Abstract
Human papillomavirus (HPV) DNA loads of six oncogenic HPV types were measured by real-time PCR in cervical scrapes of human immunodeficiency virus (HIV)-infected and uninfected women. In both groups, HPV loads increased with the grade of cervical disease. HIV infection did not affect HPV loads in low-grade lesions but was associated with significantly higher HPV loads in severe dysplasia; highest loads were found in advanced HIV disease. Our data reflect the aggressive course of HPV infection in HIV-positive women.
High-risk human papillomaviruses (HPV) induce cervical squamous intraepithelial lesions (SIL) classified as low grade (LSIL) or high grade (HSIL) in severity, which may progress to cervical cancer (21, 32, 47). Human immunodeficiency virus (HIV) infection is associated with a higher prevalence of HPV, a higher incidence of SIL, and an increased risk for cervical cancer (1, 7, 9, 13, 15, 18, 19, 28-30, 33, 34, 38, 39). Several studies of HIV-negative patients have indicated that higher HPV DNA loads in cervical scrapes are predictive of the severity of the underlying cervical lesion (3, 11, 16, 41, 42). HPV16 viral loads were shown to be positively correlated with the risk for developing cervical carcinoma in situ (23, 46). This could not be confirmed by Lorincz et al. when measuring cumulative loads of high-risk HPV types by Hybrid Capture without normalization on input cells (26).
We designed type-specific real-time PCR protocols for the quantification of the commonest high-risk HPV types (HPV16, -18, -31, -33, -45, and -56), which prevail in more than 80% of cervical cancers worldwide (5, 44). To evaluate the influence of HIV infection on HPV DNA load, we comparatively analyzed 305 cervical scrapes of HIV-positive and HIV-negative women with defined cervical disease stages (Table 1). All patients had a complete gynecologic examination. Cervical scrapes for cytology and HPV load determination were taken by one investigator (A.M.F.). Colposcopy was performed on the patients' first visit and at following visits if the first cytology had been above normal. For HIV-positive patients, the number of CD4 cells per microliter, the HIV type 1 RNA load in plasma (Cobas Amplicor HIV-1 Monitor 1.5; Roche, Mannheim, Germany), and Centers for Disease Control and Prevention stage (8) were determined. DNA isolation from cervical scrapes (QIAamp-DNA Mini-Kit; Qiagen, Hilden, Germany), β-globin gene PCR (2), HPV screening-PCR (45), and HPV typing (22) were performed as previously described. Sample characteristics and HPV DNA prevalence data are given in Table 1. Quantification of HPV DNA was performed with the LightCycler (Roche) system as described before (45), with addition of 5% dimethyl sulfoxide to the PCR buffer. Sequences of type-specific primers and probes as well as PCR parameters are given in Table 2. HPV DNA copy numbers were determined in duplicate by using standard curves, generated in the same PCR run with HPV-plasmid dilutions in a human placental DNA solution (40 ng/μl) (4, 6, 12, 25, 31, 37). Sensitivity was 5 to 10 copies of HPV DNA. Quantification was linear from 10 to 109 copies for all HPV types. Type specificity could be shown by testing 500,000 copies of heterologous HPV types (HPV6, -11, -16, -18, -31, -33, -45, and -56) instead of the target type, which never yielded signals above background. Reliability of quantification in the presence of other HPV types was tested by spiking six identical samples containing 1,000 copies of the target HPV type with 100,000 copies of frequent genital HPV types (HPV6, -11, -16, -18, -31, -33, -45, and -56), respectively. The test was designated reliable when spiked samples yielded copy numbers within the threefold standard deviation of six unspiked samples. To correct for PCR efficiency and DNA integrity and to determine the number of input cell equivalents, the single-copy-number gene β-globin was quantified by using the LightCycler Control Kit DNA (Roche). HPV DNA load was defined as the number of HPV DNA copies/β-globin gene copy. Statistical analyses (17) were performed with SPSS 10.0.7 (SPSS Inc., Chicago, Ill.). Odds ratios (OR), 95% confidence intervals (CI), Fisher's exact tests, and Mann-Whitney U tests were based only on one sample per patient (the first HPV-positive sample collected).
TABLE 1.
Patient data and HPV DNA prevalence in cervical scrapes
| HIV status | No. of patients analyzed | No. of samples analyzedb | Patients' mean age (yr) at 1st visit (range) | Cytology resultc (no. of positive samples/no. of samples analyzed [%])e
|
No. of scrapes with multiple infectionsd/no. of HPV+ samples (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal | ASCUS | LSIL | HSIL | Cervical cancer | All | |||||
| HIV+ | 152 | 353 | 32.5 (17.0-64.2; SD = 6.7) | 122/212 (57.5) | 11/18 (61.1) | 33/37 (89.2) | 83/85 (97.6) | 1/1 (100) | 250/353 (70.8) | 145/250 (58.0) |
| 53/122 (43.4) | 5/11 (45.5) | 25/33 (75.8) | 68/83 (81.9) | 1/1 (100) | 152/250 (60.8) | |||||
| HIV−a | 285 | 349 | 33.2 (15.8-72.9; SD = 9.7) | 89/196 (45.4) | 22/32 (68.8) | 27/33 (81.8) | 82/85 (96.5) | 3/3 (100) | 223/349 (63.9) | 99/223 (44.4) |
| 45/89 (50.6) | 13/22 (59.1) | 24/27 (88.9) | 68/82 (82.9) | 3/3 (100) | 153/223 (68.6) | |||||
HIV-negative women were referred to the university hospital with suspicion of dysplasia. An HIV infection was excluded by Axsym HIV1/2 MEIA (Abbott, Wiesbaden, Germany).
For patients in whom more than one sample was collected, the time interval between sample collections was 6 to 12 months.
Cytology was classified both by the Bethesda system (shown above) and by the Papanicolaou (Pap) classification system (24, 27, 40). The majority of PapIIID/HSIL and all PapIV/HSIL and cervical cancer diagnoses were confirmed by colposcopy-directed histology.
Infections with multiple HPV types are defined as infections with ≥2 HPV types.
The second and fourth rows give the number of samples positive for HPV16, -18, -31, -33, -45, or -56/number of HPV+ samples as indicated in the first and third rows.
TABLE 2.
Primers, hybridization probes, and PCR conditions for the quantification of DNA of HPV16, -18, -31, -33, -45, and -56 by real-time PCR
| HPV type | Tann.a (°C) | [Mg2+] (mM) | Sequencesb,c (5′→3′) |
|---|---|---|---|
| 16 | 56 | 4.25 | fw: GCACAGGGCCACAATAAT |
| bw: GACCAAAATTCCAGTCCTC | |||
| So1: TCAACTGTGCAAAATAACCTTAACTGC | |||
| So2: ACGTTATGACATACATACATTCTATGA ATTCCACT | |||
| 18 | 55 | 3.5 | fw: GCACAGGGTCATAACAATGG |
| bw: CAAAGTTCCAATCCTCTAA | |||
| So1: TTGGTAGCATCATATTGCCCAGGTACA | |||
| So2: AGACTGTGTAGAAGCACATATTGTT AAATTGG | |||
| 31 | 55 | 4.5 | fw: GCTCAGGGACACAATAATGG |
| bw: CAAAATTCCAATCTTCCAAA | |||
| So1: AATTACTACTTTTAAATGTAGTATCA CTGT | |||
| So2: TGCAATTGCAGCACAAACAGACAT ATTG | |||
| 33 | 54 | 3.0 | fw: GCACAAGGTCATAATAATGG |
| bw: CAAATTGCCAATCTTCTAA | |||
| So1: ATATGTACTGTCACTAGTTACTTGTGT GCAT | |||
| So2: AGTCATATTAGTACTGCGAGTGGTATCT | |||
| 45 | 54 | 4.5 | fw: GCCCAGGGCCATAACAATGG |
| bw: CAAAATTCCAATTTTCTAA | |||
| So1: AACTTAGTAGGGTCATATGTACTTGGC | |||
| So2: CAGGATTTTGTGTAGAGGCACATAATG | |||
| 56 | 54 | 4.0 | fw: GCCCAAGGCCATAATAATGG |
| bw: CAATATTCCAGTCCTCCAG | |||
| So1: GCATCATATTTACTTAACTGTTCT GTAGC | |||
| So2: GTACTAATAGTCATGTTAGTACT TCTAGT AGTATC |
Tann., annealing temperature.
fw, forward primer; bw, backward primer; So1, 5′ hybridization probe (3′ end labeled with fluorescein); So2, 3′ hybridization probe (5′ end labeled with LightCycler-Red-640 and 3′ end phosphorylated).
Primers used were at 0.5 μM; hybridization probes were at 0.15 μM.
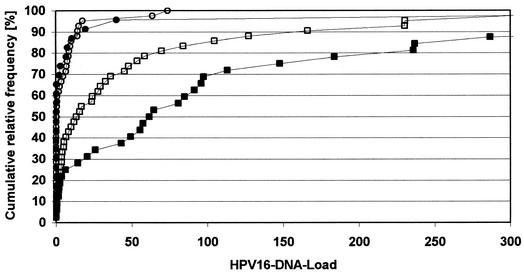
We observed an increase of median HPV16 load with the severity of cervical disease in HIV-negative and HIV-positive patients (normal cytology for HIV-negative patients/HIV-pos-itive patients, <0.1/<0.1; LSIL, 0.6/1.3; and HSIL, 14.6/63.2). For each cytological grade, strong load variations were observed. We, like others (41, 46), were therefore not able to define a clear cutoff predictive of HSIL. However, HPV16 loads that were > 1 were significantly associated with HSIL for HIV-negative (P < 0.001, OR, 8.3; CI, 2.8 to 24.5; and n = 76) and HIV-positive (P < 0.001, OR, 41.2; CI, 2.1 to 778.7; and n = 31) patients. In four scrapes from patients with cervical cancers, HPV DNA loads were above 1. The distribution of HPV16 loads in non-HSIL and HSIL scrapes is shown in Fig. 1 (non-HSIL includes normal cytology, atypical squamous cells of undetermined significance [ASCUS], and LSIL). Graphs for non-HSIL scrapes of HIV-negative and HIV-positive patients are almost identical. For HPV18, -31, -33, -45, and -56 as well, no remarkable load differences could be seen in non-HSIL scrapes of HIV-negative and HIV-positive patients. In contrast, HPV16 loads were significantly higher in HSIL scrapes from HIV-positive patients than in those from HIV-negative patients (P = 0.041; HIV+ n = 14; and HIV− n = 37) (Fig. 1). The same tendency was seen for HPV18, -31, -45, and -56 but not for HPV33 (Table 3).
FIG. 1.
Cumulative relative frequency of HPV16 DNA loads in cervical scrapes of HIV-negative and HIV-positive patients. Filled circles, HIV positive, non-HSIL (normal cytology, n = 12; ASCUS, n = 1; and LSIL, n = 10); empty circles, HIV negative, non-HSIL (normal, n = 24; ASCUS, n = 8; LSIL, n = 10); filled squares, HIV positive, HSIL (n = 27); and empty squares, HIV negative, HSIL (n = 40). HPV16 DNA loads above 300, which were only found in HSIL patients, are not shown (HIV+, n = 5; and HIV−, n = 2).
TABLE 3.
DNA loadsa of HPV16, -18, -31, -33, -45, and -56 in cervical scrapes of HIV-negative and -positive patients with HSIL
| HIV status | Type of statistic | DNA load for:
|
|||||
|---|---|---|---|---|---|---|---|
| HPV16 | HPV18 | HPV31 | HPV33 | HPV45 | HPV56 | ||
| HIV− | Median | 14.6 (42)b | <0.1 (5) | 1.6 (15) | 0.8 (6) | <0.1 (4) | 30.2 (6) |
| 80th per.c | 75.5 | 12.2 | 629.3 | 27.6 | 0.4 | 68.6 | |
| HIV+ | Median | 63.2 (32) | 15.4 (18) | 86.3 (11) | 1.1 (20) | 0.5 (15) | 266.1 (24) |
| 80th per. | 236.4 | 261.0 | 1,317.6 | 26.5 | 4,717.8 | 785.5 | |
HPV loads are defined as numbers of HPV DNA copies/β-globin gene copy.
Numbers of analyzed samples are given in parentheses.
80th per., 80th percentile HPV DNA load.
In patients infected with more than one of the investigated six high-risk types, viral loads of individual types varied by up to 3 orders of magnitude. Interestingly, median HPV16 loads found in HSIL lesions infected with more than one HPV type were twofold (HIV positive: 72.8 versus 35.0; n = 32) and fourfold (HIV negative: 23.7 versus 6.7; n = 42) higher than those in HSIL lesions infected with HPV16 alone.
Stratification by CD4 cell count, AIDS status, and HIV RNA loads (Table 4) revealed that severe immunosuppression or high HIV RNA levels were generally associated with increased HPV loads in HSIL patients. These results extend preliminary data by Heard et al. (20), who have found large HPV DNA amounts more frequently in severely immunosuppressed HIV-positive patients when comparing results from differently sensitive Southern blot and PCR analyses.
TABLE 4.
Median and 80th percentile HPV DNA loads of HPV16, -18, -31, -33, -45, and -56 in HIV-positive patients with HSIL differentiated by CD4 cell count, AIDS status, and HIV RNA load
| Patient category | DNA load for:
|
|||||
|---|---|---|---|---|---|---|
| HPV16 | HPV18 | HPV31 | HPV33 | HPV45 | HPV56 | |
| CD4 count ≥ 200/μl | 57.5a/215.1b (21)c | 1.1/12.5 (8) | 5.9/200.0 (5) | 0.6/22.2 (13) | 1.9/10,830 (8) | 227.0/785.5 (14) |
| CD4 count < 200/μl | 80.9/435.8 (11) | 157.6/3,153 (10)d | 554.6/1,746.2 (6) | 3.5/206.1 (6) | <0.1/8.0 (7) | 276.8/815.5 (10) |
| AIDS neg. (CDC A and B) | 17.5/183.8 (14) | 1.8/15.6 (7) | 119.5/239.0 (2) | 0.3/20.8 (14) | 1.7/3.3 (2) | 232.9/1,241.4 (8) |
| AIDS pos. (CDC C)e | 72.8/303.3 (18) | 132.5/2,435.7 (11) | 86.3/1,526.8 (9) | 7.0/223.6 (5) | 0.5/6,424.4 (13) | 270.9/845.8 (16) |
| HIV RNA count < 104/ml | 59.6/236.4 (22) | 5.8/277.7 (15) | 65.2/1,599.9 (8) | <0.1/6.9 (15) | 0.5/7,486.7 (11) | 272.1/584.4 (19) |
| HIV RNA count ≥ 104/ml | 85.0/2,214.5 (7)d | 132.5/182.8 (3) | 239.0/1,003.7 (3) | 153.5/241.2 (3) | 1.4/15.8 (4) | 450.8/1,764.1 (4) |
Median HPV DNA load.
Given here is 80th percentile HPV DNA load.
Numbers of analyzed samples are given in parentheses.
P < 0.05 in Mann-Whitney U test.
CDC, Centers for Disease Control and Prevention; neg., negative; and pos., positive.
Elevated HPV DNA loads found in HIV-positive patients with HSIL could result from a higher proportion of infected cells shed from larger lesions and/or from enhanced HPV replication. The impaired immunity in HIV-positive patients will lead to a less efficient elimination of HPV-infected keratinocytes (10, 36) and thus to an expansion of lesions. Increased HPV DNA replication could be the consequence of direct or indirect effects of HIV (14, 43), of synergistic effects of infections with multiple HPV types (see above), or of coinfections with other sexually transmitted agents, such as herpes simplex viruses (35). An increased risk of malignant conversion can be expected both from the larger pool of HPV-infected cells in expanded lesions and from elevated gene doses, leading to higher concentrations of viral oncoproteins. All these mechanisms could contribute to the more aggressive course of cer-vical HPV infections in HIV-positive patients. The observed elevated HPV DNA loads, due to whatever mechanism, thus reflect the increased risk for cervical cancer in HIV-positive women.
Acknowledgments
This work was supported by the Wilhelm Sander-Stiftung, project number 2000.065.1.
We thank Monika Junk for excellent technical assistance.
REFERENCES
- 1.Ahdieh, L., R. S. Klein, R. Burk, S. Cu-Uvin, P. Schuman, A. Duerr, M. Safaeian, J. Astemborski, R. Daniel, and K. Shah. 2001. Prevalence, incidence, and type-specific persistence of human papillomavirus in human immunodeficiency virus (HIV)-positive and HIV-negative women. J. Infect. Dis. 184:682-690. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, H. M., Y. Ting, C. E. Greer, J. C. Chambers, C. J. Tashiro, J. Chimera, A. Reingold, and M. M. Manos. 1991. Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA 265:472-477. [PubMed] [Google Scholar]
- 3.Bavin, P. J., J. A. Giles, A. Deery, J. Crow, P. D. Griffiths, V. C. Emery, and P. G. Walker. 1993. Use of semi-quantitative PCR for human papillomavirus DNA type 16 to identify women with high grade cervical disease in a population presenting with a mildly dyskaryotic smear report. Br. J. Cancer 67:602-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaudenon, S., D. Kremsdorf, O. Croissant, S. Jablonska, S. Wain-Hobson, and G. Orth. 1986. A novel type of human papillomavirus associated with genital neoplasias. Nature 321:246-249. [DOI] [PubMed] [Google Scholar]
- 5.Bosch, F. X., M. M. Manos, N. Munoz, M. Sherman, A. M. Jansen, J. Peto, M. H. Schiffman, V. Moreno, R. Kurman, K. V. Shah, et al. 1995. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J. Natl. Cancer Inst. 87:796-802. [DOI] [PubMed] [Google Scholar]
- 6.Boshart, M., L. Gissmann, H. Ikenberg, A. Kleinheinz, W. Scheurlen, and H. H. zur Hausen. 1984. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 3:1151-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardillo, M., R. Hagan, J. Abadi, and M. A. Abadi. 2001. CD4 T-cell count, viral load, and squamous intraepithelial lesions in women infected with the human immunodeficiency virus. Cancer 93:111-114. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1993. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA 269:729-730. [PubMed] [Google Scholar]
- 9.Conti, M., A. Agarossi, F. Parazzini, M. L. Muggiasca, A. Boschini, E. Negri, and E. Casolati. 1993. HPV, HIV infection, and risk of cervical intraepithelial neoplasia in former intravenous drug abusers. Gynecol. Oncol. 49:344-348. [DOI] [PubMed] [Google Scholar]
- 10.Crowley-Nowick, P. A., J. H. Ellenberg, S. H. Vermund, S. D. Douglas, C. A. Holland, and A. B. Moscicki. 2000. Cytokine profile in genital tract secretions from female adolescents: impact of human immunodeficiency virus, human papillomavirus, and other sexually transmitted pathogens. J. Infect. Dis. 181:939-945. [DOI] [PubMed] [Google Scholar]
- 11.Cuzick, J., G. Terry, L. Ho, T. Hollingworth, and M. Anderson. 1992. Human papillomavirus type 16 in cervical smears as predictor of high-grade cervical intraepithelial neoplasia. Lancet 339:959-960. [DOI] [PubMed] [Google Scholar]
- 12.Delius, H., and B. Hofmann. 1994. Primer-directed sequencing of human papillomavirus types. Curr. Top. Microbiol. Immunol. 186:13-31. [DOI] [PubMed] [Google Scholar]
- 13.Delmas, M. C., C. Larsen, B. van Benthem, F. F. Hamers, C. Bergeron, J. D. Poveda, B. Anzen, A. van den Hoek, F. Meier, J. M. Pena, H. Savonius, D. Sperandeo, B. Suligoi, P. Vernazza, J. B. Brunet, et al. 2000. Cervical squamous intraepithelial lesions in HIV-infected women: prevalence, incidence and regression. AIDS 14:1775-1784. [DOI] [PubMed] [Google Scholar]
- 14.Dolei, A., S. Curelli, P. Marongiu, A. Pierangeli, E. Gomes, M. Bucci, C. Serra, and A. M. Degener. 1999. Human immunodeficiency virus infection in vitro activates naturally integrated human papillomavirus type 18 and induces synthesis of the L1 capsid protein. J. Gen. Virol. 80:2937-2944. [DOI] [PubMed] [Google Scholar]
- 15.Ellerbrock, T. V., M. A. Chiasson, T. J. Bush, X. W. Sun, D. Sawo, K. Brudney, and T. C. Wright. 2000. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA 283:1031-1037. [DOI] [PubMed] [Google Scholar]
- 16.Flannelly, G., G. Jiang, D. Anderson, W. Melvin, E. Mann, and H. Kitchener. 1995. Serial quantitation of HPV-16 in the smears of women with mild and moderate dyskaryosis. J. Med. Virol. 47:6-9. [DOI] [PubMed] [Google Scholar]
- 17.Fleiss, J. L. 1961. Statistical methods for rates and proportions. John Wiley & Sons, New York, N.Y.
- 18.Frisch, M., R. J. Biggar, and J. J. Goedert. 2000. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J. Natl. Cancer Inst. 92:1500-1510. [DOI] [PubMed] [Google Scholar]
- 19.Frisch, M., R. J. Biggar, E. A. Engels, and J. J. Goedert. 2001. Association of cancer with AIDS-related immunosuppression in adults. JAMA 285:1736-1745. [DOI] [PubMed] [Google Scholar]
- 20.Heard, I., J. M. Tassie, V. Schmitz, L. Mandelbrot, M. D. Kazatchkine, and G. Orth. 2000. Increased risk of cervical disease among human immunodeficiency virus-infected women with severe immunosuppression and high human papillomavirus load. Obstet. Gynecol. 96:403-409. [DOI] [PubMed] [Google Scholar]
- 21.Holoway, P., A. B. Miller, T. Rohan, and T. To. 1999. Natural history of dysplasia of the uterine cervix. J. Natl. Cancer Inst. 91:252-258. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs, M. V., P. J. F. Snijders, A. J. C. van den Brule, T. J. M. Helmerhorst, C. J. L. M. Meijer, and J. M. M. Walboomers. 1997. A general primer GP5+/GP6+-mediated PCR-enzyme immunoassay method for rapid detection of 14 high-risk and 6 low-risk human papillomavirus genotypes in cervical scrapings. J. Clin. Microbiol. 35:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Josefsson, A. M., P. K. Magnusson, N. Ylitalo, P. Sorensen, P. Qwarforth-Tubbin, P. K. Andersen, M. Melbye, H. O. Adami, and U. B. Gyllensten. 2000. Viral load of human papilloma virus 16 as a determinant for development of cervical carcinoma in situ: a nested case-control study. Lancet 355:2189-2193. [DOI] [PubMed] [Google Scholar]
- 24.Kurman, R. J., D. E. Henson, A. L. Herbst, K. L. Noller, M. H. Schiffman, et al. 1994. Interim guidelines for management of abnormal cervical cytology. JAMA 271:1866-1869. [PubMed] [Google Scholar]
- 25.Lorincz, A. T., W. D. Lancaster, and G. F. Temple. 1986. Cloning and characterization of the DNA of a new human papillomavirus from a woman with dysplasia of the uterine cervix. J. Virol. 58:225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorincz, A. T., P. E. Castle, M. E. Sherman, D. R. Scott, A. G. Glass, S. Wacholder, B. B. Rush, P. E. Gravitt, J. E. Schussler, and M. Schiffman. 2002. Viral load of human papillomavirus and risk of CIN3 or cervical cancer. Lancet 360:228-229. [DOI] [PubMed] [Google Scholar]
- 27.Luff, R. D., et al. 1992. The Bethesda System for reporting cervical/vaginal cytologic diagnoses: report of the 1991 Bethesda workshop. Hum. Pathol. 23:719-721. [DOI] [PubMed] [Google Scholar]
- 28.Luque, A. E., L. M. Demeter, and R. C. Reichman. 1999. Association of human papillomavirus infection and disease with magnitude of human immunodeficiency virus type 1 (HIV-1) RNA plasma level among women with HIV-1 infection. J. Infect. Dis. 179:1405-1409. [DOI] [PubMed] [Google Scholar]
- 29.Maiman, M., R. G. Fruchter, A. Sedlis, J. Feldman, P. Chen, R. D. Burk, and H. Minkoff. 1998. Prevalence, risk factors, and accuracy of cytologic screening for cervical intraepithelial neoplasia in women with the human immunodeficiency virus. Gynecol. Oncol. 68:233-239. [DOI] [PubMed] [Google Scholar]
- 30.Massad, L. S., K. A. Riester, K. M. Anastos, R. G. Fruchter, J. M. Palefsky, R. D. Burk, D. Burns, R. M. Greenblatt, L. I. Muderspach, P. Miotti, et al. 1999. Prevalence and predictors of squamous cell abnormalities in Papanicolaou smears from women infected with HIV-1. J. Acquir. Immune Defic. Syndr. 21:33-41. [DOI] [PubMed] [Google Scholar]
- 31.Naghashfar, Z. S., N. B. Rosenshein, A. T. Lorincz, J. Buscema, and K. V. Shah. 1987. Characterization of human papillomavirus type 45, a new type 18-related virus of the genital tract. J. Gen. Virol. 68:3073-3079. [DOI] [PubMed] [Google Scholar]
- 32.Ostor, A. G. 1993. Natural history of cervical intraepithelial neoplasia: a critical review. Int. J. Gynecol. Pathol. 12:186-192. [PubMed] [Google Scholar]
- 33.Palefsky, J. M. 1998. Human papillomavirus infection and anogenital neoplasia in human immunodeficiency virus-positive men and women. J. Natl. Cancer Inst. 23:15-20. [DOI] [PubMed] [Google Scholar]
- 34.Palefsky, J. M., H. Minkoff, L. A. Kalish, A. Levine, H. S. Sacks, P. Garcia, M. Young, S. Melnick, P. Miotti, and R. Burk. 1999. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women. J. Natl. Cancer Inst. 91:226-236. [DOI] [PubMed] [Google Scholar]
- 35.Schmitt, J., J. R. Schlehofer, K. Mergener, L. Gissmann, and H. zur Hausen. 1989. Amplification of bovine papillomavirus DNA by N-methyl-N-nitro-N-nitrosoguanidine, ultraviolet irradiation, or infection with herpes simplex virus. Virology 172:73-81. [DOI] [PubMed] [Google Scholar]
- 36.Scott, M., M. Nakagawa, and A. B. Moscicki. 2001. Cell-mediated immune response to human papillomavirus infection. Clin. Diagn. Lab. Immunol. 8:209-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seedorf, K., G. Krammer, M. Durst, S. Suhai, and W. G. Rowekamp. 1985. Human papillomavirus type 16 DNA sequence. Virology 145:181-185. [DOI] [PubMed] [Google Scholar]
- 38.Serraino, D., P. Carrieri, C. Pradier, E. Bidoli, M. Dorrucci, E. Ghetti, A. Schiesari, R. Zucconi, P. Pezzotti, P. Dellamonica, S. Franceschi, and G. Rezza. 1999. Risk of invasive cervical cancer among women with, or at risk for, HIV infection. Int. J. Cancer 82:334-337. [DOI] [PubMed] [Google Scholar]
- 39.Six, C., I. Heard, C. Bergeron, G. Orth, J. D. Poveda, P. Zagury, P. Cesbron, C. Crenn-Hebert, R. Pradinaud, M. Sobesky, C. Marty, M. L. Babut, J. E. Malkin, A. Odier, S. Fridmann, J. P. Aubert, J. B. Brunet, and I. de Vincenzi. 1998. Comparative prevalence, incidence, and short-term prognosis of cervical squamous intraepithelial lesions amongst HIV-positive and HIV-negative women. AIDS 12:1047-1056. [PubMed] [Google Scholar]
- 40.Soost, H. J., S. Baur, and H. Smolka. 1990. Gynaekologische Zytodiagnostik. Thieme, Stuttgart, Germany.
- 41.Swan, D. C., R. A. Tucker, G. Tortolero-Luna, M. F. Mitchell, L. Wideroff, E. R. Unger, R. A. Nisenbaum, W. C. Reeves, and J. P. Icenogle. 1999. Human papillomavirus (HPV) DNA copy number is dependent on grade of cervical disease and HPV type. J. Clin. Microbiol. 37:1030-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Duin, M., P. J. Snijders, H. F. Schrijnemakers, F. J. Voorhorst, L. Rozendaal, M. A. Nobbenhuis, A. J. van den Brule, R. H. Verheijen, T. J. Helmerhorst, and C. J. Meijer. 2002. Human papillomavirus 16 load in normal and abnormal cervical scrapes: an indicator of CIN II/III and viral clearance. Int. J. Cancer 98:590-595. [DOI] [PubMed] [Google Scholar]
- 43.Vernon, S. D., C. E. Hart, W. C. Reeves, and J. P. Icenogle. 1993. The HIV-1 tat protein enhances E2-dependent human papillomavirus 16 transcription. Virus Res. 27:133-145. [DOI] [PubMed] [Google Scholar]
- 44.Walboomers, J. M., M. V. Jacobs, M. M. Manos, F. X. Bosch, J. A. Kummer, K. V. Shah, P. J. Snijders, J. Peto, C. J. Meijer, and N. Munoz. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189:12-19. [DOI] [PubMed] [Google Scholar]
- 45.Wieland, U., S. Jurk, S. Weissenborn, T. Krieg, H. Pfister, and A. Ritzkowsky. 2000. Erythroplasia of queyrat: coinfection with cutaneous carcinogenic human papillomavirus type 8 and genital papillomaviruses in a carcinoma in situ. J. Investig. Dermatol. 115:396-401. [DOI] [PubMed] [Google Scholar]
- 46.Ylitalo, N., P. Sorensen, A. M. Josefsson, P. K. Magnusson, P. K. Andersen, J. Ponten, H. O. Adami, U. B. Gyllensten, and M. Melbye. 2000. Consistent high viral load of human papillomavirus 16 and risk of cervical carcinoma in situ: a nested case-control study. Lancet 355:2194-2198. [DOI] [PubMed] [Google Scholar]
- 47.zur Hausen, H. 2000. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J. Natl. Cancer Inst. 92:690-698. [DOI] [PubMed] [Google Scholar]