Abstract
A 58-year-old man with an acute stroke suffered from splenic rupture. Streptococcus bovis was found in blood cultures, and gram-negative cocci were found in the infarcted spleen. Hemorrhagic transformation of the stroke occurred. Echocardiography showed aortic endocarditis. Cardiac surgery was not performed because of concern about cerebral bleeding. The patient died due to cerebral rehemorrhage after 3 weeks.
Splenic rupture in cases of ischemic stroke may be due to trauma, hematological disorder, malignancy, vasculitis, or systemic infection. Splenic rupture may also be caused by splenic infarction due to embolism. Cases of splenic rupture and stroke that are both due to embolism from infective endocarditis, as in the following report, have not been described previously.
Case report.
A 58-year-old man was hospitalized, because of an acute stroke in the supply area of the right middle cerebral artery with left-sided hemiparesis, 20 h after the onset of symptoms. For the previous 10 months, the patient had suffered from recurrent pharyngitis and tympanitic effusions. Eight weeks before admission, sinusitis ethmoidalis with fever had occurred and was treated with oral amoxicillin at 1,000 mg/day for 1 week. Despite antibiotic therapy, subfebrile temperatures, malaise, and night sweats persisted. At that time, laboratory tests revealed a blood sedimentation rate of 80 mm/h, a leukocyte count of 8.9/nl, an erythrocyte count of 3.67/pl, a hemoglobin level of 121 g/liter, a hematocrit of 0.34, and microhematuria. Five weeks before admission, the patient complained of sudden-onset dyspnea and fatigue. A chest X ray and computed tomography of the lung, performed 3 days before admission, showed infiltrates in both lungs, which were interpreted as pneumonia. The patient's history revealed that he had suffered from pleuritis and pulmonary embolism after cholecystectomy 22 years before and from arterial hypertension for the past 3 years. He smoked 20 cigarettes/day. He was on regular medication with terazosin, fosinopril, and hydro-chlorothiazide.
At admission, clinical neurologic examination showed left-sided central facial palsy, weakness of the left upper limb (Medical Research Council grade 1) and left lower limb (Medical Research Council grade 0), and left-sided hemihypesthesia. Clinical cardiological examination revealed pulmonary rales, a systolic murmur along the left sternal border extending to the carotid arteries, and pretibial edema. Blood pressure was 130/60 mm Hg, body temperature was 38°C, and body weight was 90 kg. The electrocardiogram was normal except for sinus tachycardia of 118/min. The leukocyte count was 8.7/nl (normal counts, 6.0 to 9.0/nl), the erythrocyte count was 3.15/pl (normal counts, 4.2 to 5.5/pl), the hemoglobin level was 93 g/liter (normal levels, 136 to 172 g/liter), the hematocrit was 0.27 (normal, 0.4 to 0.5), the C-reactive protein level was 115 mg/liter (normal, ≤6 mg/liter), the sodium level was 128 mmol/liter (normal, 135 to 150 mmol/liter), the serum iron concentration was 2.15 μmol/liter (normal, 11 to 29 μmol/liter), transferrin saturation was 0.04 (normal, 0.16 to 0.46), the γ-glutamyl transpeptidase level was 155 U/liter (normal, 6 to 28 U/liter), the alkaline phosphatase level was 384 U/liter (normal, 60 to 170 U/liter), the cholinesterase level was 2,765 U/liter (normal, 3,500 to 8,500 U/liter), the albumin level was 0.55 (normal, 0.58 to 0.70), the gamma globulin level was 0.23 (normal, 0.10 to 0.19), and the blood sedimentation rate was 68 mm/h (normal, <20 mm/h). Cerebral computed tomography showed a diffuse hypodense lesion in the posterior supply area of the right middle cerebral artery (Fig. 1).
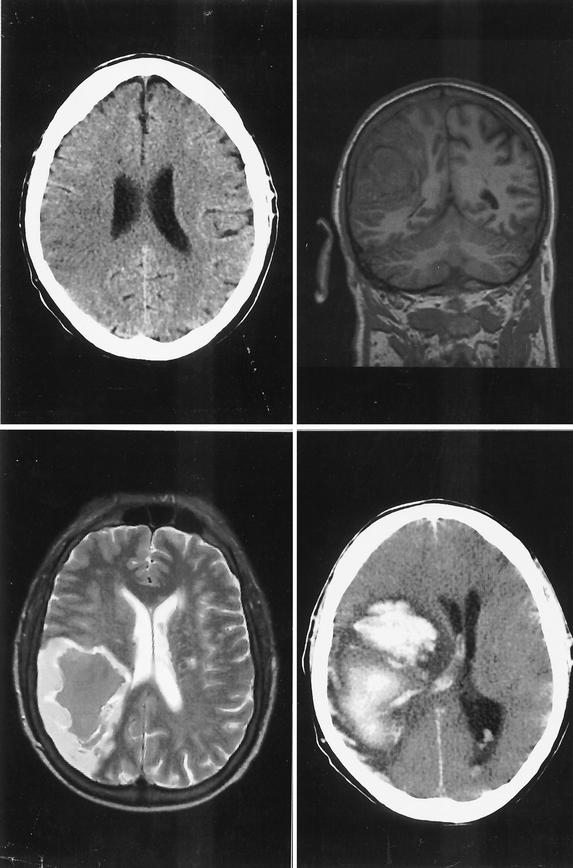
FIG. 1.
(Upper left) Cerebral computed tomography 21 h after onset of symptoms shows a hypodense area with loss of differentiation between gray and white matter in the posterior supply area of the right middle cerebral artery. (Upper right and lower left) Seven days later, a coronal T1-weighted (upper right) and an axial T2-weighted (lower left) (where T stands for relaxation time) cerebral magnetic resonance image show hemorrhagic transformation of the ischemic area. (Lower right) Thirteen days later, cerebral computed tomography shows a space-occupying hemorrhage with blood in the ventricles.
The patient received pentoxifylline (800 mg/day), acetylsalicylic acid (100 mg/day), and low-molecular-weight heparin (10,000 IU/day). After two blood cultures had been taken, levofloxacin (500 mg/day), amoxicillin (6 g/day), and clavulanic acid (600 mg/day) were started. The patient received two packs of red blood cells. Because the patient became confused and hypotensive and developed bloody diarrhea during the following 2 days, he was transferred to the intensive care unit 60 h after admission. Emergency transthoracic echocardiography showed small cardiac cavities, suggesting hypovolemia. The cardiac valves were not adequately visualized in the emergency situation. Because of a simultaneous fall of the hematocrit to 0.16, acute hemorrhage was suspected. Abdominal ultrasound and computed tomography showed blood within the peritoneal cavity. The patient underwent emergency laparatomy 3 days after admission, at which time a splenic rupture was found. Splenectomy was performed. Postoperatively, the heparin dose was reduced to 5,000 IU/day, and metronidazole (1,500 mg/day) was added to the antibiotic therapy regimen. The patient was extubated on the 1st postoperative day. The postoperative course was complicated by recurrent pulmonary edema, interpreted as due to hypertension. The two blood cultures were positive for Streptococcus bovis. No other organism was isolated from the blood cultures. The strain was sensitive to penicillin, aminopenicillin, amoxicillin, cefazolin, erythromycin, clindamycin, and vancomycin and was resistant to tobramycin, tetracycline, and levofloxacin. On the 5th postoperative day, a single generalized tonic-clonic seizure occurred, followed by respiratory insufficiency. The patient had to be reintubated and mechanically ventilated. Secondary hemorrhage in the area of the recent ischemic stroke was found upon cerebral magnetic resonance imaging (Fig. 1). On the 6th postoperative day, a high blood pressure amplitude of 180/40 mm Hg led to the suspicion of aortic insufficiency. Transthoracic and transesophageal echocardiography showed mobile vegetations on the aortic cusps and severe aortic insufficiency (Fig. 2). Aortic endocarditis was diagnosed on the basis of clinical, echocardiographic, blood chemistry, and bacteriological findings. Histological examination of the resected spleen revealed a splenic infarct with a destroyed arterial wall and intravascular fibrin thrombi, containing gram-positive cocci consistent with S. bovis and surrounding inflammatory cellular infiltrates with neutrophilic granulocytes (Fig. 3). Acute cardiac surgery was considered but was refused at the time because of concern about further cerebral bleeding and was planned for in 5 weeks. The further course was complicated by pneumonia. Repeated blood and sputum cultures did not show growth of any bacteria. Colonoscopy, performed to look for an entry portal of S. bovis, revealed an ulcus of the rectal mucosa, sigmoid diverticula, and a colonic polyp at 25 cm. Twenty-one days after the operation, the pupils widened acutely and became areactive bilaterally. A computed tomography scan of the brain showed a new massive hemorrhage (Fig. 1). The patient died on the next day. The autopsy confirmed the diagnosis of aortic valve endocarditis.
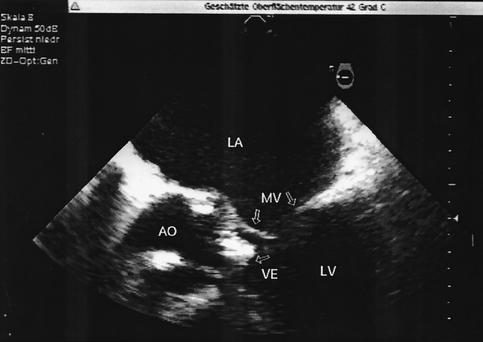
FIG. 2.
Transesophageal echocardiogram 6 days after splenectomy shows thickened aortic cusps (AO) with a mobile vegetation (VE) within the left ventricular outflow tract. LA, left atrium; LV, left ventricle; MV, mitral valve.
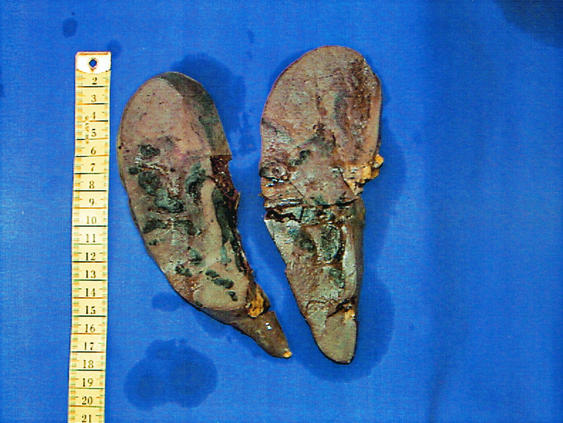
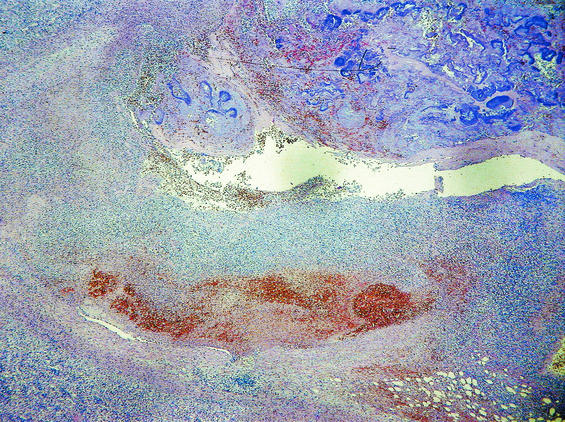
FIG.3.
(Top) Pathological specimen of the resected spleen shows a large infarct and multiple hemorrhagic areas. (Bottom) Histological examination shows a splenic infarct with a destroyed arterial wall and intravascular fibrin thrombi, containing bacteria and surrounding inflammatory cellular infiltrates with neutrophilic granulocytes.
The stroke and splenic infarction were most probably due to embolization of infectious material from the aortic cusps during the period of untreated infection. Because S. bovis bacteremia is often associated with bowel pathology, the most probable means of infection was migration of S. bovis, a member of the human gut flora in 10 to 16% of healthy people, through an intestinal lesion into the bloodstream (10). The colonic polyp, the ulcus in the rectal mucosa, or the diverticula may have served as an entry portal. S. bovis can persist for years in the human body, despite antibiotic therapy (10). This organism, which is known to occur more often in patients without preexisting cardiac pathologies than in those with pathologies, finally affected the aortic valve, destroyed the cusps, and led to aortic insufficiency (1, 5, 12). Generally, high rates of valve destruction, embolic episodes, and neurological complications are reported for patients with S. bovis endocarditis (1, 5, 6). The hemorrhagic transformation in both organs was most probably due to induction of pyogenic arterial wall necrosis and mycotic aneurysms by S. bovis (3, 9). Possibly the antithrombotic therapy with acetylsalicylic acid and heparin enhanced the propensity to bleeding (4). Ischemic and hemorrhagic strokes occur in 25 to 35% of patients with endocarditis and are clustered within the period of untreated infection (2, 7, 8). Secondary intracerebral hemorrhage has been identified as a predictor of mortality in patients with endocarditis and neurological deficits (2). Accordingly, repeated bleeding into the ischemic stroke area with consequent irreversible brain damage was considered responsible for the fatal outcome for our patient.
S. bovis endocarditis is a severe illness because of the frequent involvement of multiple valves and the frequent occurrence of hemodynamically relevant valvular insufficiency, necessitating cardiac surgery for 70 to 73% of patients (1, 5). Whether surgery would have changed the fatal course in our patient remains speculative. Patients with recent strokes undergoing cardiac surgery pose a difficult management problem. There is always the risk that cardiopulmonary bypass and heparinization may cause the neurological condition to deteriorate (11). For our patient, the situation was even more difficult because of hemorrhagic transformation of the ischemic infarcts (11). In the case presented, however, embolic complications might have been prevented, and a more favorable outcome might have been achieved, if endocarditis had been diagnosed earlier and if infection had been treated appropriately. Typically, only embolic events lead to hospitalization and diagnosis (7, 8).
We conclude that splenic rupture in a stroke patient should always lead to a search for infective endocarditis as the common pathomechanism. Endocarditis should be considered as an alternative diagnosis if treatment of subfebrile chronic infections does not lead to recovery. Even previous pulmonary disease and pathological findings upon chest X rays should not exclude endocarditis.
REFERENCES
- 1.Ballet, M., G. Gevigney, J. P. Garé, F. Delahaye, J. Etienne, and J. P. Delahaye. 1995. Infective endocarditis due to Streptococcus bovis. A report of 53 cases. Eur. Heart J. 16:1975-1980. [DOI] [PubMed] [Google Scholar]
- 2.Bitsch, A., R. Nau, R. A. Hilgers, R. Verheggen, G. Werner, and H. W. Prange. 1996. Focal neurologic deficits in infective endocarditis and other septic diseases. Acta Neurol. Scand. 94:279-286. [DOI] [PubMed] [Google Scholar]
- 3.Corbi, P., H. Manic, E. Donal, F. Roblot, J. P. Richer, D. Coisne, and P. Menu. 1999. Mycotic aneurysm of the splenic artery. A rare complication of surgically treated infectious endocarditis at the same time as the causal cardiac lesion. Arch. Mal. Coeur Vaiss. 92:1221-1224. [PubMed] [Google Scholar]
- 4.Delahaye, J. P., P. Poncet, V. Malquarti, J. Beaune, J. P. Gare, and J. M. Mann. 1990. Cerebrovascular accidents in infective endocarditis: role of anticoagulation. Eur. Heart J. 11:1074-1078. [DOI] [PubMed] [Google Scholar]
- 5.Duval, X., V. Papastamopoulos, P. Longuet, C. Benoit, C. Perronne, C. Leport, and J. L. Vilde. 2001. Definite Streptococcus bovis endocarditis: characteristics in 20 patients. Clin. Microbiol. Infect. 7:3-10. [DOI] [PubMed] [Google Scholar]
- 6.Gergaud, J. M., J. P. Breux, P. Roblot, R. Gil, and B. Becq-Giraudon. 1995. Complications neurologiques de l'endocardite infectieuse. Ann. Med. Interne (Paris) 146:413-418. [PubMed] [Google Scholar]
- 7.Hart, R. G., J. W. Foster, M. F. Luther, and M. C. Kanter. 1990. Stroke in infective endocarditis. Stroke 21:695-700. [DOI] [PubMed] [Google Scholar]
- 8.Heiro, M., J. Nikoskelainen, E. Engblom., E. Kotilainen, R. Marttila, and P. Kotilainen. 2000. Neurologic manifestations of infective endocarditis. A 17-year experience in a teaching hospital in Finland. Arch. Intern. Med. 160:2781-2787. [DOI] [PubMed] [Google Scholar]
- 9.Krapf, H., M. Skalej, and K. Voigt. 1999. Subarachnoid hemorrhage due to septic embolic infarction in infective endocarditis. Cerebrovasc. Dis. 9:182-184. [DOI] [PubMed] [Google Scholar]
- 10.Mühlemann, K., S. Graf, and M. G. Täuber. 1999. Streptococcus bovis clone causing two episodes of endocarditis 8 years apart. J. Clin. Microbiol. 37:862-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parrino, P. E., I. L. Kron, S. D. Ross, K. S. Shockey, A. M. Kron, M. A. Towler, and C. G. Tribble. 1999. Does a focal neurologic deficit contraindicate operation in a patient with endocarditis? Ann. Thorac. Surg. 67:59-64. [DOI] [PubMed] [Google Scholar]
- 12.Selton-Suty, C., B. Hoen, F. Delahaye, F. Lacassin, V. Goulet, J. Etienne, S. Briançon, and C. Leport. 1996. Comparison of infective endocarditis in patients with and without previously recognized heart disease. Am. J. Cardiol. 77:1134-1137. [DOI] [PubMed] [Google Scholar]