Abstract
Respiratory syncytial virus (RSV) is an important childhood pathogen of acute lower respiratory infections in developed and developing countries. The molecular epidemiology of RSV in India is largely unknown. The present study was undertaken to standardize and evaluate reverse transcription-PCR (RT-PCR) for the rapid and simultaneous detection of RSV groups A and B in clinical samples and to study intragroup genetic variability. RT-PCR was evaluated by comparing the results of seminested RT-PCR with centrifugation-enhanced cultures on 200 nasopharyngeal aspirates from children with acute lower respiratory infections. RSV was isolated in 34 nasopharyngeal aspirates by centrifugation-enhanced cultures and identified in 45 samples by RT-PCR. In 15 samples RSV was identified by seminested RT-PCR alone and in four by centrifugation-enhanced cultures alone. Of the 45 samples positive for RSV by nested PCR, 15 belonged to group A, 29 to group B, and one sample suggested a mixed infection. Group B RSV predominated in both years of the 2-year study. Genetic variability within RSV groups was studied by restriction fragment analysis of 35 PCR products. Among both group A and group B RSV, two different composite patterns were observed. Thus, RSV was found to be a major pathogen of acute lower respiratory tract infections in India, as it was detected in 24.5% of children by RT-PCR. RT-PCR provides a sensitive method for detection and typing of RSV group A and B viruses in clinical samples as well as a means to study intragroup variations. However, a higher sensitivity of detection of RSV in clinical samples can be obtained by its combination with additional techniques, such as virus cultivation.
Respiratory syncytial virus (RSV) is one of the major respiratory tract viral pathogens throughout the world, causing acute lower respiratory infections among infants and young children (7). Two major antigenic groups of RSV, A and B, have been identified based on monoclonal antibodies to the major structural glycoproteins G and F (1, 20). Considerable antigenic and genetic differences exist in the attachment glycoprotein G between the two major groups (22). Group A and B RSV may show temporal and geographical clustering, though cocirculation in a single epidemic is often observed (2, 10, 26). The clinical impact of these two groups appears to be relatively similar, but infection with RSV group A may produce disease with slightly greater severity than infection with group B RSV (18).
Reinfections due to RSV occur throughout life (24, 25), reflecting incomplete immunity to the virus. Children initially infected with a group A RSV are relatively protected against group A infection, and reinfections are more likely to be due to the heterologous group B RSV (19). In addition to major group differences, extensive G protein variations are seen within each group of viruses (4, 24). This intragroup diversity may contribute to the ability of RSV to cause repeated infections. Hospital-based studies from developing countries have documented the importance of RSV as a frequent cause of acute lower respiratory infections, and it is responsible for 27 to 96% of hospitalized cases of acute lower respiratory infections (29). Data on genetic variability among RSV isolates from developing countries are, however, limited.
In India, RSV has been identified as an important cause of lower respiratory tract infections (17 to 32%) in the pediatric age group (12, 15), but there are no reports from India on the molecular epidemiology of RSV. The ability to differentiate RSV into groups and subgroups is useful for epidemiological purposes as well as to assess the role of genetic variations in reinfections. The current study was designed to detect and type RSV by molecular techniques and to assess the contribution of RSV to hospitalizations due to lower respiratory tract infections (pneumonia) in our setting.
MATERIALS AND METHODS
Nasopharyngeal aspirates were collected from 200 children attending the Department of Pediatrics at All India Institute of Medical Sciences with signs and symptoms of acute lower respiratory infection (11). Study subjects included children of either sex from 7 months to 60 months of age.
Virus stocks.
Standard strains of RSV group A (A2 strain) and group B (8/60 strain) were grown in HEp-2 cells and titrated by 50% tissue culture infectious dose assays. These stocks were used for standardization of RT-PCR, centrifugation-enhanced cultures, and positive controls in both methods. Uninfected HEp-2 cells were used as a negative control.
Isolation of RSV from nasopharyngeal aspirates with centrifugation-enhanced culture.
Virus isolation by centrifugation-enhanced culture on HEp-2 cells was carried out on all 200 clinical specimens. The nasopharyngeal aspirates were inoculated in duplicate onto 24-well tissue culture plates with coverslips (12 mm) containing monolayer of HEp-2 cells. Plates were centrifuged at 1,000 rpm for 1 h and incubated at 33°C in a 5% CO2 atmosphere. At 48 h postinoculation, the viral pathogen was identified by immunofluorescent staining with a blend of monoclonal antibodies to the fusion (F) and attachment (G) proteins (Chemicon Inc.) of RSV. This method has been described earlier by our laboratory; the samples characterized in the previous publication were analyzed by PCR in this investigation (15).
Viral RNA extraction.
To standardize the RT-PCR and to determine the sensitivity, tissue culture-grown standard strains (8/60 and A2) of RSV of a known 50% tissue culture infectious dose were taken, and serial log dilutions were made from 10−1 to 10−8. Viral RNA was extracted from 400 μl of tissue culture lysate or clinical samples (nasopharyngeal aspirates) by the guanidinium isothiocyanate method (3) followed by ethanol precipitation. Four units of RNasin (Promega Corp.) and 10 μg (1 μg/μl) of glycogen (Sigma Chemicals) were added to samples prior to RNA extraction. The RNA pellet was vacuum dried and suspended in 10 μl of 0.1% diethyl pyrocarbonate-treated water and used for cDNA synthesis.
Oligonucleotides.
The oligonucleotides for external PCR and seminested PCR amplification were selected from conserved regions of the G and F protein genes based on primers described by Sullender et al. (24) as well as published sequences. Primer F164 was used as the antisense primer for both external and seminested PCR. The G32 primer was used to amplify both the genogroups in external PCR, and G267 and G399 were used as internal primers for genogroups A and B, respectively (16). The sequence, location, and specificity of primers were as follows: F164(AS)164-186 (F gene), 5′-GTT ATG ACA CTG GTA TAC CAA CC-3′, group A and B; G32(S)10-30 (G gene), 5′-GCA ACC ATG TCC AAA CAC AAG-3′, group A and B; G267(S)247-267 (G gene), 5′-GAT GCA ACA AGC CAG ATC AAG-3′, group A; and G399(S)378-399 (G gene), 5′-AAT ACA AAA TCA GAA ACA CAC C-3′, group B.
cDNA synthesis and PCR amplification.
Reverse transcription was performed by taking 2.5 μl of viral RNA in a final volume of 20 μl. The reaction mixture contained RT buffer, 200 U of Superscript RNase H reverse transcriptase (Gibco BRL, Life Technologies), 200 mM deoxynucleoside triphosphate mix, and 200 ng of random primer per μl (PdN6). The reaction mixture was incubated for 90 min at 37°C, followed by 5 min of enzyme inactivation at 95°C.
External PCR amplification was performed by taking 10 μl of cDNA in a final volume of 50 μl. The reaction volume contained 50 pmol each of primers F164 and G32, 200 mM deoxynucleoside triphosphate mix, 2 mM MgCl2, and 2.5 U of Taq polymerase (Gibco BRL, Life Technologies). The reaction was carried out for 32 cycles of amplification (denaturation at 94°C for 1 min, annealing at 55°C for 1.5 min, extension at 72°C for 1.5 min, followed by final extension for 7 min). The amplified product of ≈1.1 kb was visualized on a 1% agarose gel stained with ethidium bromide.
Seminested PCR.
The diluted external PCR products were used for seminested PCR. Fifty microliters of the final volume of PCR mix contained 2.5 U of Taq polymerase (Gibco BRL, Life Technologies), 1.5 mM MgCl2, 200 mM deoxynucleoside triphosphate mix, and 50 pmol of primer G267 for group A or G399 for group B and antisense primer F164. Amplification for 28 cycles was done with the same PCR profile as used for external PCR. The amplified products of the group A and B RSVs were ≈0.9 kb and ≈0.78 kb in length, respectively.
Restriction endonuclease digestion.
The seminested PCR amplicons were purified with a Nanosep microconcentrator (Pall Filtron) and subjected to restriction endonuclease digestion with RsaI, PstI, HincII, and AluI enzymes (21). Approximately 0.5 to 1.0 μg of DNA was subjected to digestion, and the fragments were analyzed by electrophoresis in 2.5% agarose gels. Within each virus group, the restriction patterns were assigned letter and number designations to facilitate comparisons among the viruses studied. The first letter of the designation represents the restriction enzyme, the second letter represents the group of RSV, and the number represents the pattern number.
RESULTS
Sensitivity and specificity of RT-PCR.
The RT-PCR was first standardized with RNA obtained from tissue culture-grown RSV group A (A2) and group B (8/60) viruses. A 1.1-kb band was obtained with both groups with primers F164 and G32. The specificity of primers was determined by using heterologous RNA from influenza virus A and parainfluenza viruses 1, 2, and 3 as well as nucleic acid from uninfected HEp-2 cells. No amplicons were obtained with the heterologous templates. The detection limit of external PCR for both group A and B RSV was 0.1 TCID50.
Detection and typing of A and B strains of RSV by RT-PCR and seminested PCR.
RSV was detected in 21 out of 200 nasopharyngeal aspirates by external PCR. However, by seminested RT-PCR, 45 out of 200 nasopharyngeal aspirates were positive for RSV (Fig. 1). Of these 45, 15 were typed as group A and 29 as group B. In one sample, a presumed dual infection was detected, as both the 0.9- and 0.78-kb bands were visualized and the results were confirmed thrice by repeated testing.
FIG. 1.
(A) Agarose gel showing the results of external RT-PCR on clinical samples (nasopharyngeal aspirates). lane M, φX174 HaeIII digest (molecular weight marker); lane 1, negative control; lane 2, positive control; lanes 3 to 7, clinical samples. A band of 1.1 kb is observed in lanes 2, 3, 5, and 7. (B) Agarose gel showing the results of seminested PCR on clinical samples lane M, φX174 HaeIII digest (molecular weight marker); lane 1, negative control; lane 2, group A positive control; lane 3, group B positive control; lanes 3 to 9, clinical samples. A band of 0.9 kb of group A RSV is seen in lanes 2, 8, and 9, and a band of 0.78 kb of group B RSV is seen in lanes 3, 5 and 6. No bands were seen in lanes 4, 7, and 10.
Comparison of seminested PCR with centrifugation-enhanced culture.
RSV was detected in 34 of 200 nasopharyngeal aspirate samples by centrifugation-enhanced culture followed by indirect immunofluorescence (15). On comparison of seminested PCR with centrifugation-enhanced culture in clinical samples, it was found that the results of 30 samples were concordant, whereas discordance was observed in 19 samples. In 15 samples which were positive only by seminested RT-PCR, a repeat seminested PCR confirmed the results. Four samples were negative by seminested PCR, but virus was isolated by centrifugation-enhanced culture (Table 1). With centrifugation-enhanced culture and seminested PCR, RSV could be detected in 49 of 200 nasopharyngeal aspirates from children with acute lower respiratory infections; of these, 34 (70%) were from children less than 1 year of age.
TABLE 1.
Comparison of RT-PCR and centrifugation-enhanced culture (CEC) results
| CEC result | No. of samples giving RT-PCR result:
|
||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 30 | 4 | 34 |
| Negative | 15 | 151 | 166 |
| Total | 45 | 155 | 200 |
Intratypic variations within RSV groups by restriction endonuclease digestion.
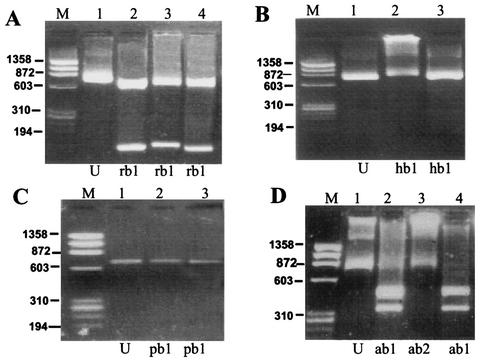
A total of 35 RSV strains, (10 group A and 25 group B) were studied. Examples of the restriction fragment patterns from the clinical and prototype samples are provided (Fig. 2 and 3). The group A strains yielded two distinct patterns after digestion with RsaI (ra1 and ra2), two patterns with PstI (pa1 and pa2), and one pattern with HincII (ha1) (Table 2). Group B yielded a single pattern (rb1) after digestion with RsaI which was similar to the standard strain and two patterns with AluI (ab1 and ab2), i.e., some strains showed digestion, whereas others remained uncut. All group B RSV strains remained uncut with PstI and HincII (pb1 and hb1, respectively) (Table 3). Overall, restriction fragment analysis revealed two distinct composite patterns with group A and group B RSV strains (Fig. 4). Of 10 group A strains, nine were similar to the standard strain of group A (A2), while one had a distinct pattern. Ten out of 25 group B strains had a pattern similar to that of the group B standard strain (8/60), while the remaining 15 had a different pattern.
FIG. 2.
Agarose gel showing restriction enzyme pattern of group A RSV with RsaI, HincII, and PstI enzymes. Panel A (digestion with RsaI): lane M, φX174 HaeIII digest (molecular weight marker); lanes 1 and 2, 0.9-kb uncut amplicon and digested amplicon of positive control of group A, respectively, lanes 3 to 6, digested amplicons of group A sample strains. The ra1 pattern was seen in lanes 2 to 5, and the ra2 pattern was seen in lane 6. Panel B (digestion with HincII): lane M, φX174 HaeIII digest (molecular weight marker); lanes 1 and 2, 0.9-kb uncut amplicon and digested amplicon of positive control of group A, respectively; lanes 3 to 5, digested amplicons of group A sample strains. The ha1 pattern was seen in lanes 2 to 5. Panel C (digestion with PstI): lane M, φX174 HaeIII digest (molecular weight marker); lanes 1 and 2, 0.9-kb uncut amplicon and digested amplicon of positive control of group A, respectively; lanes 3 to 6, digested amplicons of group A sample strains. The pa1 pattern was seen in lanes 2 to 5, and the pa2 pattern was seen in lane 6.
FIG. 3.
Agarose gel showing restriction enzyme pattern of group B RSV with RsaI, HincII, PstI, and AluI. Panel A (digestion with RsaI): lane M, φX174 HaeIII digest (molecular weight marker); lanes 1 and 2, 0.78-kb uncut amplicon and digested amplicon of positive control of group B, respectively; lanes 3 and 4, digested amplicons of group B sample strains. The rb1 pattern was seen in lanes 2 to 4. Panel B (digestion with HincII): lane M, φX174 HaeIII digest (molecular weight marker); lanes 1 and 2, 0.78-kb uncut amplicon and digested amplicon of positive control of group B, respectively; lane 3, digested amplicons of group B sample strain. The hb1 pattern (uncut) was seen in lanes 2 and 3. Panel C (digestion with PstI): lane M, φX174 HaeIII digest (molecular weight marker); lanes 1 and 2, 0.78-kb uncut amplicon and digested amplicon of positive control of group B, respectively; lane 3, digested amplicons of group B sample strain. The pb1 pattern (uncut) was seen in lanes 2 and 3. Panel D (digestion with AluI): lane M, φX174 HaeIII digest (molecular weight marker), lanes 1 and 2, 0.78-kb uncut amplicon and digested amplicon of positive control of group B, respectively; lanes 3 and 4, digested amplicons of group B sample strains. The ab1 pattern was seen in lanes 2 and 4, and the ab2 (uncut) pattern was seen in lane 3.
TABLE 2.
Restriction enzyme analysis of group A RSV strains
| Strain(s) | Restriction pattern
|
Composite patterns | Pattern | ||
|---|---|---|---|---|---|
| RsaI | PstI | HincII | |||
| A2 | ra1 | pa1 | ha1 | ra1pa1ha1 | 1A |
| 90, 107, 121, 127, 146, 170, 171, 186, 210, 220 (9 isolates) | ra1 | pa1 | ha1 | ra1pa1ha1 | 1A |
| 221 (1 isolate) | ra2 | pa2 | ha1 | ra2pa2ha1 | 2A |
TABLE 3.
Restriction enzyme analysis of group B RSV strains
| Strain | Restriction pattern
|
Composite patterns | Pattern | |||
|---|---|---|---|---|---|---|
| RsaI | AluI | PstI | HincII | |||
| 8/60 | rb1 | ab1 | pb1 | hb1 | rb1ab1pb1hb1 | 1B |
| 51, 57, 63, 77, 102, 106, 139, 187, 199, 217 (10 isolates) | rb1 | ab1 | pb1 | hb1 | rb1ab1pb1hb1 | 1B |
| 53, 69, 96, 109, 130, 134, 137, 151, 160, 183, 201, 206, 215, 231 (15 isolates) | rb1 | ab2 | pb1 | hb1 | rb1ab2pb1hb1 | 2B |
FIG. 4.
Schematic diagram of restriction enzyme patterns of amplicons of seminested PCR. Lane M, øX174 molecular weight markers; ua, undigested amplicon of group A RSV; ra1, restriction endonuclease pattern of group A strains with RsaI; ra2, restriction endonuclease pattern of group A strains with RsaI; ha1, restriction endonuclease pattern of group A strains with HincII; pa1, restriction endonuclease pattern of group A strains with PstI; pa2, restriction endonuclease pattern of group A strains with PstI; ub, undigested amplicon of group B RSV; rb1, restriction endonuclease pattern of group B strains with RsaI; hb1, restriction endonuclease pattern of group B strains with HincII; pb1, restriction endonuclease pattern of group B strains with PstI; ab1, restriction endonuclease pattern of group B strains with AluI; ab2, restriction endonuclease pattern of group B strains with AluI.
DISCUSSION
RSV is the major cause of respiratory infections among children in India (12, 15). Information on the genetic heterogeneity of RSV from developing countries is, however, extremely limited. In this study, genetic variability was characterized among RSV strains identified from children with acute lower respiratory infections in a large referral hospital in India over a 2-year period. RSV were identified and grouped into the major antigenic groups A and B by a seminested PCR protocol, and intragroup variations were studied by restriction fragment analysis with RT-PCR-amplified products of the G protein gene.
Direct immunofluorescence immunoassay is a rapid technique for identification of respiratory viruses, but it is less sensitive than culture, and negative specimens may need to be confirmed by culture. Centrifugation-enhanced culture is more rapid and sensitive than standard culture (13, 17). RT-PCR provides a sensitive tool for both detection and typing of RSV into groups (6, 8). In addition, PCR products can be further characterized to study RSV molecular epidemiology. The primers F164 and G 32 (24) were used as external primers to amplify RSV strains. In a previous study, the G32 primer was found to be specific for group B RSV (24). In this application for direct detection of viruses in clinical samples, we sought increased sensitivity. Differences from the earlier assay included lowering of the annealing temperature and the inclusion of a random hexamer primer in reverse transcription. However, under these conditions the G32 primer could amplify both group A and B RSV, yielding a product of 1.1 kb. Therefore we used a seminested approach with the specific primers for group A (G267) (24) and B (G399) (16).
RSV was detected in 30 samples by both centrifugation-enhanced culture and RT-PCR; in 15 samples virus was detected by RT-PCR alone; and in four samples centrifugation-enhanced culture detected virus but RT-PCR was negative. The samples which were RT-PCR positive and culture negative might reflect a false-positive result from RT-PCR testing. However, earlier studies with RT-PCR on clinical samples have suggested that RT-PCR may be more sensitive than culture for detection of virus in clinical samples (5, 28). Seminested PCR failed to detect viral RNA in four samples that were positive by centrifugation-enhanced culture. These false-negative results might have occurred for a variety of reasons, including viral genetic variability, degradation of template RNA, or inhibitory substances in the samples (21). A combination of the two techniques was of value in increasing the sensitivity of detection.
Both group A and B RSV were found during the 2-year study period, with a predominance of group B strains. Most of the other studies on prevalence of group A and B RSV have shown predominance of group A viruses (8, 9). Recent studies from developing countries have begun to define RSV disease burden and epidemiology. In Mozambique, as in this study, group B viruses were found to have a higher prevalence than group A viruses (23). In a rural district hospital in Mozambique, RSV was identified in 8.6% of children presenting to the outpatient department with cough or nasal secretions and 10.6% of children admitted to the hospital with lower respiratory infection (14). Involvement of the lower respiratory tract (59.7%) and hospital admission (18.1%) occurred more often than described in developed countries.
When RSV from rural South African clinics were compared to those from hospitalized patients in Soweto, most viruses were not significantly different between the two locations (28). In Gambia, the incidence rate per 100 infants for acute lower respiratory infections was 9.6 cases per year and 0.83 for severe RSV-associated illness. RSV accounted for 19% of all hospital admissions for acute lower respiratory infections (30). The identification of RSV as the etiologic agent of 24.5% of acute lower respiratory infections in our study exceeds that reported above for Mozambique and the Gambia. This may reflect the heightened sensitivity provided by a combination of culture and molecular detection techniques compared to antigen detection by enzyme-linked immunosorbent assay or immunofluorescence in the other studies.
With the screening technique of restriction fragment analysis, genetic heterogeneity was observed among both group A and B RSV. Two composite patterns were observed in both groups. Earlier studies reported less genetic variability among group B viruses compared to group A RSV (8, 24). However, we observed less variability among the group A viruses than described earlier (1, 2, 4) The restriction fragment analysis employed here was used as a screening tool for genetic variability and is not expected to reveal the full extent of genetic differences. Nucleotide sequence analysis of these samples should provide precise determination of the molecular differences among these viruses.
A detailed understanding of the epidemiology of RSV in India will require prospective, longitudinal, and community- and hospital-based investigations combined with more detailed genetic analyses of the circulating viruses. These approaches will provide insight into the role of viral genetic variability in reinfections. This is the first study from the Indian subcontinent on the use of RT-PCR on clinical samples for detection and typing of RSV. This is also the first report on genetic heterogeneity among group A and B RSV from the Indian subcontinent. In this study, we demonstrate that RT-PCR appears to be more sensitive than centrifugation-enhanced cultures for detection of RSV in clinical samples. Future studies of RSV epidemiology in health care settings and in the community will lay a foundation for efforts to define and ultimately reduce the burden of disease due to RSV in India.
Acknowledgments
This work was supported through the Indo-U.S. Vaccine Action Program (grant no. BT/MB/VAP/3/18/98) by the Department of Biotechnology, Govt. of India, and University Grants Commission, India, and by U.S. Public Health Service National Institutes of Health grant AI 50693.
REFERENCES
- 1.Anderson, L. J., J. C. Hierholzer, C. Tsou, R. M. Hendry, B. F. Fernie, Y. Stone, et al. 1985. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J. Infect. Dis. 151:626-633. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, L. J., R. M. Hendry, L. T. Pierik, C. Tsou, and K. McIntosh. 1991. Multicentre study of strains of respiratory syncytial virus. J. Infect. Dis. 163:687-692. [DOI] [PubMed] [Google Scholar]
- 3.Chomczynski, P., and N. Sacchi. 1987. Singe step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 4.Coggins, W. B., E. J. Lefkowitz, and W. M. Sullender. 1998. Genetic variability among group A and group B respiratory viruses in children hospitals. J. Clin. Microbiol. 12:3552-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falsey, A. R., M. A. Formica, and E. E. Walsh. 2002. Diagnosis of respiratory syncytial virus infection: comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J. Clin. Microbiol. 40:817-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghildyal, R., G. Hogg, and J. Meanger. 1997. Detection and sub grouping of respiratory syncytial virus directly from nasopharyngeal aspirates. Clin. Microbiol. Infect. 3:120-123. [DOI] [PubMed] [Google Scholar]
- 7.Glezen, W. P., and F. W. Denny. 1973. Epidemiology of acute lower respiratory diseases in children. N. Engl. J. Med. 288:498-505. [DOI] [PubMed] [Google Scholar]
- 8.Gottschalk, J., R. Zbinden, L. Kaempf, and I. Heinze. 1996. Discrimination of respiratory syncytial virus subgroups A and B by reverse transcription-PCR. J. Clin. Microbiol. 34:41-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall, C. B., E. E. Walsh, K. C. Schnabel, C. E. Long, K. M. McConnochie, S. W. Hildreth, et al. 1990. Occurrence of groups A and B of respiratory syncytial virus over 15 years: associated epidemiological and clinical characteristics in hospitalized and ambulatory children. J. Infect. Dis. 162:1283-1290. [DOI] [PubMed] [Google Scholar]
- 10.Hendry, R. M., A. L. Tails, E. Godfrey, L. J. Anderson, B. F. Fernie, and K. McIntosh. 1986. Concurrent circulation of antigenically distinct strains of respiratory syncytial virus during community outbreaks. J. Infect. Dis. 153:291-297. [DOI] [PubMed] [Google Scholar]
- 11.Hijazi, Z., A. Pacsa, S. Eisa, A. E. Shazil, and R. A. El-Salam. 1996. Laboratory diagnosis of acute lower respiratory tract viral infections in children. J. Trop. Pediatr. 42: 276-280. [DOI] [PubMed] [Google Scholar]
- 12.John, J. T., T. Cherian, M. C. Steinhoff, E. A. F. Simoes, and M. John. 1991. Etiology of acute respiratory infection in children in tropical southern India. Rev. Infect. Dis. 13(Suppl. 6):S463-S469. [DOI] [PubMed] [Google Scholar]
- 13.Ling, A. E., and S. Doraisingham. 1988. Comparison of tube cultures of Madin Darby canine kidney cells with shell-vial cultures after low-speed centrifugation for influenza virus isolation. Pathology 20:346-348. [DOI] [PubMed] [Google Scholar]
- 14.Loscertales M-P., A. Roca, P. J. Ventura, F. Abacassamo, F. Dos Santos, M. Sitaube, C. Menendez, B. M. Greenwood, J. C. Saiz, and P. L. Alonso. 2002. Epidemiology and clinical presentation of respiratory syncytial virus infection in a rural area of southern Mozambique. Pediatr. Infect. Dis. J. 21:148-155. [DOI] [PubMed] [Google Scholar]
- 15.Maitreyi, R. S., S. Broor, S. K. Kabra, M. Ghosh, P. Seth, L. Dar, and A. K. Prasad. 2001. Rapid detection of respiratory viruses by centrifugation-enhanced cultures from children with acute lower respiratory tract infections. J. Clin. Virol. 16:41-47. [DOI] [PubMed] [Google Scholar]
- 16.Maitreyi, R. S., S. Broor, M. Ghosh, S. K. Kabra, M. Singh, L. Dar, P. Seth, and A. K. Prasad. 1999. Rapid detection methods for diagnosis of acute lower respiratory tract infections due to respiratory syncytial virus. Ind. J. Med. Microbiol. 17:10-13. [Google Scholar]
- 17.Matthey, S., D. Nicholson, S. Ruhs, B. Alden, M. Knock, K. Schultz, and A. Schmuecker. 1992. Rapid detection of respiratory viruses by shell vial cultures and direct staining by using pooled and individual monoclonal antibodies. J. Clin. Microbiol. 30:540-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McConnochie, K. M., C. B. Hall, E. E. Walsh, and K. T. Roghmann. 1990. Variation in severity of respiratory syncytial virus infections with subtype. J. Pediatr. 117:52-62. [DOI] [PubMed] [Google Scholar]
- 19.Mufson, M. A., B. Belshe, C. Orvell, and E. Norrby. 1987. Subgroup characteristics of respiratory syncytial virus strains recovered from children with two consecutive infections. J. Clin. Microbiol. 25:1535-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mufson, M. A., C. Orvel, B. Rafnar, and E. Norrby. 1985. Two distinct subtypes of human respiratory syncytial virus. J. Gen. Virol. 66:2111-2124. [DOI] [PubMed] [Google Scholar]
- 21.Paton, A. W., J. C. Paton, A. J. Lawrence, P. N. Goldwater, and R. J. Harris. 1992. Rapid detection of respiratory syncytial virus on nasopharyngeal aspirates by reverse transcription-polymerase chain reaction amplification. J. Clin. Microbiol. 30:901-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reese, P. E., and X. Marchette. 1991. Respiratory syncytial virus infection and prevalence of subgroup A and B in Hawaii. J. Clin. Microbiol. 29:2614-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roca, A., M. P. Loscertales, L. Quinto, P. Perez-Brena, and N. Vaz. 2001. Genetic variability among group A and B respiratory syncytial viruses in Mozambique: identification of a new cluster of group B isolates. J. Gen. Virol. 82:103-111. [DOI] [PubMed] [Google Scholar]
- 24.Sullender, W. M., L. Sun, and L. J. Anderson. 1993. Analysis of respiratory syncytial virus genetic variability with amplified cDNAs. J. Clin. Microbiol. 31:1224-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullender, W. M., L. J. Anderson, M. A. Mufson, and G. W. Wertz. 1991. Genetic diversity of the attachment protein of subgroup B respiratory syncytial viruses. J. Virol. 65:5425-5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullender, W. M. 2000. Respiratory syncytial virus genetic and antigenic diversity. Clin. Microbiol. Rev. 13:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Elden, L. J. R., M. G. J. van Kraaji, M. Nijhuis, K. A. W. Hendriksen, A. W. Dekker, M. Rozenberg-Arska, and A. M. van Loon. 2002. Polymerase chain reaction is more sensitive than viral culture and antigen testing for the detection of respiratory viruses in adults with hematological cancer and pneumonia. Clin. Infect. Dis. 34:177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venter, M., M. Collinson, and B. D. Schoub. 2002. Molecular epidemiological analysis of community circulating respiratory syncytial virus in rural South Africa: comparison of viruses and genotypes responsible for different disease manifestations. J. Med Virol. 68:452-461. [DOI] [PubMed] [Google Scholar]
- 29.Weber, M. W., E. Kim Mulholland, and B. M. Greenwood. 1998. Respiratory syncytial virus infection in tropical and developing countries. Trop. Med. Int. Health 3:268-280. [DOI] [PubMed] [Google Scholar]
- 30.Weber, M. W., P. Milligan, M. Sanneh, A. Awemoyi, R. Dakour, G. Schneider, A. Palmer, M. Jallow, A. Oparaogu, H. Whittle, E. K. Mulholland, and B. M. Greenwood. 2002. An epidemiological study of RSV infection in the Gambia. Bull. W.H.O. 80:562-568. [PMC free article] [PubMed] [Google Scholar]