Abstract
No standardized method for susceptibility testing of Brachyspira spp. is currently available. A broth dilution procedure was evaluated and used to test the activities of six antimicrobial agents for 108 isolates of Swedish porcine Brachyspira spp. representing biochemical groups I, II, and III. Group I corresponds to Brachyspira hyodysenteriae, group II corresponds to B. intermedia, and group III corresponds to B. murdochii and B. innocens. A panel was designed with the antimicrobial agents dried in tissue culture trays with wells that allowed a liquid volume of 0.5 ml in each and agitation of the broth when incubated on a shaker. The MICs were determined by using brain heart infusion broth with 10% fetal calf serum. For 10 isolates, the results obtained in broth were compared to the MICs obtained on two different types of agar. Different inoculum densities and incubation times were also compared. The concentrations at which 90% of the B. hyodysenteriae isolates (n = 72) were inhibited in the broth dilution test by tiamulin (0.25 μg/ml), tylosin (>256 μg/ml), erythromycin (>256 μg/ml), clindamycin (>4 μg/ml), virginiamycin (4 μg/ml), and carbadox (0.06 μg/ml) were determined. The MICs tended to be lower in broth than on agar. Differences in inoculum densities and incubation times had little influence on the MICs. The evaluated broth dilution test was simple to perform, the end points were easily read, and the results were reproducible and reliable. No isolates with decreased susceptibility to tiamulin were found among the Swedish isolates tested.
Swine dysentery is a common disease causing major production losses among pigs worldwide (7). This severe diarrheal disease is caused by an anaerobic spirochete, Brachyspira hyodysenteriae (formerly Serpulina hyodysenteriae [8a]). The other porcine Brachyspira spp. that have been described are B. intermedia and B. murdochii (27), B. innocens (13), and B. pilosicoli (29). In this study, all porcine Brachyspira spp., with the exception of B. pilosicoli, were included. The pathogenic potential of B. intermedia is controversial, whereas B. murdochii and B. innocens are not considered to be pathogens.
Antimicrobial agents are widely used for extended periods to control swine dysentery in affected herds. The antimicrobial agents most frequently used in Sweden and many other countries for the treatment of the disease are tylosin and tiamulin. Widespread resistance to tylosin, a 16-membered macrolide antibiotic, in B. hyodysenteriae has been reported in several countries (2, 14, 17, 23). This resistance is caused by a point mutation in the 23S rRNA gene (9). After exposure to low concentrations of tylosin, susceptible strains become macrolide resistant in vitro in less than 2 weeks. More troubling, Brachyspira sp. isolates resistant to tiamulin, the most important agent for the treatment of swine dysentery in many countries, have been reported in Australia, Finland, the United Kingdom, and Hungary (2, 5, 6, 17).
Brachyspira spp. are fastidious organisms, and currently there is no standardized antimicrobial susceptibility testing method for the genus. The purpose of this study was to develop and evaluate a dilution procedure suitable for the susceptibility testing of these organisms. A panel with six antibiotics and with broth and agar was tested with recent isolates of Brachyspira spp. from pig herds throughout Sweden.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The porcine Brachyspira spp. can be differentiated according to a biochemical classification system (4). The biochemical groups generally correspond to the recognized species as follows: group I, B. hyodysenteriae; group II, B. intermedia; group III, B. murdochii (IIIa) and B. innocens (IIIb/c); and group IV, B. pilosicoli. The Brachyspira spp. used in this study were 108 Swedish isolates from 90 farms representing biochemical groups I, II, IIIa, and IIIb/c. The bacteria were isolated, identified according to the biochemical classification system, and stored in liquid nitrogen as described previously (4). Thawed isolates were grown on fastidious anaerobe agar (National Veterinary Institute, Uppsala, Sweden) in an anaerobic atmosphere provided by gas generator envelopes (BBL GasPak Plus; Becton Dickinson, Cockeysville, Md.) in jars for 3 days at 39 to 40°C. The purity of all isolates was checked by phase-contrast microscopy. The following reference and type strains were included: B. hyodysenteriae B204 (ATCC 31212), B. hyodysenteriae B78T (ATCC 27164T), B. intermedia PWS/AT (ATCC 51140T), and B. murdochii 56-150T (ATCC 51284T).
Antimicrobial agents.
The following six antimicrobial agents were used in this study: tiamulin hydrogen fumarate (Lövens, Copenhagen, Denmark), tylosin tartrate (Sigma-Aldrich, Stockholm, Sweden), erythromycin (Sigma-Aldrich), clindamycin hydrochloride (Upjohn AB, Partille, Sweden), virginiamycin (Pfizer AB, Rixensart, Belgium), and carbadox (Pfizer AB). Tiamulin, tylosin, virginiamycin, and carbadox were chosen because they are or have been used in Sweden for the treatment of swine dysentery. Erythromycin was included as a prototypical antibiotic of the macrolide group. Clindamycin served as a representative of the lincosamide group and also as an agent with accepted MIC ranges for antimicrobial susceptibility testing of anaerobic bacteria, according to NCCLS standards (18). The compounds were dissolved and diluted according to the manufacturers ' recommendations. The diluted antimicrobial agents were stored at −70°C.
Antibiotic panels.
A panel for susceptibility testing of the six antimicrobial agents was designed. Twofold serial dilutions (for the range of concentrations, see Table 2) of the antimicrobial agents were dried in tissue culture trays with 48 wells (NunclonΔ Multidishes; Nunc, Roskilde, Denmark). The panels with dried antimicrobial agents were packaged in foil pouches with a desiccant and stored at room temperature. The packages were hermetic, and 60 to 66% of the air was evacuated with a vacuum pump (Vacuumpack; Howden).
TABLE 2.
Differences in MICs for 44 isolates of B. hyodysenteriae tested in triplicate for comparisons of tests 2 and 3 with test 1
| Antimicrobial agent | MIC range tested (μg/ml) | No. of MICsf
|
% of MICs within ±1 dilution | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −2 | −1 | Same | +1 | +2 | +3 | +4 | +5 | |||
| Tiamulin | 0.016-2 | 0 | 13 | 48 | 27 | 0 | 0 | 0 | 0 | 100 |
| Tylosin | 2-256 | 0 | 7 | 68a | 13 | 0 | 0 | 0 | 0 | 100 |
| Erythromycin | 2-256 | 0 | 8 | 55b | 15 | 7 | 2 | 0 | 1 | 89 |
| Clindamycind | 0.03-4 | 2 | 13 | 45c | 24 | 2 | 0 | 0 | 0 | 95 |
| Virginiamycin | 0.25-16 | 3 | 12 | 54 | 19 | 0 | 0 | 0 | 0 | 97 |
| Carbadoxe | 0.004-0.5 | 6 | 15 | 25 | 21 | 9 | 1 | 1 | 0 | 78 |
For 19 isolates, MICs were outside the range of the panel in all three tests.
For 22 isolates, MICs were outside the range of the panel in all three tests.
For 11 isolates, MICs were outside the range of the panel in all three tests.
Forty-three isolates were compared (one isolate was excluded due to skipped wells).
Thirty-nine isolates were compared (five isolates were excluded due to skipped wells or contamination).
Values are numbers of MICs that were identical or differed within a range of −2 to +5 dilution steps.
Control of performance and shelf life of the antibiotic panels.
Each new batch of antibiotic dilutions was tested twice on different occasions with two anaerobic control strains recommended by NCCLS standards for the antimicrobial susceptibility testing of anaerobic bacteria (18): Eubacterium lentum (ATCC 43055) and Bacteroides fragilis (ATCC 25285). Each new lot of panels was also tested with two aerobic control strains: Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 29213). The anaerobic control strains were tested in brain heart infusion broth (Difco) supplemented with 10% fetal calf serum (National Veterinary Institute) (BHIS broth). The inoculation procedure for the panels and the growth conditions were the same as those for Brachyspira spp. (see below), except that the incubation time for B. fragilis was 48 h. The tests with the aerobic control strains followed NCCLS standards for broth microdilution (20), except that the inoculation volume was 0.5 ml of Mueller-Hinton broth (Difco) in each well and the incubation temperature was 37°C instead of 35°C. Packages with panels were stored for shelf life tests. To date, one test has been performed with E. coli (ATCC 25922) and S. aureus (ATCC 29213) after 1.5 years if storage. The panels were also tested regularly with B. hyodysenteriae B78T (ATCC 27164T) during storage. Strains of Brachyspira spp. suggested for the control of performance are B. hyodysenteriae B78T (ATCC 27164T), which is tiamulin and tylosin susceptible; reference strain B. hyodysenteriae B204 (ATCC 31212), which is tylosin resistant and tiamulin susceptible; and B. hyodysenteriae 84193-2x/99 (CCUG 47386), which originated from an outbreak of swine dysentery in Germany and for which the MIC of tiamulin is elevated (see Table 3).
TABLE 3.
In vitro activities (MICs) of six antimicrobial agents for control strains and for reference and type strains of Brachyspira spp. tested in the broth dilution panela
| Strain | Medium | MIC (μg/ml) of:
|
|||||
|---|---|---|---|---|---|---|---|
| Tiamulin | Tylosin | Erythromycin | Clindamycin | Virginiamycin | Carbadox | ||
| Bacteroides fragilis (ATCC 25285) | BHIS | 1-4 (9) | ≤2 (9) | 2-4 (9) | 0.25b-1 (9) [0.5-2c] | 4-8 (9) | ≥0.5 (7) |
| Eubacterium lentum (ATCC 43055) | BHIS | ≥2 (9) | ≤2 (9) | ≤2 (9) | 0.125-0.25d (9) [0.03-0.12c] | 1-4 (9) | ≥0.5 (7) |
| Escherichia coli (ATCC 25922) | MH | >2 (9) | >256 (9) [>64e] | 64-128 (9) | >4 (9) | >16 (9) | >0.5 (5) |
| Staphylococcus aureus (ATCC 29213) | MH | 1 (12) [0.5-2f] | ≤2 (12) [1-2e] | ≤2 (12) [0.25-1g] | 0.125-0.25 (12) [0.06-0.25g] | 1-2 (12) | >0.5 (5) |
| B. hyodysenteriae B78T (ATCC 27164T) | BHIS | 0.03-0.06 (7) | 4-8 (7) | 4-32 (6) | 0.06-0.125 (7) | 1-2 (7) | 0.016-0.06 (7) |
| B. hyodysenteriae B204 (ATCC 31212) | BHIS | 0.03-0.06 (6) | 64->256 (6) | >256 (6) | 1->4 (6) | 1-2 (6) | 0.016-0.03 (5) |
| B. hyodysenteriae 84193-2x/99 (CCUG 47386) | BHIS | 8-16 (5) | >128 (2) | NT | NT | NT | NT |
| B. intermedia PWS/AT (ATCC 51140T) | BHIS | 0.03-0.06 (3) | 4-8 (3) | 2-16 (3) | 0.03-0.06 (3) | 1 (3) | 0.008-0.03 (3) |
| B. murdochii 56-150T (ATCC 51284T) | BHIS | 0.06-0.125 (3) | >256 (3) | >256 (3) | ≥4 (3) | 1-2 (3) | 0.03-0.06 (3) |
Numbers in parentheses represent numbers of tests performed. Numbers in brackets represent MIC ranges. MH, Mueller-Hinton broth. NT, not tested.
Eight of nine tests were within the range.
Approved by the NCCLS (18).
Five of nine tests were within the range.
Proposed by Odland et al. (21).
Approved by the NCCLS (19).
Approved by the NCCLS (20).
Broth dilution procedure.
Bacteria harvested from agar plates were suspended in BHIS broth to a concentration of between 1 × 108 and 5 × 108 CFU/ml. The optical density of the suspension was measured spectrophotometrically (Secomam S.250 spectrophotometer; 620 nm, 5-mm path length) and correlated with the population density by viable cell counts. From this suspension, 300 μl was transferred to 30 ml of BHIS broth to obtain a final inoculum concentration of 1 × 106 to 5 × 106 CFU/ml. Each well in the panels was filled with 0.5 ml of the inoculum. The panels were incubated in square GENbox anaerobic jars with GENbox Anaer generator sachets (bioMérieux, Lyon, France). The panels were covered with plastic lids, with a maximum of four panels per jar. After 4 days of incubation on a rotary shaker (60 to 80 rpm) at 37°C, the MIC was read as the lowest concentration of the antimicrobial agent that prevented visible growth. One well in each panel contained no drug and was used as a growth control and for visual comparison with growth in the other wells. The reading was made with the assistance of a viewing device with a mirror to obtain indirect light. The MIC was determined in triplicate for 91, in duplicate for 11, and once for 6 isolates.
Rate of growth in BHIS broth.
To determine the rate of growth in the medium used, the growth of B. hyodysenteriae B78T (ATCC 27164T) and one field isolate of B. hyodysenteriae, AN 2420:97, in BHIS broth was monitored spectrophotometrically. To imitate the susceptibility testing conditions, a suspension of bacteria was prepared and incubated in the same way as for the broth dilution test. Viable counts were determined at the end of the exponential phase.
Comparison of different inoculum densities and different incubation times.
To evaluate the effect of the inoculum density on the MIC, bacterial suspensions from 10 isolates were tested. Three inoculum densities of each isolate were tested in BHIS broth (optical density at 620 nm [OD620], 0.102 to 0.864). Three panels with the same isolate were tested simultaneously in the same batch of media and were incubated together in one jar. Viable cell counts of bacterial suspensions with low, medium, and high densities of B. hyodysenteriae B78T (ATCC 27164T) and one field isolate of B. hyodysenteriae, AN 4225:99, were determined in duplicate.
The effect of different incubation times in BHIS broth was investigated for four isolates. Four panels were prepared from the same inoculum of each isolate and placed in different jars. The jars were opened sequentially, and the MICs were read on days 2, 3, 4, and 5. Carbadox and virginiamycin were not included in this test.
Comparison of broth dilution and agar dilution.
Ten isolates were tested twice in BHIS broth and once on two different agar media, Trypticase soy agar with 5% ox blood (TSA agar; National Veterinary Institute) and Wilkins-Chalgren agar with 5% defibrinated horse blood (WC agar; National Veterinary Institute). The same panels were used for agar as described above for broth, and 0.5 ml of agar was poured into each well. Each agar-filled well was inoculated with 2 μl (1 × 105 to 5 × 105 CFU/spot) of a suspension identical to that prepared for broth dilution. After 4 days of incubation at 37°C, the MIC obtained with agar dilution was read as the lowest concentration of the antimicrobial agent that prevented visible growth or hemolysis. Control strains E. coli ATCC 25922 and S. aureus ATCC 29213 were used to test the agar panels. The inoculum of each control strain was prepared as for broth dilution and diluted to 1 × 103 to 1 × 104 CFU/spot. The panels were uncubated for 16 to 20 h at 37°C.
RESULTS
Antimicrobial susceptibility.
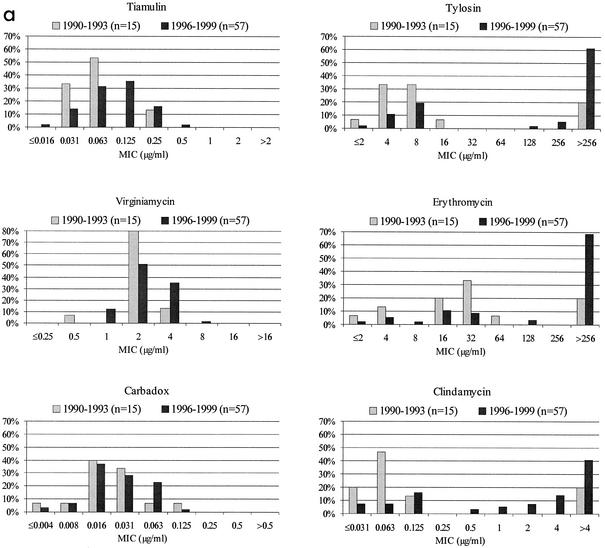
The results of the susceptibility testing of the Swedish field isolates of Brachyspira spp. are shown in Table 1 as the concentrations at which 90 and 50% of the isolates were inhibited (MIC90 and MIC50, respectively). The population distribution of the MICs of the six antimicrobial agents tested is shown in Fig. 1. When the tests were carried out in triplicate, the value used in the population distribution diagrams and for the MIC90 and the MIC50 was either the most common value obtained or the middle value, whereas the highest value was used when the isolates were tested in duplicate.
TABLE 1.
MICs of six antimicrobial agents for Swedish field isolates of Brachyspira spp.
| Groupa | No. of isolates | Yr of collection | MIC (μg/ml) of:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tiamulin
|
Tylosin
|
Erythromycin
|
Clindamycin
|
Virginiamycin
|
Carbadox
|
|||||||||||||||
| 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Range | |||
| I | 15 | 1990-1993 | 0.03 | 0.25 | ≤0.016-0.25 | 8 | >256 | ≤2->256 | 16 | >256 | ≤2->256 | 0.06 | >4 | ≤0.03->4 | 2 | 4 | 0.5-4 | 0.016 | 0.06 | ≤0.004-0.125 |
| I | 57 | 1996-1999 | 0.125 | 0.125 | 0.03-0.25 | >256 | >256 | 4->256 | >256 | >256 | ≤2->256 | 4 | >4 | ≤0.03->4 | 2 | 4 | 1-16 | 0.03 | 0.06 | ≤0.004-0.125 |
| II | 20 | 1990-1999 | 0.06 | 0.25 | ≤0.016-2 | 4 | >256 | ≤2->256 | 8 | >256 | ≤2->256 | 0.06 | 4 | ≤0.03->4 | 1 | 2 | 0.5-4 | 0.008 | 0.03 | 0.008-0.06 |
| III | 16 | 1990-1998 | 0.06 | 2 | 0.03->2 | 8 | 16 | ≤2->256 | 32 | 64 | ≤2->256 | 0.125 | 4 | ≤0.03->4 | 2 | 8 | 0.5-16 | 0.016 | 0.03 | 0.008-0.06 |
See the text.
FIG. 1.
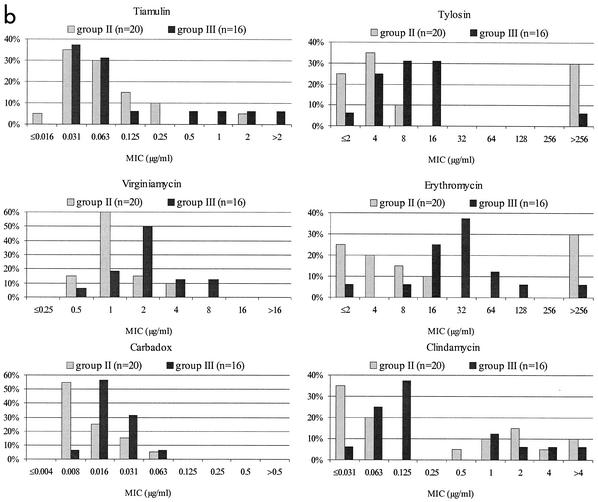
MIC distributions. (a) Distribution of MICs of six antimicrobial agents for 72 Swedish field isolates of B. hyodysenteriae. (b) Distribution of MICs of six antimicrobial agents for 36 Swedish field isolates of Brachyspira sp. biochemical groups II and III.
The tests were carried out in triplicate for 91 of the 108 isolates. For 255 of these tests, the inoculum density was measured and varied between OD620s of 0.177 and 0.695 (corresponding to a final inoculum density of 1 × 106 to 5 × 106 CFU/ml). The MICs of tylosin and tiamulin in these repeated tests did not differ more than two twofold dilutions for any of the 91 isolates. For virginiamycin, clindamycin, erythromycin, and carbadox, the difference between the highest and the lowest MICs was more than two twofold dilutions for 1, 2, 7, and 14 isolates, respectively.
The MICs for all B. hyodysenteriae isolates tested in triplicate and for which an optical density was obtained for the inoculum in all tests were compared. For these isolates (n = 44), the MICs obtained in the second and third tests were compared with those obtained in the first test (Table 2). The inoculum density varied between OD620s of 0.177 and 0.679 (corresponding to a final inoculum density of 1 × 106 to 5 × 106 CFU/ml). For four antimicrobial agents, 95% or more of the MICs were within ±1 twofold dilution. For about one-half of the isolates tested, the tylosin and erythromycin MICs were above the range of the concentrations used in all three tests, and the same was true for about one-fourth of the isolates tested with clindamycin.
Control of performance and shelf life.
The results obtained with all control strains and all proposed or accepted ranges available for the test organisms used are shown in Table 3. For all strains, the difference in test results was less than two twofold dilutions, except for four test series with Brachyspira spp. In shelf life tests, the MICs of all six antimicrobial agents were either within the available accepted ranges or within ranges obtained from previous repeated tests with the control strains.
Rate of growth in BHIS broth.
The optical densities of the initial suspensions of B. hyodysenteriae B78T (ATCC 27164T) and field isolate AN 2420:97 were 0.305 and 0.398, respectively; those after two 10-fold dilutions were 0.007 and 0.008, respectively. The growth curve for the field isolate was analogous to that for B78T (ATCC 27164T), and the end of the log phase was reached within 48 h. The viable counts for both isolates at 48 h were 5 × 108 CFU/ml.
Different inoculum densities and incubation times.
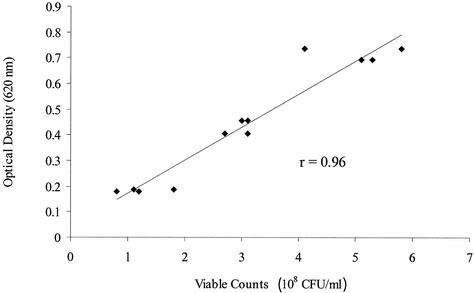
For tiamulin, tylosin, clindamycin, and virginiamycin, the MIC was at most one twofold dilution higher with a higher inoculum density (Table 4). The MICs of erythromycin differed by two twofold dilutions for one isolate, and those of carbadox differed by three twofold dilutions for three isolates. Again, for some isolates, the MICs were not within the ranges tested for tylosin, erythromycin, and clindamycin. Of the MICs within the test ranges, 36% were the same for all three inoculum densities tested. There was a high correlation between the duplicate determinations of viable counts and the optical densities of two isolates suspended at three different inoculum densities (Fig. 2). Differences in incubation time had little influence on the MICs. In all cases but one, the variation was never more than one twofold dilution (Table 5).
TABLE 4.
Influence of inoculum density on MICs of six antibiotics for 10 B. hyodysenteriae isolates tested in BHIS broth
| Isolate | Inoculum densitya | MIC (μg/ml) of:
|
|||||
|---|---|---|---|---|---|---|---|
| Tiamulin | Tylosin | Erythromycin | Clindamycin | Virginiamycin | Carbadox | ||
| AN 117:99 | 0.190 | 0.125 | >256 | >256 | >4 | 2 | 0.016 |
| 0.375 | 0.125 | >256 | >256 | >4 | 4 | 0.06 | |
| 0.488 | 0.125 | >256 | >256 | >4 | 4 | 0.125 | |
| AN 2343:99 | 0.207 | 0.125 | >256 | >256 | >4 | 4 | 0.016 |
| 0.362 | 0.125 | >256 | >256 | >4 | 4 | 0.016 | |
| 0.446 | 0.125 | >256 | >256 | >4 | 4 | 0.016 | |
| AN 756:99 | 0.179 | 0.125 | >256 | >256 | 4 | 2 | 0.016 |
| 0.458 | 0.125 | >256 | >256 | >4 | 2 | 0.016 | |
| 0.719 | 0.250 | >256 | >256 | >4 | 4 | 0.06 | |
| AN 949:99 | 0.213 | 0.125 | >256 | >256 | >4 | 4 | 0.016 |
| 0.475 | 0.250 | >256 | >256 | >4 | 4 | 0.06 | |
| 0.864 | 0.250 | >256 | >256 | >4 | 4 | 0.125 | |
| AN 872:99 | 0.205 | 0.125 | >256 | >256 | 4 | 2 | 0.008 |
| 0.313 | 0.125 | >256 | >256 | 4 | 2 | 0.008 | |
| 0.650 | 0.250 | >256 | >256 | >4 | 4 | 0.03 | |
| AN 702:99 | 0.102 | 0.06 | >256 | >256 | 4 | 2 | 0.016 |
| 0.322 | 0.125 | >256 | >256 | >4 | 2 | 0.03 | |
| 0.582 | 0.125 | >256 | >256 | >4 | 2 | 0.016 | |
| AN 1482:99 | 0.166 | 0.25 | 8 | 8 | 0.25 | 2 | 0.008 |
| 0.368 | 0.25 | 8 | 16 | 0.5 | 2 | 0.016 | |
| 0.662 | 0.5 | 8 | 32 | 0.5 | 4 | 0.06 | |
| AN 2331:99 | 0.116 | 0.03 | 4 | 16 | 0.125 | 1 | 0.008 |
| 0.248 | 0.06 | 8 | 32 | 0.125 | 2 | 0.016 | |
| 0.460 | 0.06 | 8 | 32 | 0.125 | 2 | 0.03 | |
| AN 799:99 | 0.149 | 0.03 | 4 | 16 | 0.06 | 2 | 0.016 |
| 0.352 | 0.03 | 4 | 16 | 0.06 | 2 | 0.016 | |
| 0.558 | 0.06 | 4 | 32 | 0.06 | 2 | 0.03 | |
| AN 2224:99 | 0.222 | 0.25 | >256 | >256 | >4 | 4 | 0.016 |
| 0.416 | 0.25 | >256 | >256 | >4 | 4 | 0.016 | |
| 0.612 | 0.25 | >256 | >256 | >4 | 4 | 0.016 | |
Optical density measured spectrophotometrically.
FIG. 2.
Correlation of optical densities and viable counts for suspensions of B. hyodysenteriae B78T (ATCC 27164T) and a field isolate of B. hyodysenteriae. r is the Pearson correlation coefficient.
TABLE 5.
Comparison of different incubation times for a panel similar to that used in the main study but with alterations of antibiotics and rangesa
| Isolate | Incubation time (days) | MIC (μg/ml) of:
|
|||
|---|---|---|---|---|---|
| Tylosin | Tiamulin | Erythromycin | Clindamycin | ||
| AN 640:00 | 2 | 4 | 0.03 | 8 | ≤0.125 |
| 3 | 4 | 0.03 | 8 | ≤0.125 | |
| 4 | 4 | 0.03 | 8 | ≤0.125 | |
| 5 | 4 | 0.03 | 16 | ≤0.125 | |
| AN 809:00 | 2 | >256 | 0.06 | >256 | 4 |
| 3 | >256 | 0.125 | >256 | 8 | |
| 4 | >256 | 0.125 | 256 | 8 | |
| 5 | >256 | 0.125 | >256 | 8 | |
| AN 817:00 | 2 | >256 | 0.125 | >256 | 4 |
| 3 | >256 | 0.125 | >256 | 8 | |
| 4 | >256 | 0.125 | >256 | 8 | |
| 5 | >256 | 0.125 | >256 | 8 | |
| AN 780:00 | 2 | >256 | 0.125 | >256 | >16 |
| 3 | >256 | 0.125 | >256 | >16 | |
| 5 | >256 | 0.25 | >256 | 16 | |
The panels were prepared from the same inoculum and incubated in four different jars, opened sequentially on days 2, 3, 4, and 5. Carbadox and virginiamycin were not included.
Comparison of broth dilution and agar dilution.
For some of the isolates, the end points were difficult to read on agar. Often, growth was not visible to the naked eye, and the reading had to rely on the occurrence of hemolysis. For all antimicrobial agents except carbadox, the MICs tended to be higher on agar than in broth. The greatest discrepancies were seen for erythromycin, clindamycin, and carbadox (Table 6).
TABLE 6.
MICs of six antibiotics for 10 B. hyodysenteriae isolates and two control strains tested with broth dilution and agar dilutiona
| Isolate or strain | Medium | MIC (μg/ml) of:
|
|||||
|---|---|---|---|---|---|---|---|
| Tiamulin | Tylosin | Erythromycin | Clindamycin | Virginiamycin | Carbadox | ||
| AN 174:92 | BHIS 1 | 0.03 | 4 | 4 | ≤0.03 | 2 | 0.03 |
| BHIS 2 | 0.03 | 4 | ≤2 | ≤0.03 | 2 | 0.016 | |
| WC | 0.03 | 4 | 4 | 0.06 | 2 | 0.008 | |
| TSA | 0.06 | 4 | 4 | 0.06 | 4 | ≤0.004 | |
| AN 2156:98 | BHIS 1 | 0.03 | 128 | >256 | 1 | 1 | 0.008 |
| BHIS 2 | 0.06 | >256 | >256 | 1 | 1 | 0.008 | |
| WC | 0.125 | >256 | >256 | >4 | 2 | 0.008 | |
| TSA | 0.06 | >256 | >256 | >4 | 4 | ≤0.004 | |
| AN 2157:98 | BHIS 1 | 0.06 | 256 | >256 | 2 | 1 | ≤0.004 |
| BHIS 2 | 0.06 | >256 | >256 | 1 | 1 | ≤0.004 | |
| WC | 0.25 | >256 | >256 | >4 | 4 | 0.008 | |
| TSA | 0.125 | >256 | >256 | 4 | 4 | ≤0.004 | |
| AN 460:98 | BHIS 1 | 0.03 | 8 | 4 | 0.06 | 2 | 0.06 |
| BHIS 2 | ≤0.016 | 4 | ≤2 | ≤0.03 | 1 | 0.008 | |
| WC | 0.06 | 8 | 32 | 0.125 | 4 | 0.016 | |
| TSA | 0.03 | 8 | 16 | ≤0.03 | 4 | 0.008 | |
| AN 487:98 | BHIS 1 | 0.125 | >256 | >256 | 2 | 4 | 0.016 |
| BHIS 2 | 0.06 | >256 | >256 | 1 | 1 | 0.008 | |
| WC | 0.125 | >256 | >256 | 4 | 4 | 0.016 | |
| TSA | 0.06 | >256 | >256 | 2 | 8 | ≤0.004 | |
| AN 522:98 | BHIS 1 | 0.125 | >256 | >256 | >4 | 4 | 0.125 |
| BHIS 2 | 0.03 | >256 | >256 | 1 | 2 | 0.008 | |
| WC | 0.06 | >256 | >256 | 4 | 4 | 0.008 | |
| TSA | 0.06 | >256 | >256 | 2 | 8 | ≤0.004 | |
| AN 613:98 | BHIS 1 | 0.06 | >256 | >256 | 4 | 4 | 0.016 |
| BHIS 2 | 0.06 | >256 | >256 | 1 | 2 | 0.008 | |
| WC | 0.125 | >256 | >256 | >4 | 8 | 0.016 | |
| TSA | 0.06 | >256 | >256 | 4 | 8 | 0.008 | |
| AN 724:98 | BHIS 1 | 0.03 | 4 | 4 | ≤0.03 | 2 | 0.03 |
| BHIS 2 | ≤0.016 | ≤2 | ≤2 | ≤0.03 | 1 | 0.008 | |
| WC | 0.06 | 16 | 64 | 0.06 | 2 | 0.016 | |
| TSA | 0.06 | 8 | 4 | 0.06 | 4 | 0.008 | |
| AN 371:98 | BHIS 1 | 0.06 | >256 | >256 | 2 | 4 | 0.03 |
| BHIS 2 | 0.06 | >256 | >256 | 2 | 2 | 0.016 | |
| WC | 0.125 | >256 | >256 | >4 | 4 | 0.016 | |
| TSA | 0.06 | >256 | >256 | >4 | 4 | 0.008 | |
| AN 903:98 | BHIS 1 | 0.03 | 4 | ≤2 | ≤0.03 | 1 | 0.008 |
| BHIS 2 | 0.03 | 4 | ≤2 | ≤0.03 | 2 | 0.03 | |
| WC | 0.03 | 8 | 32 | ≤0.03 | 4 | 0.008 | |
| TSA | 0.03 | 4 | ≤2 | ≤0.03 | 2 | 0.008 | |
| Escherichia coli ATCC 25922 | BHIS | >2 | >256 | 64 | >4 | >16 | >0.5 |
| WC | >2 | >256 | 128 | >4 | >16 | >0.5 | |
| TSA | >2 | >256 | 64 | >4 | >16 | >0.5 | |
| Staphylococcus aureus ATCC 29213 | BHIS | 1 | ≤2 | ≤2 | 0.25 | 1 | >0.5 |
| WC | 1 | ≤2 | ≤2 | 0.25 | 2 | >0.5 | |
| TSA | 1 | ≤2 | ≤2 | 0.25 | 1 | >0.5 | |
The inocula of B. hyodysenteriae isolates were 1 × 106 to 5 × 106 CFU/ml in broth and 1 × 105 to −5 × 105 CFU/spot on agar. The inocula of the control strains were approximately 5 × 105 CFU/ml in broth and 1 × 104 CFU/spot on agar.
DISCUSSION
Most reports on susceptibility testing of Brachyspira spp. have used agar dilution. No widely accepted or standardized method for susceptibility testing of these organisms is currently available. The test conditions vary considerably and include type of medium, supplements, incubation time, and inoculum density, making it difficult to compare results.
The solid medium most commonly used for the antimicrobial susceptibility testing of B. hyodysenteriae is Trypticase soy agar with 5% ox or ssheep blood (8, 14, 23, 26, 29). In general, the MIC is reported as the lowest concentration of the antimicrobial agent that prevents growth or hemolysis. However, reading end points by means of hemolysis can be subjective because subinhibitory concentrations of some antimicrobial agents may prevent hemolysis in certain bacterial species (24, 25). With regard to Brachyspira spp., this situation is a problem because growth on agar after inoculation with 105 CFU/spot is difficult to detect for many isolates and the absence of hemolysis is the only means of observing growth inhibition. Because of this problem, it is difficult to follow the NCCLS recommendations to read the end point at which a marked change occurs in the appearance of growth compared to the growth on the control plate (18). Furthermore, reading end points is even more difficult for the weakly hemolytic species of Brachyspira.
Given the impact of swine dysentery on the pig industry, a method for the susceptibility testing of the causative agent, B. hyodysenteriae, that can provide reliable results that are comparable between laboratories is essential. In many countries, the antimicrobial arsenal available against swine dysentery has been reduced to only a few substances, because of decreased susceptibility and withdrawal of drugs authorized for the treatment of pigs. For example, to date in Sweden, tiamulin and tylosin are the only antimicrobial agents licensed for the treatment of swine dysentery and formulated for medication via feed or water. To prevent a scenario in which there are no efficacious antimicrobial agents for use against swine dysentery, the monitoring of antimicrobial resistance in B. hyodysenteriae is important. This monitoring is essential for the early detection of resistance and subsequent intervention against the spread of resistance. The use of tiamulin only under strict conditions would also help to avoid this situation. In addition to a standardized method, interpretation of the results of susceptibility tests needs to be standardized. To achieve comparability of results between countries, a common approach with uniform breakpoints for resistance is desirable.
A clinical breakpoint for tiamulin resistance of 4 μg/ml has been proposed (23). However, this breakpoint is based solely on pharmacokinetic data. With this breakpoint, all isolates of B. hyodysenteriae tested in this study would be designated susceptible to tiamulin, in agreement with the distribution of the MICs and indicating a tightly grouped population of isolates for which the MICs were below 0.5 μg/ml (Fig. 1a). The proposed clinical breakpoint is considerably higher than the MICs for the normal susceptible population. To monitor a gradual decrease in susceptibility to tiamulin among Brachyspira species isolates, a much lower microbiological breakpoint of, for example, 0.5 μg/ml is more relevant. Thus, isolates for which the MICs of tiamulin are above this microbiological breakpoint should be reported as having reduced susceptibility or possibly as resistant.
A tendency toward higher tiamulin, tylosin, erythromycin, and clindamycin MICs was seen for the B. hyodysenteriae isolates from 1996 to 1999 than for the isolates from 1990 to 1993 (Fig. 1a). Studies from Poland and Finland have shown a gradual decrease in susceptibility to tiamulin over time (1, 5). In two recent studies on B. hyodysenteriae isolates from Germany and the Czech Republic, this decrease was even more obvious. These studies show an increase in tiamulin MICs corresponding to four or five doubling dilutions for the whole population tested over the last 5- to 6-year period (3, 11). Thus, even if tiamulin resistance in B. hyodysenteriae does not develop rapidly, in due course exposure to the drug will result in decreased susceptibility. For some isolates, this decrease most likely will be sufficient to cause treatment failure. In vitro resistance following subculturing in the presence of tiamulin also develops slowly and stepwise (10).
About two-thirds of the B. hyodysenteriae isolates from 1996 to 1999 were resistant to tylosin. Further, all those isolates also showed cross-resistance to erythromycin and clindamycin (Fig. 1a). This resistance is caused by a point mutation at position 2058 (E. coli numbering) in the 23S rRNA gene (9). Mutation or methylation of the equivalent position causes macrolide, lincosamide, and streptogramin B resistance in several bacterial genera (22, 30, 31). Virginiamycin is a combination of streptogramins A and B; therefore, the mutation at position 2058 will not affect activity. This conclusion is supported by the virginiamycin MICs presented, which showed the distribution of a susceptible population (Fig. 1). The high frequency of tylosin resistance reported for B. hyodysenteriae in many countries (2, 14, 17, 23) is not surprising in view of selective pressure due to the wide use of tylosin as a therapeutic agent and as a growth promoter in swine production.
The distribution of MICs for group II and III isolates showed a few differences from that for other B. hyodysenteriae isolates. Isolates with decreased susceptibility to tiamulin were found; most were group III isolates. The MICs of virginiamycin and carbadox also were higher for group III isolates than for group II isolates. On the other hand, very few group III isolates and only one-third of group II isolates were resistant to tylosin. However, it is difficult to draw any conclusions due to the limited number of isolates investigated.
There are few anaerobic control strains for use in antimicrobial susceptibility tests. For example, three strains are recommended by the NCCLS, and the list of antimicrobial agents with accepted ranges includes no antimicrobial agents used only in veterinary medicine. Lincomycin is the lincosamide used therapeutically in pigs, but we chose clindamycin instead to include in the panel at least one antimicrobial agent with accepted ranges for the anaerobic control strains. The B. fragilis strain used in this study grew well with clear, stable end points, whereas E. lentum grew weakly in broth with 10% fetal calf serum as a supplement even after 4 days on a shaker. The weak growth of E. lentum in broth makes it difficult to read the end points, and so the strain is not suitable as a control organism for this broth method. Thus, it would be desirable to have more internationally available and recommended anaerobic control strains that give reproducible results and for which there are accepted ranges for drugs used in veterinary medicine.
The inoculum used here was prepared from bacteria in stationary phase by removing 3-day-old culture material from solid media and suspending this material in broth. After 3 days of incubation on agar, some spirochetes coil or form spherical bodies, which may not be as viable as spirochetes with a normal morphology. Because of this situation, we chose viable cell counts instead of direct microscope counts to estimate the inoculum bacterial concentration. On the other hand, Brachyspira cells tend to aggregate, and viable counting may result in a lower number (CFU) than direct counting of individual cells (28).
As a general rule, MICs determined by both broth macrodilution and broth microdilution are often one twofold dilution lower for anaerobes than are those obtained with agar dilution tests (18). This situation was also seen in this study when the results from broth tests were compared to the results from agar tests. An exception was carbadox, for which lower MICs were recorded on agar than in broth. When broth dilution with prepared panels was chosen as a method for this study, the advantages over agar dilution included having a standardized reproducible test which was easy to perform at short notice and with easily read end points. Different types of broth have been shown to support the growth of Brachyspira spp. (12, 15, 28). In the present study, BHIS broth supported growth well, as also reported by others (28), and it has likewise been proposed in an NCCLS approved standard for the susceptibility testing of anaerobes (18). When Lemcke et al. compared the rates of growth of B. hyodysenteriae in broth supplemented with different sera, they found that rabbit serum supported the greatest population density, but fetal calf serum was only slightly inferior (16).
Because there is a high correlation between optical densities and viable counts, the measure of absorbance may be a convenient method for estimating the number of viable organisms when one is preparing inocula of Brachyspira spp. However, both the different inoculum densities and the different incubation times tested in this study had little influence on the MICs. Other factors, such as the condition of the isolate and the duration of exposure to oxygen, may have a greater influence on the results.
In conclusion, the present investigation demonstrates a broth dilution method for the antimicrobial susceptibility testing of porcine Brachyspira spp. The method is easy to standardize and to perform, and the MIC end points are easily read. Further, this broth dilution method appears to be a suitable tool for monitoring resistance in Brachyspira spp.
Acknowledgments
This work was supported by the Swedish Council for Forestry and Agricultural Research.
We thank Margareta Horn af Rantzien for excellent technical assistance.
REFERENCES
- 1.Binek, M., U. Wojcik, Z. Synkiewicz, T. Jakubowski, P. Poomvises, and P. Ingkaninun. 1994. Dynamics of susceptibility of Serpulina hyodysenteriae to different chemotherapeutics in-vitro, p. 203. In P. Poomvises and P. Ingkaninum (ed.), Proceedings of the 13th International Pig Veterinary Society Congress. Chulalongkorn University Faculty of Veterinary Science, Bangkok, Thailand.
- 2.Buller, N. B., and D. J. Hampson. 1994. Antimicrobial susceptibility testing of Serpulina hyodysenteriae. Aust. Vet. J. 71:211-214. [DOI] [PubMed] [Google Scholar]
- 3.Cizek, A., D. Lobova, and J. Smola. 2002. In vitro susceptibility of Brachyspira hyodysenteriae strains isolated in the Czech Republic from 1996 to 2001, p. 191. In Proceedings of the 17th International Pig Veterinary Society Congress. IPVS, Perry, Iowa.
- 4.Fellström, C., and A. Gunnarsson. 1995. Phenotypical characterisation of intestinal spirochaetes isolated from pigs. Res. Vet. Sci. 59:1-4. [DOI] [PubMed] [Google Scholar]
- 5.Fossi, M., T. Saranpää, and E. Rautiainen. 1999. In vitro sensitivity of the swine Brachyspira species to tiamulin in Finland 1995-1997. Acta Vet. Scand. 40:355-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gresham, A. C., B. W. Hunt, and R. W. Dalziel. 1998. Treatment of swine dysentery—problems of antibiotic resistance and concurrent salmonellosis. Vet. Rec. 143:619. [PubMed] [Google Scholar]
- 7.Harris, D. L., D. J. Hampson, and R. Glock. 1999. Swine dysentery, p. 579-600. In B. E. Straw, S. D'Allaire, W. L. Mengeling, and D. J. Taylor (ed.), Diseases of swine, 8th ed. Iowa State University Press, Ames.
- 8.Hommez, J., F. Castryck, C. Miry, A. Lein, L. A. Devriese, and F. Haesebrouck. 1998. Susceptibility of different Serpulina species in pigs to antimicrobial agents. Vlaams Diergeneeskd. Tijdschr. 67:32-35. [Google Scholar]
- 8a.International Committee on Systematic Bacteriology.1998. Validation list no. 64. Int. J. Syst. Bacteriol. 48:327-328.
- 9.Karlsson, M., C. Fellström, M. U. Heldtander, K. E. Johansson, and A. Franklin. 1999. Genetic basis of macrolide and lincosamide resistance in Brachyspira (Serpulina) hyodysenteriae. FEMS Microbiol. Lett. 172:255-260. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson, M., A. Gunnarsson, and A. Franklin. 2001. Susceptibility to pleuromutilins in Brachyspira (Serpulina) hyodysenteriae. Anim. Health Res. Rev. 2:59-65. [PubMed] [Google Scholar]
- 11.Karlsson, M., J. Rohde, M. Kessler, and A. Franklin. 2002. Decreased susceptibility to tiamulin in German isolates of Brachyspira hyodysenteriae, p. 189. In Proceedings of the 17th International Pig Veterinary Society Congress. IPVS, Perry, Iowa.
- 12.Kinyon, J. M., and D. L. Harris. 1974. Growth of Treponema hyodysenteriae in liquid medium. Vet. Rec. 95:219-220. [DOI] [PubMed] [Google Scholar]
- 13.Kinyon, J. M., and D. L. H. Harris. 1979. Treponema innocens, a new species of intestinal bacteria, and emended description of the type strain of Treponema hyodysenteriae Harris et al. Int. J. Syst. Bacteriol. 29:102-109. [Google Scholar]
- 14.Kitai, K., M. Kashiwazaki, Y. Adachi, K. Kunugita, and A. Arakawa. 1987. In vitro antimicrobial activity against reference strains and field isolates of Treponema hyodysenteriae. Antimicrob. Agents Chemother. 31:1935-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunkle, R. A., D. L. Harris, and J. M. Kinyon. 1986. Autoclaved liquid medium for propagation of Treponema hyodysenteriae. J. Clin. Microbiol. 24:669-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemcke, R. M., J. Bew, M. R. Burrows, and R. J. Lysons. 1979. The growth of Treponema hyodysenteriae and other porcine intestinal spirochaetes in a liquid medium. Res. Vet. Sci. 26:315-319. [PubMed] [Google Scholar]
- 17.Molnar, L. 1996. Sensitivity of strains of Serpulina hyodysenteriae isolated in Hungary to chemotherapeutic drugs. Vet. Rec. 138:158-160. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. 1997. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 4th ed. Approved standard M11-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 19.National Committee for Clinical Laboratory Standards. 1999. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. Approved standard M31-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 21.Odland, B. A., M. E. Erwin, and R. N. Jones. 2000. Quality control guidelines for disk diffusion and broth microdilution antimicrobial susceptibility tests with seven drugs for veterinary applications. J. Clin. Microbiol. 38:453-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts, M. C., J. Sutcliffe, P. Courvalin, L. B. Jensen, J. Rood, and H. Seppala. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 43:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rønne, H., and J. Szancer. 1990. In vitro susceptibility of Danish field isolates of Treponema hyodysenteriae to chemotherapeutics in swine dysentery (SD) therapy. Interpretation of MIC results based on the pharmacokinetic properties of the antibacterial agents, p. 1126. In Proceedings of the 11th International Pig Veterinary Society Congress. Swiss Association of Swine Medicine, Berne, Switzerland.
- 24.Shibl, A. M., and C. G. Gemmell. 1983. Effect of four antibiotics on haemolysin production and adherence to human uroepithelial cells by Escherichia coli. J. Med. Microbiol. 16:341-349. [DOI] [PubMed] [Google Scholar]
- 25.Shibl, A. M., M. A. Ramadan, and A. F. Tawfik. 1994. Differential inhibition by clindamycin on slime formation, adherence to Teflon catheters and hemolysin production by Staphylococcus epidermidis. J. Chemother. 6:107-110. [DOI] [PubMed] [Google Scholar]
- 26.Smith, S. C., T. Muir, M. Holmes, and P. J. Coloe. 1991. In vitro antimicrobial susceptibility of Australian isolates of Treponema hyodysenteriae. Aust. Vet. J. 68:408-409. [DOI] [PubMed] [Google Scholar]
- 27.Stanton, T. B., E. Fournie-Amazouz, D. Postic, D. J. Trott, P. A. Grimont, G. Baranton, D. J. Hampson, and I. Saint Girons. 1997. Recognition of two new species of intestinal spirochetes: Serpulina intermedia sp. nov. and Serpulina murdochii sp. nov. Int. J. Syst. Bacteriol. 47:1007-1012. [DOI] [PubMed] [Google Scholar]
- 28.Stanton, T. B., and D. F. Lebo. 1988. Treponema hyodysenteriae growth under various culture conditions. Vet. Microbiol. 18:177-190. [DOI] [PubMed] [Google Scholar]
- 29.Trott, D. J., T. B. Stanton, N. S. Jensen, G. E. Duhamel, J. L. Johnson, and D. J. Hampson. 1996. Serpulina pilosicoli sp. nov., the agent of porcine intestinal spirochetosis. Int. J. Syst. Bacteriol. 46:206-215. [DOI] [PubMed] [Google Scholar]
- 30.Vester, B., and S. Douthwaite. 2001. Macrolide resistance conferred by base substitutions in 23S rRNA. Antimicrob. Agents Chemother. 45:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weisblum, B. 1995. Erythromycin resistance by ribosome modification. Antimicrob. Agents Chemother. 39:577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]