Abstract
The city of Mashhad is the capital of Khorasan, the northeastern province of Iran, which has been recognized as an area where human T-lymphotropic virus type 1 (HTLV-1) infection is endemic. All serum samples from blood donors are routinely screened for HTLV-1 by using enzyme-linked immunosorbent assay (ELISA). In the present study, 28,926 donors (81.86% male and 18.14% female) with a mean age of 32 years (range, 18 to 65 years) were screened in a 6 months period (July to December 1999). Of these donors in the primary screening, 228 (0.78%) tested positive by ELISA. The positive samples were confirmed by Western blot (WB) analysis. The WB results indicated that, of 228 positive ELISA specimens, 91.2% (208 specimens) were HTLV-1, 4.82% (11 specimens) were HTLV, 3.5% (8 specimens) were indeterminate, and 0.44% (1 specimen) was not confirmed. HTLV refers to samples in which the complete viral antigen banding patterns on WB strips were not present. In order to further evaluate the detection methodologies used, the HTLV-1-seropositive samples, the indeterminant samples, and/or HTLV samples were examined and confirmed by PCR. The HTLV samples were determined to be HTLV-1, the remaining samples were indeterminant, and the negative sample could not be confirmed for HTLV-1 by PCR. The prevalence of HTLV-1 infection in our study was 0.77% among blood bank donors, which reconfirms the city of Mashhad as an area where the virus is endemic compared to other regions in the world. The incidence was correlated with increasing age, and it was higher in females than in males.
Human T-lymphotropic virus type 1 (HTLV-1) was first identified in humans in 1980 (11) and 1982 (5). It is the etiologic agent of two distinct human diseases, adult T-cell leukemia or lymphoma (1) and a chronic, progressive demyelinating disorder known as HTLV-1-associated myelopathy/tropical spastic paraparesis (2). HTLV-1 is distributed worldwide, but it is endemic only in certain parts of the world such as southwestern Japan, the Caribbean basin, Africa, part of South America, southern Italy, Taiwan, and the United States (6). Routes of infection include transfusion, sharing of needles or syringes with infected individuals, sexual contact, and breast-feeding; transplacental transmission is also suspected (7, 9). Cellular blood products are the main source of transfusion-associated HTLV transmission, whereas fresh frozen plasma, cryoprecipitate, or coagulation factor concentrates appear not to cause infection (4, 10).
To avoid HTLV-1 transmission by transfusion, screening of blood donation for HTLV-1/2 infection has been mandatory in several countries: in 1986 in Japan; in 1989 in the United States; in 1990 in Canada, in 1989 in French Caribbean and in 1991 in the entire French territory; in 1993 in The Netherlands; in 1994 in Sweden, Denmark, and Iran; and more recently in Portugal and Greece. Such screening is still under debate in other countries. The present study was carried out to validate the efficacy of serological screening of blood for HTLV-1 contamination by using hybridization and PCR methods.
MATERIALS AND METHODS
Subjects.
A total of 28,926 blood donors were tested for HTLV-1/2 during July to December 1999. The donors were 83% male (23,680) and 17% female (5,246), with a mean age of 32 years (ages ranged between 18 and 65 years). All donors fulfilled the criteria for blood donation, which included a clinical examination and an interview to record the history of previous infectious diseases, surgery, blood transfusion, heart diseases, anemia, and information on foreign travel. Redonation rate during the 6-month study period was 1.5% (439 individuals). All HTLV-1-positive subjects were informed of the test result and were prohibited from redonation.
Serological assays.
Serum samples were screened for HTLV-1/2 by using enzyme-linked immunosorbent assay (ELISA; Vironostika HTLV I/II, Organon Teknica). All repeatedly positive samples were confirmed by Western blotting (WB; HTLV blot 2.4 kit; Gene Labs Diagnostics, Ltd.). Our index of HTLV-1 seropositivity was reactivity to GAG (P19 with or without P24) and two ENV (GD21 and rgp46-I) (Table 1).
TABLE 1.
Interpretation of WB pattern
| Conclusion | Patterna
|
No. of subjectsb
|
||||||
|---|---|---|---|---|---|---|---|---|
| GD21 | P19 | P24 | rgp-46I | rgp-46II | WB | PCR (tax) | PCR (LTR) | |
| HTLV-1 | + | + | +/− | + | − | 208 | 50+ | 50+ |
| HTLV-2 | + | +/− | + | − | + | 0 | 0 | 0 |
| HTLVbc | + | + | + | − | − | 11 | 11 (HTLV-1+) | 11 (HTLV-1+) |
| Indeterminant | + | − | + | − | − | 3 | Negative | Negative |
| Indeterminant | + | + | − | − | − | 5 | Negative | Negative |
rgp-46I/II, type-specific recombinant Env proteins; P19 and P24, Gag proteins; GD21, Env protein.
For PCR (tax) and PCR (LTR), of 208 positive samples, 50 were randomly tested for tax and LTR regions of the HTLV-1 genome by PCR amplification, which confirmed the WB results.
All HTLV WB-positive specimens were determined to be HTLV-1 positive by PCR amplification.
Synthesis and purification of oligonucleotides.
Oligonucleotides were synthesized on a Biosearch 8600 automated DNA synthesizer and purified by high-performance liquid chromatography and/or by polyacrylamide gel electrophoresis.
DNA purification and PCR.
The peripheral blood mononuclear cell DNA was extracted by a nonenzymatic method and then analyzed for HTLV-1 sequence. PCR amplification was performed with two primer sets, tax (forward, GGA TAC CCA GTC TAC GTG TTT G; reverse, CGG AAC ATT GGT GAG GAA GGC) and long terminal repeat (LTR; forward, CCA GAC TAA GGC TCT GAC GTC TC; reverse, CCT GAG CTC TAA ACT TAC CTA GAC G; GenBank accession no. LO3562), resulting in 522- and 394-bp products, respectively. The PCR mixture contained a 1-μg sample DNA, 10 pmol of each primer, 200 μM concentrations of each deoxynucleoside triphosphate, 50 mM KCl, 10 mM Tris (pH 8.3), 1.5 mM MgCl2, and 1 U of Thermus aquaticus (Taq) enzyme (CinnaGen, Inc., Tehran, Iran). The reaction mixture was incubated for 5 min at 94°C and then subjected to 30 cycles consisting of 1 min at 94°C, 1 min at 53°C, and 30 s at 68°C. The final annealing step was performed for 5 min at 68°C in a DNA thermal cycler (Perkin-Elmer 480). For positive control, MT-2 cell line DNA was used. The reaction mixtures were stored at 4°C until they were analyzed by agarose gel (1.5%) electrophoresis. To confirm the PCR fidelity, two blood samples were amplified and sequenced by using an automated sequencer (Applied Biosystems 377).
Statistical methods.
Descriptive results were presented as crowd frequencies, ratios, and 95% confidence intervals. The correlation between different factors was evaluated by chi-square test.
RESULTS
A total of 28,926 blood samples were analyzed for HTLV-1 contamination. In the primary screening, 228 (0.77%) samples tested positive by ELISA. All samples were assayed in duplicate, and the positive samples were confirmed by WB analysis. The WB results indicated that, of these 228 positive ELISA specimens, 91.2% (208 specimens) were HTLV-1, 4.82% (11 specimens) were HTLV, 3.5% (8 specimens) were indeterminate, and 0.44% (1 specimen) was not confirmed. HTLV refers to samples in which the complete viral antigen banding patterns on WB strips were not present.
The prevalence of the infection was 0.77% (after correction for the redonation rate of 1.5% [439 of 28,926]) among blood bank donors. In order to address the issues of false negatives, 150 samples (previously tested negative by ELISA) were randomly selected and analyzed by using WB. These analyses also tested negative for HTLV contamination of selected samples. Similarly, to validate the methodology used and to determine the possibility of false positives, 20 samples of HTLV-1 ELISA and WB positives were randomly selected and examined by PCR. All of the samples were confirmed by PCR.
In order to confirm and determine the HTLV strains, the HTLV-1-seropositive samples, indeterminant samples, and HTLV samples were examined by PCR. The 11 HTLV-positive samples were determined to be HTLV-1. The PCR products corresponding to the tax and LTR regions (Fig. 1) of the HTLV-1 genome were sequenced and resulted in a complete homology with the cosmopolitan strain of HTLV-1. The eight indeterminants and the one negative sample tested negative for HTLV-1 by PCR.
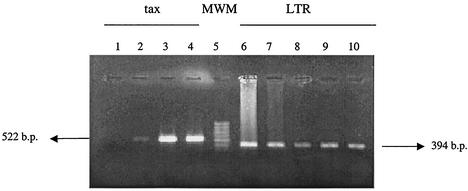
FIG. 1.
Amplification of tax and LTR regions in blood donors. All 522- and 394-bp fragments were amplified from the tax and LTR regions of HTLV-1 genome, respectively. PCR products were electrophoresed in a 1.5% agarose gel, stained in ethidium bromide and photographed. Lane 1, negative control; lanes 2 and 3, blood donors; lane 4, MT-2 cell line DNA; lane 5, molecular weight marker; lanes 6 to 10, blood donors.
A significant correlation exists between increasing age and incidence of infection (P value of correlation, 0.0001 for men and 0.0002 for women). It is also concluded that seroprevalence rate in females is higher than males (Fig. 2).
FIG. 2.
HTLV-1 seroprevalence by age group (in years) and gender among Mashhad blood donors. Lightly shaded bars, male; darkly shaded bars, female.
DISCUSSION
There is no defined treatment for patients infected with HTLV-1, but the accurate knowledge of seroprevalence rates in different population groups may be helpful in establishing prophylactic measures to reduce rates of viral transmission from infected individuals. The overall 0.77% HTLV-1 seroprevalence rate found in Mashhad blood donors is greater than that seen in similar studies in the United States (0.004%), France (0.004%), and Brazil (0.42%). Higher seroprevalence in blood donors has been found in Jamaica (2.1%) (12). Such comparisons must be made cautiously because screening tests, specificity in marker levels, and medical selection of blood donors can vary from one study to another, but we have attempted to accomplish standard screening tests. The present study confirms, by using both serological and PCR detection methodologies, that Mashhad is a region where HTLV-1 infection is endemic. In a previous study, seropositivity was reported to be 3% among the patients reporting to the clinic with HTLV-1-associated disease symptoms in Iran (13). ELISA kits have high sensitivity and low specificity; thus, it may not be a reliable screening tool. Therefore, positive ELISA results should be confirmed by WB or PCR. The WB seropositivity parameters used (HTLV blot 2.4 kit) a recombinant spiked WB assay, which is more stringent than those previously used with whole-virus lysate WB. The epidemiology of HTLV-1/2 has been largely defined through the use of antibody testing. PCR has also been a useful tool for facilitating epidemiological studies for distinguishing virus type and for quantifying viral presence (3). Age and sex relationships have been identified as contributing factors to HTLV-1 seroprevalence in all areas where this virus is highly endemic (8). Female predominance could be related to a preferential sexual transmission from husband to wife. Our study also revealed a strong age-dependent rise in seroprevalence rate. This pattern is also well documented in previous studies, which could be explained by a cohort effect (14) and by cumulative effect of infections occurring over the lifetime of individuals, such as by heterosexual transmission. The age-dependent rise in HTLV-1 prevalence could also be explained by a birth cohort effect (12).
Acknowledgments
We thank Antoine Gessain, Fata Kashanchi, and Absar Alum for useful insights. We are also appreciative of the great efforts made on behalf of this project by the personnel of the blood bank center in Mashhad.
REFERENCES
- 1.Blattner, W. A., K. Takatsuki, and R. C. Gallo. 1983. Human T-cell leukemia-lymphoma virus and adult T-cell leukemia. JAMA 250:1074-1080. [PubMed] [Google Scholar]
- 2.Gessain, A., E. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-1 in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 3.Heneine, W., R. F. Khabbaz, R. B. Lal, and J. E. Kaplan. 1992. Sensitive and specific polymerase chain reaction assay for diagnosis of human T-cell lymphotropic virus type I (HTLV-I) and HTLV-II infection in HTLV-I/II-seropositive individuals. J. Clin. Microbiol. 30:1605-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hjelle, B., R. Mills, G. Mertz, and S. Swenson. 1990. Transmission of HTLV-1 via blood transfusion. Vox Sang. 59:119-122. [DOI] [PubMed] [Google Scholar]
- 5.Kalyanaraman, V. S., M. G. Sarngadharan, B. Poiesz, F. W. Ruscetti, and R. C. Gallo. 1981. Immunological properties of a type C retrovirus isolated from cultured human T-lymphoma cells and comparison to other mammalian retroviruses. J. Virol. 38:906-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meytes, D., B. Schochat, et al. 1990. Serological and molecular survey for HTLV-1 infection in a high-risk Middle Eastern group. Lancet 336:1533-1535. [DOI] [PubMed] [Google Scholar]
- 7.Monplaisir, N., V. C. Neisson, M. Bouillot, et al. 1993. HTLV-1 maternal transmission in Martinique using serology and polymerase chain reaction. AIDS Res. Hum. Retrovir. 9:869-874. [DOI] [PubMed] [Google Scholar]
- 8.Mueller, N., N. Tachibana, S. O. Stuver, et al. 1990. Epidemiological perspectives of HTLV-1, p. 281-293. In W. A. Blattner (ed.), Human retrovirology: HTLV-1. Raven Press, Inc., New York, N.Y.
- 9.Murphy, E. L., J. P. Figueroa, W. N. Gibbs, A. Brathwaite, M. Holding-Cobham, D. Waters, B. Cranston, B. Hanchard, and W. A. Blattner. 1989. Sexual transmission of human T-lymphotropic virus type I (HTLV-1). Ann. Intern. Med. 111:555-560. [DOI] [PubMed] [Google Scholar]
- 10.Okochi, K., H. Sato, and Y. Hinuma. 1984. A retrospective study on transmission of adult T-cell leukemia virus by blood transfusion: seroconversion in recipients. Vox Sang. 46:245-253. [DOI] [PubMed] [Google Scholar]
- 11.Poiesz, B. J., F. W. Ruscetti, A. F. Gadzar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type c retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rouet, F., C. Foucher, M. Rabier, I. Gawronski, D. Taverne, B. Chancerel, O. Casman, and M. Strobel. 1999. Human T-lymphotropic virus type I among blood donors from Guadeloupe. Transfusion 39:1-6. [DOI] [PubMed] [Google Scholar]
- 13.Safai, B., R. Farid, J. L. Hung, E. Boeri, J. Raafat, et al. 1996. Prevalence of HTLV type I infection in Iran: a serological and genetic study. AIDS Res. Hum. Retrovir. 12:1185-1190. [DOI] [PubMed] [Google Scholar]
- 14.Takezaki, T., K. Tajima, H. Komoda, and J. Imal. 1995. Incidence of human T lymphotropic virus type I seroconversion after age 40 among Japanese residents in an area where the virus is endemic. J. Infect. Dis. 171:559-565. [DOI] [PubMed] [Google Scholar]