.9%) paired cervicovaginal samples from 188 HSV-2-seropositive, nonpregnant childbearing-aged human immunodeficiency virus-seronegative women living in Gabon. These observations demonstrate that cervicovaginal washing is more suitable than endocervicovaginal swabbing for detecting and quantifying HSV-2 DNA by PCR in female genital secretions.
The ability to assess viral burden in genital secretions is essential in understanding the mechanisms of heterosexual, as well as mother-to-child, transmissions of herpes simplex virus (HSV) infection and in predicting the effect of antiviral therapy at the level of the genital compartment (4, 6). Furthermore, determination of HSV genital shedding has become a major biological marker to evaluate the role of genital herpes as a possible cofactor of human immunodeficiency virus (HIV) sexual transmission (11). HSV type 2 (HSV-2) genital shedding is also used as a biological endpoint in intervention trials used to assess the role of HSV-2 as a cofactor of HIV heterosexual transmission (15). Evaluation of genital viral burden by means of HSV DNA nucleic acid or signal amplification techniques in cervicovaginal secretions is not yet well standardized (8, 11, 12). A convenient collection procedure of genital fluids and further well-standardized and reproducible qualitative and quantitative assays are mandatory prerequisites for the evaluation of HSV genital shedding. In the present study, two sample collection procedures of female genital secretions were compared to estimate the genital shedding of HSV-2 in childbearing-aged, HIV-seronegative African women.
(Evelyne Avoune presented the results of this study as part of her requirements for an M.D., January 2002, at the Université des Sciences de la Santé, Libreville, Gabon.)
A total of 329 childbearing-aged women (mean age, 26.2 years; range, 14 to 44 years) at the Gynecology and Obstetrical Department of the Centre Hospitalier de Libreville and the Maternité Joséphine Bongo in Libreville, the capital city of Gabon, for family planning (n = 39) or common gynecological evaluation (n = 290 [including 134 women suffering from vaginal discharge]) were prospectively recruited on a volunteer basis to evaluate their HSV-2 genital shedding. All of the women were nonpregnant. The research protocol followed the ethical recommendations of the Ministry of Health of Gabon, and informed verbal consent was obtained from all participants. Type-specific serum antibodies to HSV-2 were assessed by a enzyme-linked immunosorbent assay developed from a radioimmunoassay with monoclonal antibodies to HSV-2 and validated against Western-blotted sera from the United Kingdom and Africa as described previously (7).
Genital secretions were collected successively by two different procedures. Sterile dry swabs were first used to collect genital secretions on the endocervix. The swabs were gently applied on the cervical os, and a slight pressure was applied by partly rotating the swabs, without any mucosal trauma. All of the swab samples were rapidly stored at −80°C until use. After genital swab sampling, whole vaginal secretions were collected by a standardized 60-s vaginal washing with 3 ml of phosphate-buffered saline (pH 7.2) as previously described (2). The vaginal washing consisted of three successive aspiration discharges against the vaginal walls and the cervix by using a flexible plastic pipette. The total mixture of vaginal secretions diluted in washing buffer was recovered from the posterior vaginal fornix by a final aspiration. The collection of vaginal secretions obtained by vaginal washing introduced a mean dilution of genital secretions of about 1:10 (2). After centrifugation of the endocervical lavage sample at 2,000 × g for 15 min, the cell-free supernatant and the cellular pellet were collected and stored separately at −80°C. Contaminating semen in vaginal secretions was investigated by detecting the Y chromosome by using PCR assay in DNA extracted from the cellular pellet of cervicovaginal secretions, as previously described (3). Traces of hemoglobin in vaginal secretions were detected by spectrophotometry of the acellular fraction of genital fluids, as previously described (14).
To detect and quantify HSV-2 DNA from the swab samples, the tips of the swabs were first treated for 20 min at 56°C in 400 μl of lysis buffer containing DNase- and RNase-free reagent obtained from the QiaAmp DNA minikit (Qiagen, Courtaboeuf, France). Then, the following steps of DNA extraction by using a classical fixation-elution procedure onto a silicated column (Qiagen) were carried out according to the manufacturer's instructions. Extracted DNA was further diluted in 200 μl of specimen diluent. To quantify HSV-2 DNA levels in the cervicovaginal washing samples, 1,000 μl of the acellular part of vaginal secretions were initially precipitated by centrifugation at 23,000 × g for 60 min at 4°C, and the total DNA was extracted from the resulting pellet by using the QiaAmp DNA minikit as described above. The quality of DNA and the absence of substantial PCR inhibitors were assessed by determining the positivity of β-globin PCR detection (13) in all extracted samples. Extracted DNA was further diluted in 200 μl of specimen diluent. HSV-2 DNA was detected and quantified in 5 μl of DNA extracted from swabs or from the paired acellular fractions of cervicovaginal secretions lavage samples by real-time PCR in the HSV DNA polymerase gene by using the LightCycler technology, as previously described (10). This previously published procedure has a threshold of sensitivity of 10 HSV DNA copies per reaction (10). The results were expressed as the number of HSV-2 DNA copies/milliliter for cervicovaginal washing samples and as the number of HSV-2 DNA copies/microgram of total DNA (quantitated by spectrophotometry at 260 nm) for endocervical swabbing samples. Strain differentiation for HSV-1 and HSV-2 types was performed by restriction fragment length polymorphism analysis, as described previously (15). Individuals with a quantity below this were deemed “nonshedders,” and their genital HSV-2 DNA load was considered negative. Correlations between viral loads were assessed by using the Spearman rank correlation coefficient. Fisher exact and Spearman rank order correlation tests were used for statistical analyses. Linear regression analysis on log10-transformed values was performed by using the software StatView SE 1 (Abacus Concepts, Inc., Berkeley, Calif.).
The prevalence of seropositivity for HSV-2 was determined to be 57.1% in the study population. The subgroup of 188 HSV-2-seropositive, nonpregnant women (mean age, 24.7 years; range, 18 to 48 years) was further selected for inclusion in the study. All women were clinically healthy and showed no evidence of sexual transmitted diseases, including recurrences of genital herpes. None showed semen and hemoglobin traces in their vaginal-secretion samples.
The detection threshold for real-time PCR quantitation of HSV-2 DNA shedding was estimated as 400 copies/ml for vaginal washing samples and as 400 copies/μg for endocervical swabbing samples. A woman was considered an HSV shedder when her vaginal secretions were found to be positive for HSV DNA whatever the collection procedures used to collect the genital secretions. A total of 25 (13.3%) of 188 HSV-2-seropositive women were found to be shedding HSV DNA. The genital HSV were determined to be HSV-2 by restriction fragment analysis.
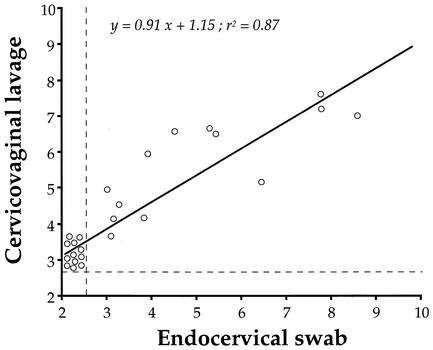
When cervicovaginal secretions were collected by endocervical swabbing, HSV-2 DNA was detected in only 13 (6.9%) cervical samples. When cervicovaginal secretions were collected by washing, HSV-2 DNA was detected in 25 (13.3%) cervicovaginal lavage samples. All cervicovaginal secretions collected by vaginal washing and found to be positive for HSV-2 DNA were also found to be positive by swabbing. Furthermore, the prevalence of detection of HSV-2 DNA was higher when the genital secretions were collected by vaginal washing than when they were collected by endocervical swabbing (P = 0.036). The levels of HSV-2 DNA in cervicovaginal lavage samples ranged from none to 8.1 × 108 copies/ml, and the mean level of HSV-2 DNA-positive vaginal lavage samples was (6.31 ± 4.03) × 107 copies/ml. The levels of HSV-2 DNA in the endocervical mucus ranged from none to 4.0 × 108 copies/μg, and the mean level of HSV-2 DNA-positive vaginal lavage samples was (2.11 ± 1.61) × 107 copies/μg of total DNA. Figure 1 shows the distribution of the HSV-2 DNA levels according to the sampling procedures. The levels of HSV-2 DNA in cervicovaginal lavage samples were highly correlated with those in paired endocervical mucus (Spearman r = 0.87; P < 0.0001).
FIG. 1.
Distribution of the genital HSV-2 DNA levels as assessed by real-time PCR, according to the collection procedures of the genital secretions, in 25 HSV-2-seropositive, nonpregnant, childbearing-aged African women. Total cervicovaginal secretions were collected by standardized vaginal washing with 3 ml of phosphate-buffered saline, and the levels of HSV-2 DNA are expressed as log10 DNA copies per milliliter of washing fluid. Endocervical samples were colleted by using dry swabs, and the levels of HSV-2 DNA in the swab samples are expressed as log10 HSV-2 DNA copies per microgram of total DNA. The vertical and horizontal dashed lines indicate the positivity thresholds of detection in cervicovaginal lavages samples (2.6 log10 copies/ml) and in whole DNA extracted from eluted endocervical swab samples (2.6 log10 copies/μg DNA). The linear regression curve is depicted.
In the present study, collection procedures of cervicovaginal secretions by cervicovaginal washing and endocervical swabbing were compared to detect and quantify HSV-2 DNA in HSV-2-seropositive women. In order to reduce possible methodological bias in data interpretation, we carefully selected childbearing-aged African women on the basis of clinical and laboratory inclusion criteria. All women in the study were clinically asymptomatic and were not being treated by antiviral drugs. Criteria for the selection of the patients included the lack of genital infection and particularly genital ulcer or inflammation, allowing for an evaluation of genital samples as close as possible to the natural history of genital herpes when the infection is asymptomatic and when HSV-2-shedding women may transmit the virus to exposed sexual male partners. We furthermore checked for the absence of semen traces by a sensitive Y PCR (3), excluding the risk of vaginal receptacle contamination by heterologous semen HSV-2 DNA coming from a possible HSV-2-shedding male sexual partner.
The cervicovaginal washing procedure was much more sensitive than the endocervicovaginal swabbing procedure for qualitatively detecting HSV-2 DNA in genital fluids. In the present series, nearly half of the cervicovaginal secretion lavage samples found to be positive for HSV-2 DNA were negative when endocervical swabbing samples were used to detect HSV-2. Furthermore, the measured levels of HSV-2 DNA were higher when genital secretions were collected by vaginal washing than when they were collected by swabbing. These findings clearly indicate that endocervical swabbing is not suitable for detecting and quantifying genital HSV-2 in women. Swabbing collection techniques may be useful for the determination of HSV-2 DNA detection in different anatomic sites of the genital tract (5). In contrast, cervicovaginal washing appears to be highly sensitive for detecting HSV-2 in female genital secretions, probably because asymptomatic genital excretion of HSV-2 is the result of mucosal replication of the virus in a mucosal territory largely exceeding the endocervix (5). Collection of cervicovaginal secretions by the vaginal washing technique may also be useful for immunological investigations, mainly by using the acellular part of the genital secretions, in which humoral mucosal immunity may be easily studied (2). However, the vaginal washing procedure introduces a dilution factor that is seemingly difficult to estimate (2). When the simultaneous determinations of HIV-1- and HSV-2 genital shedding in HIV-1- and HSV-2-coinfected women (11) are combined, the collection procedures of genital secretions by vaginal washing may also be useful for quantitative analysis. Indeed, cervicovaginal washing and endocervical swabbing are both suitable for detecting and quantifying HIV-1 RNA in genital secretions (1, 9). Taken together, our findings suggest that cervicovaginal washing may be used to collect genital secretions in women included in intervention trials, in which biological endpoints such as genital levels of HIV-1 or HSV-2 are chosen (16).
One major feature of the present study is the demonstration of asymptomatic genital excretion of HSV-2 in approximately one of eight HSV-2-seropositive childbearing-aged women. Such a high prevalence of genital shedding of HSV-2 raises concerns not only about the risk of sexual transmission of HSV-2 to an exposed sexual male partner but also about perinatal HSV infection in the population of African women of reproductive age because of the risk of transmission of the virus to their babies during pregnancy, with potentially devastating consequences to the fetus.
Acknowledgments
We thank the midwives of the Service de Gynécologie et Obstétrique, Centre Hospitalier de Libreville, and of the Maternité Joséphine Bongo, Libreville, for assistance with the volunteers, and we thank the technical staff of the Département de Microbiologie, Université des Sciences de la Santé, Libreville, Gabon, for help in sample processing. We also thank David Brown of the Public Health Laboratory Service, Colindale, London, United Kingdom, for having kindly carried out the HSV-2 serologies.
This work was supported by the Agence Nationale de Recherches sur le SIDA (ANRS 12-12), Paris, France.
REFERENCES
- 1.Baron, P., J. Bremer, S. S. Wasserman, N. Mareck, B. Driscoll, B. Polsky, A. Kovacs, and P. S. Reichelfelder. 2000. Detection and quantification of human immunodeficiency virus type 1 in the female genital tract. J. Clin. Microbiol. 38:3822-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bélec, L., D. Meillet, M. Levy, A. Georges, C. Tevi-Benissan, and J. Pillot. 1995. Dilution assessment of cervicovaginal secretions obtained by vaginal washing for immunological assays. Clin. Diagn. Lab. Immunol. 2:57-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chomont, N., G. Gresenguet, M. Levy, H. Hocini, P. Becquart, M. Matta, J. Tranchot-Diallo, L. Andreoletti, M.-P. Carreno, M. D. Kazatchkine, and L. Belec. 2001. Polymerase chain reaction for Y chromosome to detect semen in cervicovaginal fluids: a prerequisite to assess HIV-specific vaginal immunity and HIV genital shedding. AIDS 15:801-802. [DOI] [PubMed] [Google Scholar]
- 4.Corey, L., and H. H. Handsfield. 2000. Genital herpes and public health: addressing a global problem. JAMA 283:791-794. [DOI] [PubMed] [Google Scholar]
- 5.Corey, L., and A. Wald. 1999. Genital herpes, p. 285-312. In K. K. Holmes, P. F. Sparling, P.-A. Mardh, S. M. Lemon, W. E. Stamm, P. Piot, and J. N. Wasserheit (ed.), Sexually transmitted diseases, 3rd ed. McGraw-Hill Book Co., New York, N.Y.
- 6.Fleming, D. T., and J. N. Wasserheit. 1999. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex. Transm. Infect. 75:3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gopal, R., T. Gibbs, M. J. Slomka, J. Whitworth, L. M. Carpenter, A. Vyse, and D. W. G. Brown. 2000. A monoclonal blocking EIA for herpes simplex virus type 2 (HSV-2) antibody: validation for seroepidemiological studies in Africa. J. Virol. Methods 87:71-80. [DOI] [PubMed] [Google Scholar]
- 8.Hobson, A., A. Wald, N. Wright, and L. Corey. 1997. Evaluation of a quantitative competitive PCR assay for measuring herpes simplex virus DNA content in genital tract secretions. J. Clin. Microbiol. 35:548-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.John, G. C., H. Sheppard, D. Mbori-Ngacha, R. Nduati, D. Maron, M. Reiner, and J. Kreiss. 2001. Comparison of techniques for HIV-1 detection and quantitation in cervicovaginal secretions. J. Acquir. Immune Defic. Syndr. 26:170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessler, H. H., G. Muhlbauer, B. Rinner, E. Stelzl, A. Berger, H. W. Dorr, B. Santner, E. Marth, and H. Rabenau. 2000. Detection of herpes simplex virus DNA by real-time PCR. J. Clin. Microbiol. 38:2638-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mbopi-Kéou, F.-X., G. Gresenguet, P. Mayaud, H. A. Weiss, R. Gopal, M. Matta, J.-L. Paul, D. W. G. Brown, R. J. Hayes, D. C. W. Mabey, and L. Bélec. 2000. Interactions between herpes simplex virus type 2 and HIV infection in African women: opportunities for intervention. J. Infect. Dis. 182:1090-1096. [DOI] [PubMed] [Google Scholar]
- 12.Ryncarz, A. J., J. Goddard, A. Wald, M. L. Huang, B. Roizman, and L. Corey. 1999. Development of a high-throughput quantitative assay for detecting herpes simplex virus DNA in clinical samples. J. Clin. Microbiol. 37:1941-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saiki, R. K., S. Scharf, F. Faloona, K. B. Mullis, G. T. Horn, H. A. Erlich, and N. Arnheim. 1985. Enzymatic amplification of β-globin genomic sequences and restriction site analysis for the diagnosis of sickle-cell anemia. Science 230:1350-1354. [DOI] [PubMed] [Google Scholar]
- 14.Si-Mohamed, A., M. D. Kazatchkine, I. Heard, C. Goujon, T. Prazuck, G. Aymard, G. Cessot, Y. H. Kuo, M. C. Bernard, B. Diquet, J.-E. Malkin, L. Gutmann, and L. Bélec. 2000. Selection of drug-resistant variants in the female genital tract of human immunodeficiency virus type 1-infected women receiving antiretroviral therapy. J. Infect. Dis. 182:112-122. [DOI] [PubMed] [Google Scholar]
- 15.Vogel, J.-U., B. Weber, and H. W. Doerr. 1994. Typing and strain differentiation of clinical herpes simplex virus type 1 and 2 isolates by polymerase chain reaction and subsequent fragment length polymorphism analysis. Zentbl. Bakteriol. 281:502-512. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. 2001. Herpes simplex virus type 2: programmatic and research priorities in developing countries. World Health Organization, Geneva, Switzerland.