Abstract
Porcine reproductive and respiratory syndrome has been devastating the swine industry since the late 1980s. The disease has been controlled, to some extent, through the use of modified live-attenuated (MLV) vaccines once available. However, such a practice periodically resulted in isolation or detection of vaccine-like viruses from pigs as determined by a partial genomic sequencing. In this study, we developed a heteroduplex mobility assay (HMA) for quickly identifying porcine reproductive and respiratory syndrome virus (PRRSV) isolates with significant nucleotide sequence identities (≥98%) with the modified live-attenuated vaccines. The major envelope gene (ORF5) of 51 PRRSV field isolates recovered before and after the introduction of the vaccines was amplified, denatured, and reannealed with the HMA reference vaccine strains Ingelvac PRRS MLV and Ingelvac PRRS ATP, respectively. Nine of the 51 field isolates and the VR2332 parent virus of Ingelvac PRRS MLV, which were all highly related to Ingelvac PRRS MLV with ≤2% nucleotide sequence divergence as determined by sequence analysis, were all identified by the HMA to form homoduplexes with the reference Ingelvac PRRS MLV. No homoduplex-forming field isolate was identified when Ingelvac PRRS ATP was used as the HMA reference except for its parent virus JA142. Other field isolates with more than 2% nucleotide sequence divergence with the respective reference vaccine strain resulted in the formation of heteroduplexes with reduced mobility in polyacrylamide gel electrophoresis. The HMA results also correlated well with the results of phylogenetic analyses. The data indicated that the HMA developed in the study may be a rapid and efficient method for large-scale screening of potential vaccine-like PRRSV field isolates for further genetic characterization.
Porcine reproductive and respiratory syndrome, often characterized by late-term abortions and stillbirths in sows and respiratory disease in nursery pigs, has resulted in extensive economic losses in the swine industry for over a decade (19). By estimation, approximate annual losses of $236 per inventoried adult female pig are due to acute porcine reproductive and respiratory syndrome outbreaks (32).
Porcine reproductive and respiratory syndrome virus (PRRSV), the causative agent of porcine reproductive and respiratory syndrome (PRRS), is a small, enveloped, positive-stranded RNA virus consisting of eight overlapping open reading frames (ORFs) (22, 26). The virus is genetically, antigenically, and pathogenically heterogenic (19). Substantial sequence divergence exists between the European and North American genotypes of the virus, showing only about 70% nucleotide sequence identity (1, 11, 14-19, 23-26). Among the North American isolates, the PRRSV genomic sequences also vary significantly (10, 16, 17, 25).
The heterogeneity of the virus has made it difficult to design effective vaccines based on a single PRRSV strain (19). Modified live-attenuated vaccines (MLV) are currently used for the protection against PRRS mainly by providing protection against clinical disease (20, 31). These modified live-attenuated vaccines, such as Ingelvac PRRS MLV, have reduced the incidence and severity of PRRS outbreaks on many farms. A severe form of PRRS, designated acute or atypical PRRS, has recently been reported in the midwestern United States. Many of these acute outbreaks occurred in PRRSV MLV-vaccinated herds, suggesting that the commonly used modified live-attenuated vaccines are not fully effective. The occurrence of the acute syndrome in vaccinated pigs resulted in the recent introduction of another MLV, Ingelvac PRRS ATP, to the market in February 2000.
Other concerns about the modified live-attenuated vaccines pertain to their safety. In Danish swine herds, Ingelvac PRRS MLV vaccine virus has been shown to be capable of reverting to a pathogenic phenotype (5, 29, 33). Additionally, Mengeling et al. (21) confirmed that numerous vaccine-like field isolates, which contained the same restriction site marker that is found in the Ingelvac PRRS MLV vaccine virus, were capable of causing disease more severe than any clinical signs induced by the MLV. The restriction site marker was not identified in any isolates collected prior to the introduction of the Ingelvac PRRS MLV vaccine except for the parent strain ATCC VR2332. Other vaccine-like strains have also been reported (11, 19, 30) and some of these isolates were shown to be mildly to moderately pathogenic in pigs (30).
Due to the widespread use of Ingelvac PRRS MLV and the periodic identification of vaccine-like isolates, there is a demand for a rapid assay that can be used routinely for identifying and differentiating these vaccine-like isolates from field isolates of PRRSV. The current methods for differentiating PRRSV isolates include PCR amplification, subsequent sequencing and sequence analyses, or PCR-restriction fragment length polymorphism (35). Because these assays are costly and time-consuming, they are not very well suited for routine large-scale screening of viruses. The heteroduplex mobility assay (HMA) is a rapid and inexpensive method of differentiating viral isolates. Delwart et al. (8) originally developed the assay for genetic typing of human immunodeficiency virus. More recently, HMA techniques have been applied to the study of other viruses such as influenza virus (38), feline immunodeficiency virus (2), measles virus (12), poliovirus (7), Newcastle disease virus (4), and hepatitis C virus (9, 36). The assay relies on the formation of mismatched base pairs when two closely related DNA molecules are combined, denatured, and reannealed. The mismatches cause structural distortions in the newly formed DNA molecule, resulting in heteroduplexes with reduced mobility on a polyacrylamide gel. It has been shown that the reduction in mobility is proportional to the degree of divergence between the two sequences (8).
In this study, we report the development of an HMA and its use in the identification of PRRSV field isolates with ≥98% nucleotide sequence identities with the modified live-attenuated vaccines.
MATERIALS AND METHODS
Viruses.
A total of 51 field isolates of PRRSV were analyzed by sequence and phylogenetic analyses and by HMA analyses using two reference strains, Ingelvac PRRS MLV and Ingelvac PRRS ATP. Ingelvac PRRS MLV was introduced to the swine industry in early 1995, and Ingelvac PRRS ATP was introduced to the swine industry in February 2000 for use in the PRRS vaccination program. Twenty of the PRRSV isolates collected during PRRS outbreaks prior to the introduction of Ingelvac PRRS MLV were obtained from Prem S. Paul's former laboratory at Iowa State University and from National Veterinary Service Laboratories in Ames, Iowa (Table 1). Fourteen PRRSV isolates were obtained from National Veterinary Service Laboratories in Ames, Iowa, from pigs in herds experiencing acute PRRS outbreaks in Iowa and North Carolina between 1997 and 1998 (Table 1). Fifteen PRRSV isolates were obtained from Iowa State University's Veterinary Diagnostic Laboratory (Table 1), and some were determined by sequencing to be vaccine-like isolates. The two parent strains to the vaccines, ATCC VR2332 (Ingelvac PRRS MLV) and JA142 (Ingelvac PRRS ATP), were also included in this study.
TABLE 1.
Correlation between nucleotide sequence identity of PRRSV isolates and HMA results when Ingelvac PRRS MLV and Ingelvac PRRS ATP vaccines were used as reference strains
| Isolate no. | Origina | Ingelvac PRRS MLV
|
Ingelvac PRRS ATP
|
||||
|---|---|---|---|---|---|---|---|
| Nucleotide sequence identity (%) | HMA resultb | After vaccine introduced | Nucleotide sequence identity (%) | HMA result | After vaccine introduced | ||
| Ingelvac PRRS MLV | BIVI | 100 | + | 89 | − | N | |
| VR2332 | BIVI | 99 | + | N | 90 | − | N |
| 27011-01 | ISUVDL | 99 | + | Y | 89 | − | N |
| 44010-01 | ISUVDL | 99 | + | Y | 90 | − | N |
| 47450-01 | ISUVDL | 99 | + | Y | 90 | − | N |
| 16727-01 | ISUVDL | 99 | + | Y | 89 | − | N |
| 18571A-01 | ISUVDL | 99 | + | Y | 89 | − | N |
| 93-41462 | NVSL | 99 | + | N | 89 | − | N |
| 19139-01 | ISUVDL | 98 | + | Y | 89 | − | N |
| 21373A-01 | ISUVDL | 98 | + | Y | 89 | − | N |
| 18087B-01 | ISUVDL | 98 | + | Y | 89 | − | N |
| 30603-22-01 | ISUVDL | 97 | − | Y | 88 | − | N |
| 12711-3-01 | ISUVDL | 97 | − | Y | 89 | − | N |
| ISU22 | ISUPSP | 97 | − | N | 89 | − | N |
| PDV9502 | NVSL | 97 | − | N | 91 | − | N |
| 98-37120-2 | ∗NVSL | 97 | − | Y | 89 | − | N |
| 15102 44.43 | ISUVDL | 96 | − | Y | 88 | − | N |
| ISU1894 | ISUPSP | 96 | − | N | 89 | − | N |
| 93-4506 | NVSL | 96 | − | N | 89 | − | N |
| 98-27687 | ∗NVSL | 96 | − | Y | 89 | − | N |
| 35019-01 | ISUVDL | 95 | − | Y | 88 | − | N |
| 18070-01 | ISUVDL | 95 | − | Y | 87 | − | N |
| ISU79 | ISUPSP | 94 | − | N | 88 | − | N |
| ISU30262 | ISUPSP | 93 | − | N | 90 | − | N |
| 89-47361 | NVSL | 93 | − | N | 90 | − | N |
| 89-46489 | NVSL | 93 | − | N | 90 | − | N |
| 89-47363 | NVSL | 93 | − | N | 90 | − | N |
| ISU28 | ISUPSP | 92 | − | N | 90 | − | N |
| 93-30352 | NVSL | 92 | − | N | 92 | − | N |
| 1765-2-01 | ISUVDL | 91 | − | Y | 88 | − | N |
| ISU 3927 | ISUPSP | 91 | − | N | 91 | − | N |
| ISU33 | ISUPSP | 91 | − | N | 88 | − | N |
| 92-1205 | NVSL | 91 | − | N | 87 | − | N |
| 93-36048 | NVSL | 91 | − | N | 89 | − | N |
| 93-45010 | NVSL | 91 | − | N | 91 | − | N |
| ISU55 | ISUPSP | 91 | − | N | 89 | − | N |
| ISU4 | ISUPSP | 91 | − | N | 91 | − | N |
| 98-21995-1 | ∗NVSL | 90 | − | Y | 95 | − | N |
| 98-31701 | ∗NVSL | 90 | − | Y | 91 | − | N |
| 97-27796-4 | ∗NVSL | 90 | − | Y | 96 | − | N |
| JA142 | BIVI | 90 | − | Y | 99 | + | N |
| Ingelvac PRRS ATP | BIVI | 89 | − | Y | 100 | + | |
| 4519-01 | ISUVDL | 89 | − | Y | 88 | − | N |
| 97-27796-2 | ∗NVSL | 89 | − | Y | 90 | − | N |
| 98-5579 | ∗NVSL | 89 | − | Y | 91 | − | N |
| 98-4236 | ∗NVSL | 89 | − | Y | 90 | − | N |
| VR2385 | ISUPSP | 89 | − | N | 89 | − | N |
| 98-13795-3 | ∗NVSL | 88 | − | Y | 90 | − | N |
| 98-6470 | ∗NVSL | 88 | − | Y | 89 | − | N |
| 98-13795-1 | ∗NVSL | 88 | − | Y | 89 | − | N |
| 98-13795-5 | ∗NVSL | 88 | − | Y | 90 | − | N |
| 98-13795-10 | ∗NVSL | 88 | − | Y | 90 | − | N |
| 98-13795-13 | ∗NVSL | 88 | − | Y | 90 | − | N |
BIVI, Boehringer Ingelheim Vetmedica, Inc. Ames, Iowa; ISUVDL, Iowa State University's Veterinary Diagnostic Laboratory, Ames, Iowa; ISUPSP, Prem S. Paul (while he was at Iowa State University, Ames, Iowa); NVSL, National Veterinary Services Laboratories, Ames, Iowa (acute PRRS isolates indicated with ∗).
+, homoduplex; −, heteroduplex.
Primer design.
Two degenerate primers were designed to amplify the heterogenic open reading frame (ORF) 5 of various PRRSV isolates. The primers were constructed based on a multiple sequence alignment of the ORF5 of 29 PRRSV isolates available in the GenBank database (National Center for Biotechnology Information, National Library of Medicine, Bethesda, Md.). The sense primer AssayU (5′-GTATGTTGGRGAAATGCTTGACC-3′) began 2 bp upstream of the ORF5 start codon and includes the first 21 bp of the ORF5. The antisense primer AssayL (5′-GGACGACCCCATTGTTCCGCTG-3′) consists of the 22 bp immediately preceding the last four bases at the 3′ end of the ORF5 gene. The primers were synthesized and purified commercially (Gibco-BRL, Gaithersburg, Md.). The expected PCR product was 601 bp.
RNA extraction and RT-PCR.
RNA was extracted with Trizol reagents (Gibco-BRL) from 200 μl of each of the 51 PRRSV isolates and the two MLV reference strains. The total RNA was resuspended in 11.5 μl of DNase-free, RNase-free, and proteinase-free water. Reverse transcription was performed at 42°C for 60 min in the presence of a master mix consisting of 11.5 μl of total RNA, 0.25 μl of Superscript II reverse transcriptase, 1 μl of 10 μM antisense primer, 0.5 μl of RNase inhibitor, 1 μl of 0.1 M dithiothreitol, 4 μl of 5× RT buffer, and 1 μl of 10 mM deoxynucleoside triphosphates. The resulting cDNA was amplified by PCR with the ORF5 sense and antisense primers and AmpliTaq Gold DNA polymerase (Perkin-Elmer, Shelton, Conn.). The PCR parameters were 95°C for 9 min, followed by 39 cycles of 94°C for 1 min for denaturation, 50°C for 1 min for annealing, and 72°C for 1.5 min for extension, followed by a final incubation period at 72°C for 7 min. The PCR products were examined on an agarose gel.
Sequencing and phylogenetic analyses.
The PCR products were excised from an agarose gel and purified using the glassmilk procedure with a Geneclean kit (Bio101, Inc., Carlsbad, Calif.). The PCR products were sequenced at the Virginia Tech DNA Sequencing Facility with an automated DNA sequencer (Applied Biosystems, Inc., Foster City, Calif.). The ORF5 genes of the 15 PRRSV isolates from Iowa State University's Veterinary Diagnostic Laboratory were sequenced at the Iowa State University Nucleic Acid Facility as part of diagnostic investigation, and the procedure for sequencing and sequence analysis has been described elsewhere (6).
The ORF5 sequences of the two vaccines and their parent strains and 11 field isolates of PRRSV have been reported previously (11, 15-18, 23-25). The sequences of all isolates were analyzed by using the MacVector program (Oxford Molecular, Inc., Oxford, England).
The ORF5 genes of the 51 field isolates and the reference strains were phylogenetically analyzed by using the PAUP program (David L. Swofford, Smithsonian Institute, Washington, D.C., distributed by Sinauer Associates, Inc., Sunderland, Mass.) to create a consensus tree. Lelystad virus (22) was also included as a representative of the European genotype of PRRSV in the phylogenetic analysis.
HMA.
Five microliters of the PCR product from each reference virus was combined with 5 μl of PCR product from each of the 51 PRRSV field isolates and 1.1 μl of annealing buffer (1 M NaCl, 100 mM Tris [pH 7.8], 20 mM EDTA) in a 0.2-ml thin-walled polypropylene tube (Perkin-Elmer, Shelton, Conn.). The mixture was denatured at 95°C for 5 min and reannealed at 50°C for 30 min in a thermocycler. Heteroduplexes and homoduplexes were separated on an 8.0% nondenaturing polyacrylamide gel at 150 V for 5 h in Tris-borate-EDTA buffer. The gels were stained with ethidium bromide and visualized under UV light. Homoduplexes were identified as a single band, while more than one band with reduced mobility was classified as a heteroduplex.
Nucleotide sequence accession numbers.
The ORF5 sequences of the 38 PRRSV isolates sequenced in this study were deposited in the GenBank database under accession numbers AF537919 to AF537956.
RESULTS
Sequence analysis.
The ORF5 genes of all 51 PRRSV isolates and the two MLV reference strains were successfully amplified with the degenerate primers AssayU and AssayL. The amplified ORF5 regions of the isolates were sequenced. The sequences were compared to determine the percent nucleotide sequence identities with the corresponding sequence of each reference strain. Sequence analyses revealed that the field isolates showed 88 to 99% identity with Ingelvac PRRS MLV and 87 to 96% identity with the Ingelvac PRRS ATP (Table 1). In general, the mismatches seemed to occur throughout the ORF5 in both reference strains. The isolates ISU4 and ISU 3927 contained a three-nucleotide deletion at nucleotide positions 76 to 78 compared to both reference strains. Ingelvac PRRS MLV and Ingelvac PRRS ATP showed 99% nucleotide sequence identity with their respective parent strains, VR2332 and JA142. Ingelvac PRRS MLV and VR2332 differed by two nucleotides, while Ingelvac PRRS ATP and JA142 differed by five nucleotides in the amplified region.
HMA with Ingelvac PRRS MLV as the reference.
When Ingelvac PRRS MLV was used as the reference for the HMA, 10 of the 51 PRRSV isolates formed a homoduplex and the remaining ones formed heteroduplexes (Table 1). The 10 homoduplex-forming isolates displayed 98 to 99% nucleotide sequence identity with Ingelvac PRRS MLV. Eight of the 10 homoduplex-forming isolates were isolated after the introduction of the Ingelvac PRRS MLV vaccine to the swine industry. The two remaining homoduplex-forming isolates included isolate VR2332, which is the parent strain of Ingelvac PRRS MLV, and isolate 93-41462, which was isolated from outbreaks occurring prior to the introduction of Ingelvac PRRS MLV. The remaining isolates formed heteroduplexes with reduced mobility of variable degrees compared to the reference.
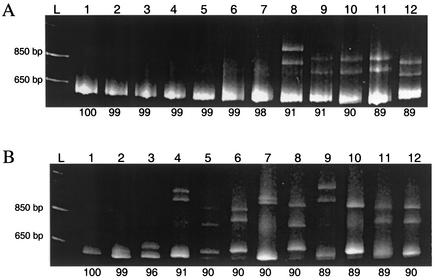
Based on surveys of the polyacrylamide gels of all of the isolates, the reduction in mobility is roughly proportional to the level of divergence between the sequences (data not shown). However, due to the presence of unpaired nucleotides at positions 76 to 78, the isolates ISU4 and ISU 3927 displayed an exaggerated reduction in mobility compared to the other isolates with the same 91% nucleotide sequence identity (Fig. 1A, lanes 8 and 9). This increased reduction in mobility due to deletions, which has been described in other HMA studies (8), was also observed in the HMA with the Ingelvac PRRS ATP vaccine as the reference.
FIG. 1.
(A) HMA results for selected PRRSV isolates when Ingelvac PRRS MLV vaccine was used as the reference. Lanes: L, 1-kb DNA ladder; lane 1, reference plus reference; lane 2, reference plus VR2332; lane 3, reference plus 16727-01; lane 4, reference plus 44010A-01; lane 5, reference plus 47450-01; lane 6, reference plus 27011-01; lane 7, reference plus 18087B-01; lane 8, reference plus ISU3927; lane 9, reference plus ISU55; lane 10, reference plus JA142; lane 11, reference plus Ingelvac PRRS ATP; lane 12, reference plus 4519-01. Percent identity of nucleotide sequence between the reference and the PRRSV isolates is listed below each lane. (B) HMA results for selected PRRSV isolates when Ingelvac PRRS ATP vaccine was used as the reference. Lanes: L, 1-kb DNA ladder; lane 1, reference plus reference; lane 2, reference plus JA142; lane 3, reference plus 97-27796-4; lane 4, reference plus ISU3927; lane 5, reference plus ISU28; lane 6, reference plus ISU30262; lane 7, reference plus 98-4236; lane 8, reference plus 98-13795-3; lane 9, reference plus 93-36048; lane 10, reference plus 93-4506; lane 11, reference plus Ingelvac PRRS MLV; lane 12, reference plus VR2332. Percent identity of nucleotide sequence between the reference and the PRRSV isolates is listed below each lane.
HMA with Ingelvac PRRS ATP vaccine as the reference.
When the Ingelvac PRRS ATP vaccine strain was used as the reference, 50 of the 51 isolates formed a heteroduplex with the reference strain. The parent strain to the vaccine, JA142, formed a near homoduplex with the reference. The band formed between Ingelvac PRRS ATP and its parent strain JA142 migrated only slightly slower than the homoduplex formed when Ingelvac PRRS ATP was combined, denatured, and reannealed with itself (Fig. 1B, lanes 1 and 2). Sequence analysis demonstrated five mismatched base pairs between the amplified regions of the Ingelvac PRRS ATP vaccine and its parental strain JA142. These mismatches, occurring at positions 103, 169, 217, 273, and 341, were located towards the middle of the ORF5 region. The remaining heteroduplex-forming isolates demonstrated various degrees of reduced mobility, which appeared to correlate with the degree of divergence between the sequences. All of the isolates used in the study were isolated prior to the introduction of the Ingelvac PRRS ATP vaccine.
Phylogenetic analysis.
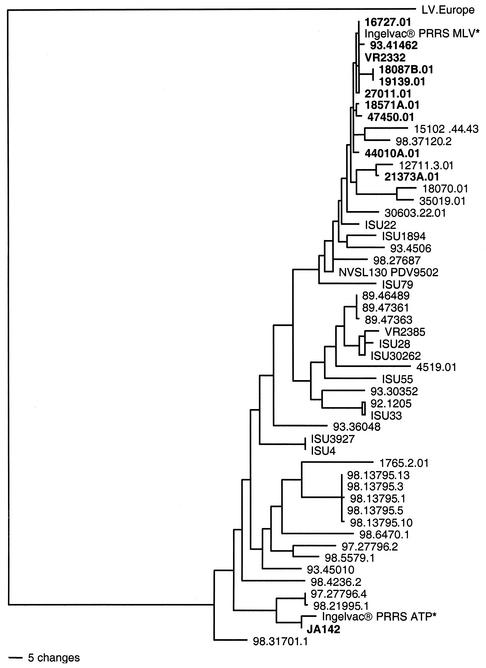
A phylogenetic tree was constructed based on the two reference strains Ingelvac PRRS MLV and Ingelvac PRRS ATP, the 51 PRRSV field isolates, including the two vaccine parental strains, and the European Lelystad virus. As illustrated in Fig. 2, the phylogram demonstrates the distinct European and North American genotypes among PRRSV isolates and that the isolates analyzed in this study all belong to the North American genotype. The phylogenetic analysis also demonstrates that the North American isolates formed several genotypic clusters, indicating the heterogeneity of the virus (Fig. 2, Table 1). All 10 isolates showing ≥98% nucleotide sequence identities and forming homoduplexes in the HMA with Ingelvac PRRS MLV clustered together in the phylogenetic tree with the Ingelvac PRRS MLV. Similarly, the JA142 strain forming a near homoduplex in the HMA and showing 99% sequence identity with Ingelvac PRRS ATP clustered together with Ingelvac PRRS ATP (Fig. 2).
FIG. 2.
Phylogenetic tree based on the ORF5 region of PRRSV isolates analyzed in the study: two MLV vaccines (Ingelvac PRRS MLV and Ingelvac PRRS ATP, designated with a *) used as the HMA references, 51 PRRSV field isolates analyzed in the study, including the parent strains of the two vaccines, VR2332 and JA142, and the European Lelystad virus (LV) as the outgroup. Homoduplex-forming isolates are shown in boldface. The tree was constructed with the PAUP program with a heuristic search option with midpoint rooting and 1,000 replicates.
DISCUSSION
Ingelvac PRRS MLV has been used as a tool for preventing and/or controlling PRRS since early 1995. Recently, numerous vaccine-like PRRSV isolates have been identified from nonsymptomatic persistently infected pigs and diseased animals. In 1996, Danish sow herds experienced clinical signs of PRRS after having been vaccinated in an extralabel fashion with Ingelvac PRRS MLV a few months prior. Through the use of monoclonal antibodies, the virus causing the outbreaks was found to be closely related to the North American phenotype. Further genetic studies confirmed that the isolates from Danish swine herds were of Ingelvac PRRS MLV vaccine origin, indicating that the vaccine virus is capable of spreading and possibly reverting to a pathogenic phenotype (5, 13, 28, 30, 33). Other North American field isolates of PRRSV have also been speculated to be vaccine related (11, 21) based on genetic analyses of the ORF5 genes, and some were found to cause mild to moderate disease in pigs (30).
In this study, a heteroduplex mobility assay was developed to rapidly screen field isolates of PRRSV for potential vaccine-like isolates. The HMA developed in this study uses the heterogenic ORF5 gene (6, 10, 19) as a focus for identifying the genetic divergence between the isolates. The ORF5 gene encodes the major envelope protein of PRRSV, which is exposed outside the virions and under immune selection pressure. Fifty-one PRRSV field isolates collected from various PRRS outbreaks were included in the study to evaluate the HMA. Sequence analyses revealed that nine of these isolates (93-41462, ISUVDL2001027011, ISUVDL2001044010, ISUVDL2001047450, ISUVDL2001016727, ISUVDL2001018571A, ISUVDL2001019139, ISUVDL2001021373A, and ISUVDL2001018087B) showed ≥98% nucleotide sequence identity with Ingelvac PRRS MLV. The VR2332 isolate with 99% sequence identity with the Ingelvac PRRS MLV is the parent strain of Ingelvac PRRS MLV vaccine. It has been shown that low levels of sequence divergence without insertions or deletions are generally associated with homoduplex formation in the HMA (3, 8). In this study, each of the nine field isolates with ≥98% sequence identity with the Ingelvac PRRS MLV and the VR2332 parent virus of the Ingelvac PRRS MLV formed a homoduplex in the HMA when combined, denatured, and reannealed with the reference strain Ingelvac PRRS MLV vaccine.
By analyzing the HMA results, the sequence data, and the time of virus isolation, we speculated on the origins of these homoduplex-forming isolates that showed ≥98% nucleotide sequence identities with the Ingelvac PRRS MLV. The isolates ISUVDL2001027011, ISUVDL2001044010, ISUVDL2001047450, ISUVDL2001016727, ISUVDL2001018571A, ISUVDL2001019139, ISUVDL2001021373A, and ISUVDL2001018087B were all isolated after the introduction of the Ingelvac PRRS MLV. Wesley et al. (35) showed that a glycine residue at position 151 of the ORF5 is rapidly substituted for an arginine upon replication in pigs, suggesting that arginine at position 151 could be a marker for cell culture adaptation of PRRSV.
Notably, five of the nine homoduplex-forming field isolates contained an arginine residue at position 151, suggesting that they may be of Ingelvac PRRS MLV vaccine origin. Two isolates (ISUVDL2001019139 and ISUVDL2001018087B) each had an isoleucine residue, one isolate (93-41462) had a valine residue, and one isolate (ISUVDL2001016727) retained the glycine residue at position 151. The other substitutions in the sequences tended to be located at the signal sequence (amino acid positions 1 to 31) and the hypervariable region (amino acid positions 32 to 39) near the N terminus. The isolate 93-41462, which was isolated prior to the use of the Ingelvac PRRS MLV and had 99% nucleotide sequence identity with both Ingelvac PRRS MLV and its parent virus VR2332, is more likely to be derived from the wild-type VR2332 instead of Ingelvac PRRS MLV.
The origins of the remaining three homoduplex-forming isolates (ISUVDL2001019139, ISUVDL2001018087B, and ISUVDL2001016727) are not known. Seven of the nine isolates with 96 to 97% nucleotide sequence identities with the Ingelvac PRRS MLV contained an arginine residue at position 151, and the remaining two isolates both contained a lysine residue. Due to the nature of rapid genetic changes of the virus in pigs (6, 37), it is logical to speculate that these isolates with more than 96% nucleotide sequence identities with Ingelvac PRRS MLV could be derived from Ingelvac PRRS MLV due to its widespread use in the vaccination program or from its parent virus, ATCC VR2332, which is likely still circulating in the pig population. However, a definitive conclusion as to the origins of these vaccine-like isolates cannot be drawn without determining the complete genomic sequence of these isolates.
Ingelvac PRRS ATP vaccine was introduced to the swine industry only in February 2000, and thus vaccine-like field isolates due to the spread of ATP vaccine occur less frequently. Except for isolate JA142, which is the parent virus of the Ingelvac PRRS ATP vaccine, all other field isolates showed less than 97% nucleotide sequence identity and formed heteroduplexes with the Ingelvac PRRS ATP vaccine reference. The isolate JA142 formed a near homoduplex with the reference strain. At 99% nucleotide sequence identity with the reference Ingelvac PRRS ATP vaccine, JA142 contained five centrally located mismatches with the vaccine virus. The significance of the location as well as the quantity of mismatches has been noted in previous HMA studies, which demonstrated that the clustering of mismatches toward the center of a fragment can have a more exaggerated effect on mobility reduction (27, 34). We speculate that concentration of the five mismatches toward the center of ORF5 explains the slight reduction in mobility of JA142 even at only 1% divergence with the reference vaccine virus. A similar phenomenon was not observed between Ingelvac PRRS MLV and its parent strain VR2332 because these isolates had only two mismatches located at the periphery of the ORF5 gene. The other isolates with 1% divergence also tended to have mismatches found mainly at the 5′ end of the ORF5.
The results of phylogenetic analyses of the 51 field isolates agreed well with both the sequence results and the HMA data. All the homoduplex-forming isolates with ≥98% sequence identity to the Ingelvac PRRS MLV vaccine are phylogenetically closely related to the Ingelvac PRRS MLV. The Ingelvac PRRS ATP vaccine and its parent strain JA142 are also clustered together in the phylogenetic tree. Genotypic relationships between some of the closely related PRRSV isolates were also clearly identifiable in this study. The group of acute PRRSV isolates, 98-13795-1, 98-13795-3, 98-13795-5, 98-13795-10, and 98-13795-13, were all isolated from the same herd outbreak in 1998, and they showed 99 to 100% nucleotide sequence identity with each other and clustered together in the phylogenetic tree.
The HMA assay developed in this study correlated well with both sequence and phylogenetic analysis results and correctly identified all field isolates with ≥98% nucleotide sequence identities with the modified live-attenuated vaccines. Therefore, the HMA developed in this study defines viruses of ≥98% nucleotide sequence identity with the modified live-attenuated vaccines. This HMA will be a useful and more efficient method for large-scale screening of potential vaccine-like isolates for further genetic characterization. With this assay, a field isolate of PRRSV within 2% sequence divergence in the ORF5 gene of the commonly used Ingelvac PRRS MLV vaccine can be readily identified. The assay is also an effective method for identifying closely related PRRSV isolates through the formation of similar or identical heteroduplex banding patterns when combined with any reference strain.
Acknowledgments
This work was supported in part by a grant from Boehringer Ingelheim Vetmedica, Inc., Ames, Iowa.
We thank Prem Paul of the University of Nebraska, Lincoln, and John Landgraf and Sabrina Swenson of the National Veterinary Services Laboratories, Ames, Iowa, for providing some of the PRRSV isolates.
REFERENCES
- 1.Allende, R., T. L. Lewis, Z. Lu, D. L. Rock, G. F. Kutish, A. Ali, A. R. Doster, and F. A. Osorio. 1999. North American and European porcine reproductive and respiratory syndrome viruses differ in non-structural protein coding regions. J. Gen. Virol. 80:307-315. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann, M. H., C. Mathiason-Dubard, G. H. Learn, A. G. Rodrigo, D. L. Sodora, P. Mazzetti, E. A. Hoover, and J. L. Mullins. 1997. Genetic diversity of feline immunodeficiency virus: dual infection, recombination, and distinct evolutionary rates among envelope sequence clades. J. Virol. 71:4241-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barlow, K. L., J. Green, and J. P. Clewley. 2000. Viral genome characterization by the heteroduplex mobility and heteroduplex tracking assays. Rev. Med. Virol. 10:321-335. [DOI] [PubMed] [Google Scholar]
- 4.Berinstein, A., H. S. Sellers, D. J. King, and B. S. Seal. 2001. Use of a heteroduplex mobility assay to detect differences in the fusion protein cleavage site coding sequence among Newcastle disease virus isolates. J. Clin. Microbiol. 39:3171-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botner, A., B. Strandbygaard, K. J. Sorensen, P. Have, K. G. Madsen, E. S. Madsen, and S. Alexandersen. 1997. Appearance of acute PRRS-like symptoms in sow herds after vaccination with a modified live PRRS vaccine. Vet. Rec. 141:497-499. [DOI] [PubMed] [Google Scholar]
- 6.Chang, C. C., K. J. Yoon, J. J. Zimmerman, K. M. Harmon, P. M. Dixon, C. M. Dvorak, and M. P. Murtaugh. 2002. Evolution of porcine reproductive and respiratory syndrome virus during sequential passages in pigs. J. Virol. 76:4750-4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chezzi, C., and B. D. Schoub. 1996. Differentiation between vaccine-related and wild-type polioviruses with a heteroduplex mobility assay. J. Virol. Methods 62:93-102. [DOI] [PubMed] [Google Scholar]
- 8.Delwart, E. L., E. G. Shpaer, J. Louwagie, F. E. McCutchan, M. Grez, H. Rubsamen-Waigmann, and J. I. Mullins. 1993. Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 env genes. Science 262:1257-1261. [DOI] [PubMed] [Google Scholar]
- 9.Gretch, D. R., S. J. Polyak, J. J. Wilson, R. L. Carithers, Jr., J. D. Perkins, and L. Corey. 1996. Tracking hepatitis C virus quasispecies major and minor variants in symptomatic and asymptomatic liver transplant recipients. J. Virol. 70:7622-7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapur, V., M. R. Elam, T. M. Pawlovich, and M. P. Murtaugh. 1996. Genetic variation in porcine reproductive and respiratory syndrome virus isolates in the midwestern United States. J. Gen. Virol. 77:1271-1276. [DOI] [PubMed] [Google Scholar]
- 11.Key, K. F., G. Haqshenas, D. K. Guenette, S. L. Swenson, T. E. Toth, and X. J. Meng. 2001. Genetic variation and phylogenetic analyses of the ORF5 gene of acute porcine reproductive and respiratory syndrome virus isolates. Vet. Microbiol. 83:249-263. [DOI] [PubMed] [Google Scholar]
- 12.Kreis, S., and T. Whistler. 1997. Rapid identification of measles virus strains by the heteroduplex mobility assay. Virus Res. 47:197-203. [DOI] [PubMed] [Google Scholar]
- 13.Madsen, K. G., C. M. Hansen, E. S. Madsen, B. Strandbygaard, A. Botner, and K. J. Sorensen. 1998. Sequence analysis of porcine reproductive and respiratory syndrome virus of the American type collected from Danish swine herds. Arch. Virol. 143:1683-1700. [DOI] [PubMed] [Google Scholar]
- 14.Mardassi, H., R. Athanassious, S. Mounir, and S. Dea. 1994. Porcine reproductive and respiratory syndrome virus: morphological, biochemical and serological characteristics of Quebec isolates associated with acute and chronic outbreaks of porcine reproductive and respiratory syndrome. Can. J. Vet. Res. 58:55-64. [PMC free article] [PubMed] [Google Scholar]
- 15.Meng, X. J., P. S. Paul, and P. G. Halbur. 1994. Molecular cloning and nucleotide sequencing of the 3′-terminal genomic RNA of the porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 75:1795-1801. [DOI] [PubMed] [Google Scholar]
- 16.Meng, X. J., P. S. Paul, P. G. Halbur, and M. A. Lum. 1995a. Phylogenetic analyses of the putative M (ORF 6) and N (ORF 7) genes of porcine reproductive and respiratory syndrome virus (PRRSV): implication for the existence of two genotypes of PRRSV in the USA and Europe. Arch. Virol. 140:745-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng, X. J., P. S. Paul, P. G. Halbur, and I. Morozov. 1995b. Sequence comparison of open reading frames 2 to 5 of low and high virulence United States isolates of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 76:3181-3188. [DOI] [PubMed] [Google Scholar]
- 18.Meng, X. J., P. S. Paul, I. Morozov, and P. G. Halbur. 1996. A nested set of six or seven subgenomic mRNAs is formed in cells infected with different isolates of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 77:1265-1270. [DOI] [PubMed] [Google Scholar]
- 19.Meng, X. J. 2000. Heterogeneity of porcine reproductive and respiratory syndrome virus: implications for current vaccine efficacy and future vaccine development. Vet. Microbiol. 74:309-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mengeling, W. L., K. M. Lager, and A. C. Vorwald. 1999. Safety and efficacy of vaccination of pregnant gilts against porcine reproductive and respiratory syndrome. Am. J. Vet. Res. 60:796-801. [PubMed] [Google Scholar]
- 21.Mengeling, W. L., A. C. Vorwald, K. M. Lager, D. F. Clouser, and R. D. Wesley. 1999. Identification and clinical assessment of suspected vaccine-related field strains of porcine reproductive and respiratory syndrome virus. Am. J Vet Res. 60:334-340. [PubMed] [Google Scholar]
- 22.Meulenberg, J. J., M. M. Hulst, E. J. De Meijer, P. L. Moonen, A. Den Besten, E. P. De Kluyver, G. Wensvoort, and R. J. Moormann. 1993. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology 192:62-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morozov, I., X. J. Meng, and P. S. Paul. 1995. Sequence analysis of open reading frames (ORFs) 2 to 4 of a U.S. isolate of porcine reproductive and respiratory syndrome virus. Arch. Virol. 140:1313-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murtaugh, M. P., M. R. Elam, and L. T. Kakach. 1995. Comparison of the structural protein coding sequences of the VR-2332 and Lelystad virus strains of the PRRS virus. Arch. Virol. 140:1451-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murtaugh, M. P., K. S. Faaberg, J. Laber, M. Elam, and V. Kapur. 1998. Genetic variation in the PRRS virus. Adv. Exp. Med. Biol. 440:787-794. [DOI] [PubMed] [Google Scholar]
- 26.Nelsen, C. J., M. P. Murtaugh, and K. S. Faaberg. 1999. Porcine reproductive and respiratory syndrome virus comparison: divergent evolution on two continents. J. Virol. 73:270-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson, J. A., S. A. Fiscus, and R. Swanstrom. 1997. Evolutionary variants of the human immunodeficiency virus type 1 V3 region characterized by with a heteroduplex tracking assay. J. Virol. 71:8750-8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen, H. S., M. B. Oleksiewicz, R. Forsberg, T. Stadejek, A. Botner, and T. Storgaard. 2001. Reversion of a live porcine reproductive and respiratory syndrome virus vaccine investigated by parallel mutations. J. Gen. Virol. 82:1263-1272. [DOI] [PubMed] [Google Scholar]
- 29.Oleksiewicz, M. B., A. Botner, K. G. Madsen, and T. Storgaard. 1998. Sensitive detection and typing of porcine reproductive and respiratory syndrome virus by RT-PCR amplification of whole viral genes. Vet. Microbiol. 64:7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Opriessnig, T., P. G. Halbur, K. J. Yoon, R. M. Pogranichniy, K. M. Harmon, R. Evans, K. F. Key, F. J. Pallares, P. Thomas, and X. J. Meng. 2002. Comparative pathogenicity of a modified live PRRSV vaccine (Ingelvac PRRS MLV), the parent strain of the vaccine (ATCC VR2332), ATCC VR2385, and two recent field isolates of PRRSV. J. Virol. 76:11837-11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osorio, F. A., F. Zuckermann, R. Wills, W. Meier, S. Christian, J. Galeota, and A. Doster. 1998. PRRSV: comparison of commercial vaccines in their ability to induce protection against current PRRSV strains of high virulence. 1998 Allen D. Leman Swine Conf. 25:176-182. [Google Scholar]
- 32.Polson, D. D., W. E. Marsh, and G. D. Dial. 1992. Financial evaluation and decision making in the swine breeding herd. Vet. Clin. North Am. Food Anim. Pract. 8:725-747. [DOI] [PubMed] [Google Scholar]
- 33.Storgaard, T., M. Oleksiewicz, and A. Botner. 1999. Examination of the selective pressures on a live PRRS vaccine virus. Arch. Virol. 144:2389-2401. [DOI] [PubMed] [Google Scholar]
- 34.Upchurch, D. A., R. Shankarappa, and J. I. Mullins. 2000. Position and degree of mismatches and the mobility of DNA heteroduplexes. Nucleic Acids Res. 28:E69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wesley, R. D., W. L. Mengeling, K. M. Lager, A. C. Vorwald, and M. B. Roof. 1999. Evidence for divergence of restriction fragment length polymorphism patterns following in vivo replication of porcine reproductive and respiratory syndrome virus. Am. J. Vet. Res. 60:463-467. [PubMed] [Google Scholar]
- 36.Wilson, J. J., S. J. Polyak, T. D. Day, and D. R. Gretch. 1995. Characterization of simple and complex hepatitis C virus quasispecies by heteroduplex gel shift analysis: correlation with nucleotide sequencing. J. Gen. Virol. 76:1763-1771. [DOI] [PubMed] [Google Scholar]
- 37.Yoon, K.-J., C.-C. Chang, J. J. Zimmerman, and K. M. Harmon. 2001. Genetic and antigenic stability of PRRS virus in persistently infected pigs: clinical and experimental prospective. Adv. Exp. Med. Biol. 494:25-30. [DOI] [PubMed] [Google Scholar]
- 38.Zou, S. 1997. A practical approach to genetic screening for influenza virus variants. J. Clin. Microbiol. 35:2623-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]