Abstract
The hemolytic ability, the presence of cyl genes, and the diagnostic accuracy of cytolysin molecular detection were investigated in the genus Enterococcus by using 164 strains from 20 different species (26 reference strains, 42 clinical isolates from human and veterinary origin, and 96 isolates from ewe cheese and milk). Hemolysis was assayed with sheep and horse erythrocytes and under aerobic or anaerobic conditions. Screening of cytolysin genes (cylLL, cylLS, cylM, cylB, and cylA) was performed with new specific primers and the anaerobic assay of beta-hemolysis was used as the “gold standard” for the evaluation of cyl gene-based PCRs. Since beta-hemolysis and cyl genes were found in 10 and 14 species, respectively, the hemolytic ability seems to be spread throughout the genus Enterococcus. Beta-hemolysis was observed in 6 of 26 (23%) reference strains, 14 of 42 (33%) clinical isolates, and 6 of 96 (6%) food isolates. The presence of cyl genes was detected in 15 of 26 (58%) reference strains, 37 of 42 (88%) clinical isolates, and 67 of 96 (70%) food isolates. These data indicate a virulence potential in food isolates, reinforcing the need of their safety assessment. Analysis of phenotypic-genotypic congruence suggests a divergent sequence evolution of cyl genes and the effect of environmental factors in the regulation of cytolysin expression. Evaluation of the diagnostic accuracy of cytolysin molecular detection points to cylLL-based PCR and cylLLLSMBA-based PCR as the most reliable approaches. Nevertheless, the low sensitivity (46%) and gene variability indicated by our study strongly recommend the phenotypic assay for the assessment of hemolytic ability in enterococci, followed by the molecular screening of cyl genes in nonhemolytic strains to evaluate their virulence potential.
Enterococci are gram-positive bacteria that belong to the normal gastrointestinal flora of mammals and other warm-blooded animals; they can also be found in soil, on plants, in water, and in several food products, such as a variety of cheeses, especially artisanal ones (1, 11, 17, 30, 33, 39).
The natural ability of enterococci to acquire, accumulate, and share extrachromosomal elements encoding virulence traits or antibiotic resistance genes in part explains their increasing importance as nosocomial pathogens (2, 7, 12, 20, 23, 41, 42, 46) and the major concerns regarding their use in food products and probiotics (9, 11). In fact, enterococci are a frequent cause of a wide variety of infection in humans, especially in the last two decades. Enterococcus faecalis causes 80 to 90% of human enterococcal infections, and E. faecium accounts for the majority of the remainder. Other enterococcal species, including E. avium, E. casseliflavus, E. durans, E. gallinarum, E. gilvus, E. hirae, E. malodoratus, E. mundtii, E. pallens, E. raffinosus, and E. solitarius, are infrequent causes of human infection (29, 30, 31, 34, 37, 44).
Despite the identification and extensive study of virulence mechanisms in E. faecalis (15), screening of virulence determinants in other enterococcal species was only performed in E. faecium and E. durans (9, 11, 15, 29, 34). Many putative enterococcal virulence factors reside on highly conserved conjugative pheromone-responsive plasmids, such as the hemolysin-encoding plasmid pAD1 (3, 16, 18, 24, 27, 29), although a pathogenicity island harboring several virulence determinants was recently found in vancomycin-resistant strains of E. faecalis (38).
Hemolysin is more commonly referred to as cytolysin because of its broad target cell range that includes both eukaryotic and prokaryotic cells. Until nowadays cytolysin is still one of the most studied virulence traits attributed to this genus (11, 29, 30, 34). The production of cytolysin has been demonstrated to contribute to the severity of enterococcal disease in a number of animal models (8, 14, 25, 28), as well as in humans (21, 22, 26). These studies demonstrated that as many as 60% of E. faecalis strains isolated from sites of infection produce cytolysin. The cytolysin has been shown to lower the 50% lethal dose in an intraperitoneal mouse model and to contribute to toxicity in experimental endocarditis and endophthalmitis (8, 25, 28). In addition, it is associated with a fivefold-increased risk of acutely terminal outcome in patients with enterococcal bacteremia (22).
Nucleotide sequence determination of the E. faecalis cytolysin operon revealed a complex determinant encoding five gene products that are necessary and sufficient for cytolysin production. The model for cytolysin expression, maturation, secretion, and activation includes two cytolysin structural subunits (coded by genes cylLL and cylLS) that are posttranslationally modified intracellularly by cylM gene product and transported out of the cell by an ATP-binding cassette transporter encoded by cylB; after externalization, cytolysin precursor components are activated by cylA gene product, an extracellular activator serine protease (16, 18, 24). A sixth gene (cylI) encodes a protein responsible for immunity of the cytolysin-producing bacteria to the cytolysin (5). All six open reading frames are clustered (cylLL, cylLS, cylM, cylB, cylA, and cylI) and arranged in the same orientation. The regulation of cytolysin expression was recently described (19), and the studies demonstrated that the products of two other genes, cylR1 and cylR2, are implicated in repressing the transcription of cytolysin genes by a new type of quorum-sensing mechanism.
In the present study we investigated the hemolytic and cytolytic ability in the genus Enterococcus by phenotypic and molecular approaches, using type strains and clinical (from human and veterinary origins) and food isolates. Hemolysis was assayed with sheep and horse erythrocytes and under both aerobic and anaerobic conditions. The strains were also screened for the presence of cytolysin genes (cylLL, cylLS, cylM, cylB, and cylA) in order to evaluate the conservation of this operon among enterococcal species. Another purpose of the present study was to evaluate the accuracy of molecular methods for the screening of cytolysin-producing strains.
MATERIALS AND METHODS
Microorganisms.
A total of 164 enterococci were used in the present study. Ninety-six strains were isolated from ewes' cheese and milk from four Portuguese Registered Designation of Origin areas, as described previously (33). Thirty-one isolates were obtained from several Portuguese hospitals; all of them were referred as human infecting strains collected over a ca. 10-year period and with any known epidemiological relationship. Eleven isolates, obtained from Lisbon Veterinarian Faculty, were reported as dog-infecting strains. When phenotypic and molecular methods (6, 33, 35) were applied in the identification of isolates, 66 were confirmed as E. faecalis, 29 were confirmed as E. durans, 21 were confirmed as E. faecium, 5 were confirmed as E. hirae, 1 was confirmed as E. casseliflavus, and 16 remained unidentified at the species level.
Twenty-five reference strains were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ; Braunschweig, Germany) and the Colección Espanola de Cultivos Tipo (CECT; Valencia, Spain): E. asini DSMZ 11492T, E. avium DSMZ 20679T, E. casseliflavus DSMZ 20680T, E. cecorum DSMZ 20682T, E. columbae DSMZ 7374T, E. dispar DSMZ 6630T, E. durans DSMZ 20633T, E. faecalis DSMZ 20478T, E. faecalis DSMZ 20376, E. faecalis CECT 795, E. faecalis CECT 184 (formerly Streptococcus faecalis subsp. liquefaciens), E. faecalis CECT 187 (formerly S. faecalis subsp. zymogenes), E. faecium DSMZ 20477T, E. faecium DSMZ 2146, E. flavescens DSMZ 7370T, E. gallinarum DSMZ 20628T, E. hirae DSMZ 20160T, E. malodoratus DSMZ 20681T, E. mundtii DSMZ 4838T, E. pseudoavium DSMZ 5632T, E. raffinosus DSMZ 5633T, E. saccharolyticus DSMZ 20726T, E. solitarius DSMZ 5634T, E. sulfureus DSMZ 6905T, and Lactococcus garvieae DSMZ 6783 (formerly E. seriolicida). E. faecalis DS16 (a kind gift of C. B. Clewell, Department of Oral Biology, School of Dentistry, University of Michigan, Ann Arbor, Mich.) was also used as a positive control.
The species E. villorum (45), E. haemoperoxidus and E. moraviensis (40), E. porcinus and E. ratti (43), and E. pallens and E. gilvus (44) were not included in the study, since they have been proposed and accepted as new species more recently.
Assay of hemolytic activity.
The production of hemolysin was determined, according to the method of Lányí (32), by streaking bacterial cultures, grown overnight at 37°C in brain heart infusion agar (Oxoid), on Columbia agar plates supplemented with 5% of sheep or horse blood (bioMérieux). Plates were incubated at 37°C for 72 h either in aerobic or anaerobic conditions, after which the plates were examined for hemolysis. The presence or absence of zones of clearing around the colonies were interpreted as beta-hemolysis (positive) or gamma-hemolysis (negative) activity, respectively. When observed, greenish zones around the colonies were interpreted as alpha-hemolysis and taken as negative for the assessment of beta-hemolytic activity.
DNA preparation.
Strains were cultured overnight at 37°C in 20 ml of brain heart infusion broth (Oxoid) and then harvested by centrifugation at 10,000 × g at 4°C. Total DNA was extracted by the guanidium thiocyanate method (36).
PCR amplification of the cyl operon genes.
The oligonucleotide primers used in the present study are listed in Table 1 and were purchased from Life Technologies (England). Primers were originally developed on the basis of GenBank nucleotide sequence for the E. faecalis cytolysin operon (accession no. L37110). PCR amplifications were performed in a Thermo RoboCycler (Stratagene, La Jolla, Calif.) in 0.2-ml reaction tubes with mixtures (25 μl each) with Life Technologies PCR buffer (pH 8.4; 2.5 mmol of MgCl2 liter−1), 0.1 mmol of deoxynucleoside triphosphates (Life Technologies) liter−1, 0.5 μmol of each primer liter−1, 2 U of Taq DNA polymerase (Life Technologies), and 250 ng of enterococcal DNA. Thermocycler reactions were as follows: initial cycle of 94°C for 3 min, 35 cycles of 94°C for 1 min, 55°C for 1 min, 72°C for 2 min, a final extension step of 72°C for 7 min, and thereafter cooled to 4°C. A 5-μl aliquot of the amplification mixture was combined with 2 μl of loading buffer, and the preparation was electrophoresed on 1% (wt/vol) agarose gel at 90 V for 2 h.
TABLE 1.
PCR primers and products for detection of cytolysin genes
| Gene and primer | Sequence (5′ to 3′) | Product size (bp) |
|---|---|---|
| cylLL | ||
| cylLL1 | 5′-GATGGAGGGTAAGAATTATGG-3′ | 253 |
| cylLL2 | 5′-GCTTCACCTCACTAAGTTTTATAG-3′ | |
| cylLs | ||
| cylLs1 | 5′-GAAGCACAGTGCTAAATAAGG-3′ | 240 |
| cylLs2 | 5′-GTATAAGAGGGCTAGTTTCAC-3′ | |
| cylM | ||
| cylM1 | 5′-AAAAGGAGTGCTTACATGGAAGAT-3′ | 2,940 |
| cylM2 | 5′-CATAACCCACACCACTGATTCC-3′ | |
| cylB | ||
| cylB1 | 5′-AAGTACACTAGTAGAACTAAGGGA-3′ | 2,020 |
| cylB2 | 5′-ACAGTGAACGATATAACTCGCTATT-3′ | |
| cylA | ||
| cylA1 | 5′-TAGCGAGTTATATCGTTCACTGTA-3′ | 1,282 |
| cylA2 | 5′-CTCACCTCTTTGTATTTAAGCATG-3′ |
Confirmation of primer specificity and identity of amplified products was performed by sequencing amplicons obtained from E. faecalis DS16, a positive control strain harboring plasmid pAD1 that encodes the cytolysin operon (16), and by restriction analysis of amplicons from selected strains (data not shown).
Data analysis.
The chi-square analysis of contingency tables (47) was used to test the statistical independence between blood agar media and incubation conditions in hemolytic assays, as well as among the occurrence of cyl genes, beta-hemolysis, and the origin and species allocation of isolates. When appropriate, odds ratios (ORs) and 95% confidence intervals (CIs) were also calculated (13). The sensitivity, specificity, and predictive values of phenotypic and molecular diagnostic approaches of hemolytic potential in enterococci were also assessed (4). The anaerobic assay of beta-hemolytic activity was used as the “gold standard” for the diagnostic accuracy of cyl gene-based PCR detection of cytolysin-producing strains.
RESULTS
Hemolytic activity.
A set of 59 strains, including all reference strains (except type strains of E. asini and E. columbae) and 35 cheese and milk isolates randomly selected, was used to compare the hemolytic activity on sheep and horse blood plates incubated in aerobiosis. Although the incidence of beta-hemolysis was quite similar on horse blood (13 of 59; 22%) and on sheep blood (10 of 59; 17%), no equivalence was found for both media (P > 0.75 by chi-square test; OR, 0.86; 95% CI, 0.16 to 4.67) since only two strains produced matching beta-hemolytic results. Since 90% of the beta-hemolytic strains on sheep blood were isolates from ewe's cheese and milk and the positive control strain harboring plasmid pAD1 was only hemolytic on horse blood, this last medium was then selected for assaying beta-hemolysis on the whole set of 164 strains, in both aerobic and anaerobic conditions (Table 2).
TABLE 2.
Summary of PCR detection of cyl genes and beta-hemolysis assay results for the enterococci analyzed in this study
| cyl genes and hemolysisa | Reference strain(s)b (n = 26) | Organism (no. of isolates)b
|
|
|---|---|---|---|
| Clinical (n = 42) | Food (n = 96) | ||
| Five cyl genes | |||
| β/β | E. faecalis DS16; CECT 187 | E. faecalis (5) | E. faecalis (2) |
| E. raffinosus DSMZ 5633T | NID (1) | ||
| −/− | E. faecalis (1) | ||
| −/− | NID (1) | E. durans (1) | |
| One to four cyl genes | |||
| β/β | E. avium DSMZ 20679T | NID (1) | |
| E. casseliflavus DSMZ 20680T | |||
| E. durans DSMZ 20633T | |||
| −/β | E. faecium (3) | E. faecalis (1) | |
| E. faecalis (2) | NID (1) | ||
| β/− | E. cecorum DSMZ 20682T | ||
| E. flavescens DSMZ 7374T | |||
| E. gallinarum DSMZ 20628T | |||
| E. malodoratus DSMZ 20681T | |||
| −/− | E. faecalis CECT 795 | E. faecalis (17) | E. faecalis (24) |
| E. hirae DSMZ 20160T | E. faecium (3) | E. durans (17) | |
| E. saccharolyticus DSMZ 20726T | E. solitarius (1) | E. faecium (12) | |
| E. solitarius DSMZ 5634T | NID (3) | E. hirae (3) | |
| L. garvieae DSMZ 6783T | NID (5) | ||
| No cyl genes | |||
| β/β | E. faecalis (1) | ||
| −/β | NID (1) | E. durans (1) | |
| β/− | E. casseliflavus (1) | ||
| E. durans (1) | |||
| NID (1) | |||
| −/− | E. faecalis DSMZ 20478T, 20376; CECT 184 | E. faecalis (1) | E. faecalis (12) |
| E. faecium DSMZ 20477T, 2146 | E. faecium (1) | E. durans (9) | |
| E. asini DSMZ 11492T | NID (1) | E. faecium (2) | |
| E. columbae DSMZ 7370T | E. hirae (2) | ||
| E. dispar DSMZ 6630T | |||
| E. mundrii DSMZ 4838T | |||
| E. pseudoavium DSMZ 563296T | |||
| E. sulfureus DSMZ 6905T | |||
Strains were grouped according to the number of cyl genes detected by PCR:β/β indicates beta-hemolysis in aerobic and anaerobic conditions, −/β indicates beta-hemolysis only in anaerobic conditions β/−, indicates beta-hemolysis only in aerobic conditions, and −/− indicates no beta-hemolytic activity in both conditions.
The number of isolates or strains is indicated in parentheses. NID, unidentified isolates.
The prevalence of beta-hemolysis was estimated to be 26 of 164 (16%) with anaerobic incubation and 23 of 164 (14%) in aerobic conditions, and a total of 90% matching results were obtained, showing a highly significant association between both assays (P < 0.001 by chi-square test; OR, 29.94; 95% CI, 10.0 to 89.64).
When hemolysis was tested in aerobic conditions, 10 of 23 (43%) of the beta-hemolytic strains were determined to be members of the species E. faecalis (6 human clinical isolates, 2 cheese isolates, and strains CECT 187 and DS16, both harboring plasmid pAD1). Members of other enterococcal species, such as type strains of E. avium, E. casseliflavus, E. cecorum, E. durans, E. flavescens, E. gallinarum, E. malodoratus, and E. raffinosus, were also found to be beta-hemolytic under these conditions. Of the remaining beta-hemolytic isolates, two were cheese isolates identified as E. durans and E. casseliflavus, respectively, and three were unidentified (one from cheese and two with human medical origin).
For hemolysis on anaerobiosis, results show that 14 of 26 (54%) of the beta-hemolytic strains belong to the species E. faecalis: 8 clinical isolates, 4 food isolates, and strains CECT 187 and DS16. Strains belonging to the species E. avium (DSMZ 20679T), E. casseliflavus (DSMZ 20680T), E. durans (DSMZ 20633T and one milk isolate), E. faecium (three clinical isolates, two from human and one from veterinary origin), and E. raffinosus (DSMZ 5633T) also produced beta-hemolysis. In spite of the highest prevalence of beta-hemolysis in E. faecalis, no significant association (P > 0.10 by chi-square test; OR, 1.61; 95% CI, 0.69 to 3.73) was found between hemolytic ability and allocation of isolates to this species.
When food isolates are compared with the group formed by clinical and reference strains, it becomes evident the lower prevalence (6 of 96 [6%]) of the anaerobic beta-hemolytic phenotype among food isolates in contrast to 14 of 42 (33%) for the other group of strains. In fact, a highly significant association (P < 0.001 by chi-square test; OR, 6.25; 95% CI, 2.35 to 16.61) was found between beta-hemolysis and the group of clinical and reference strains relative to the food isolates.
Among the beta-hemolytic discrepant strains found both in anaerobiosis (10 strains) and in aerobiosis (7 strains), all discrepant clinical isolates (4 of human origin and 2 of veterinary origin) and 4 food isolates were negative in aerobiosis, whereas the 4 discrepant type strains (E. cecorum, E. flavescens, E. gallinarum, and E. malodoratus) and 3 food isolates were negative in anaerobiosis.
When both assays were compared, the occurrence of alpha-hemolysis was only observed in aerobiosis and for all groups of strains (38 of 141, 27% of the negative results). If we assume the anaerobic assay as the gold standard, the sensitivity and the predictive value of the positive test for the aerobic assay were 62 and 70%, respectively, whereas the specificity and the predictive value of the negative test were 95 and 93%.
Molecular screening of cyl operon.
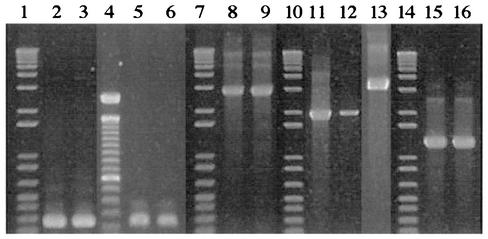
PCR amplification of the cytolysin genes cylLL, cylLS, cylM, cylB, and cylA was used to screen the cytolysin operon on reference strains and enterococcal isolates from clinical and food origins. Our set of primers generated two- to fourfold larger products for cylM, cylB, and cylA and, in the case of cylLL and cylLS, were mainly directed to intergenic flanking sequences. Sequence analysis of amplicons from positive control strain DS16 and restriction analysis of products obtained from selected isolates and type strains confirmed the specificity of this new set of primers. As illustrated in Fig. 1, amplicons from each gene showed the expected size indicated in Table 1, except for one nonhemolytic clinical isolate, for which cylB seemed to contain an insertion with ca. 1 kb.
FIG. 1.
PCR amplification products of cyl operon genes. Lanes: 1, 7, 10, and 14, 1-kb plus DNA ladder (Gibco-BRL); 4, 100-bp DNA ladder (Gibco-BRL); 2 and 3, cylLL of clinical isolates; 5 and 6, cylLS of food isolates; 8 and 9, cylM of reference strains CECT 187 and DSMZ 5633; 11, 12, and 13, cylB of clinical isolates; 15 and 16, cylA of food isolates.
If we take into account the number of cyl genes detected by PCR, the 164 enterococci analyzed could be divided into three sets (Table 2): 14 (9%) containing the whole cyl operon, 105 (64%) with one to four cytolysin genes, and 45 (27%) without cyl genes.
Among the 119 cyl-harboring strains, a total of 14 cyl genotypes were detected (Table 3), the more frequent being cylMBA+ (52%), cylBA+ (18%), and cylLLLSMBA+ (12%). With regard to species distribution, 45% (54 of 119) of the cyl+ strains belong to E. faecalis, 16% (19 of 119) belong to E. durans, 15% (18 of 119) belong to E. faecium, and 3% (4 of 119) belong to E. hirae. The type strains of E. avium, E. casseliflavus, E. cecorum, E. flavescens, E. gallinarum, E. malodoratus, E. raffinosus, E. saccharolyticus, E. solitarius, and L. garvieae also have at least one cyl determinant. When the global frequencies of cyl+ and cyl-negative genotypes were compared among species, no significant association (P > 0.50 by chi-square test) of cyl genes with a particular species was found, since the values of the cyl+ frequencies were close enough among species: 63% (19 of 30) in E. durans, 67% (4 of 6) in E. hirae, 76% (55 of 72) in E. faecalis, 78% (18 of 23) in E. faecium, and 70% (23 of 33) in the group formed by the remaining species.
TABLE 3.
Distribution of enterococcal reference strains and clinical and food isolates according to cyl genotype and anaerobic beta-hemolytic behavior
| cyl genotype | No. of strains or isolatesa
|
||||||
|---|---|---|---|---|---|---|---|
| Reference straina (n = 26)
|
Clinical isolate (n = 42)
|
Food isolate (n = 96)
|
Total (n = 164) | ||||
| β+ | β− | β+ | β− | β+ | β− | ||
| cylLLLSMBA+ | 3 | 0 | 6 | 1 | 3 | 1 | 14 |
| cylLLMBA+ | 0 | 1 | 0 | 1 | 0 | 2 | 4 |
| cylLSMBA+ | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| cylLSBA+ | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| cylMBA+ | 1 | 5 | 3 | 17 | 2 | 34 | 62 |
| cylLSM+ | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| cylLSB+ | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| cylMB+ | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| cylMA+ | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| cylBA+ | 1 | 1 | 3 | 5 | 0 | 12 | 22 |
| cylLS+ | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| cylM+ | 1 | 1 | 0 | 0 | 0 | 3 | 5 |
| cylB+ | 0 | 0 | 0 | 1 | 0 | 2 | 3 |
| cylA+ | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| cyl negative | 0 | 11 | 2 | 3 | 1 | 28 | 45 |
β+, Beta-hemolytic in anaerobic conditions; β−, no hemolytic activity in anaerobic conditions.
Although high frequencies of cyl+ genotypes were found both in food isolates (67 of 96; 70%) and in clinical strains (37 of 42; 88%), the whole cyl operon was only detected in 4 of 96 (4%) of the food isolates compared to 7 of 42 (17%) in clinical ones. A significant association (P < 0.01 by chi-square test; OR, 3.61; 95% CI, 1.32 to 9.89) was found between the occurrence of cyl genes and clinical isolates relative to the group formed by reference strains and food isolates. When a comparison between E. faecalis and non-E. faecalis strains was performed for both groups, no significant association was detected in clinical isolates (P > 0.25; OR, 2.77; 95% CI, 0.41 to 18.74) and in food isolates plus reference strains (P > 0.95; OR, 1.01; 95% CI, 0.46 to 2.21).
Results obtained for each cyl gene also revealed that those coding for cytolysin structural subunits are the least-detected genes, since cylLLLS+ was only found in 9% (14 of 164) of the strains analyzed, and both genes were not detected in 85% (140 of 164) of them. Genes cylM, cylB, and cylA were more frequent, being found in 54% (89 of 164), 67% (110 of 164), and 65% (107 of 164) of the strains, respectively.
Congruence between phenotypic and molecular screening.
To analyze the congruence between blood agar hemolysis and detection of cyl operon, a stepwise procedure based on contingency tables was applied. Taking into account the three sets of strains defined by cyl detection (whole cyl operon, one to four cyl genes, and cyl negative), the occurrence of beta-hemolysis was cross-analyzed and revealed a highly significant association (P < 0.001 by chi-square test; OR, 58.28; 95% CI, 11.83 to 287.21) of beta-hemolysis with the whole cyl operon, since 148 of 164 (90%) of the strains were congruent at phenotypic and genotypic levels: 12 of 164 (7%) beta-hemolytic and cylLLLSMBA+ genes, 94 of 164 (57%) nonhemolytic and at least without one cyl gene, and 42 of 164 (26%) nonhemolytic and cyl negative. When the occurrence of beta-hemolysis was successively cross-analyzed by using distinct partitions of the universe of strains, significant associations (P < 0.05 by chi-square test) were always obtained between beta-hemolysis and the presence of cylLL and/or cylLS (OR, 9.00; 95% CI, 3.40 to 23.79), cylMBA (OR, 2.68; 95% CI, 1.09 to 6.57), cylLL (OR, 38.57; 95% CI, 9.70 to 153.27), cylLS (OR, 9.90; 95% CI, 3.69 to 26.55), cylM (OR, 2.64; 95% CI, 1.04 to 6.67), cylB (OR, 3.13; 95% CI, 1.02 to 9.58), and cylA (OR, 3.43; 95% CI, 1.12 to 10.50). However, phenotypic-genotypic congruence (β+/cyl+ plus β−/cyl−) is variable for each genotype assayed: 90% (147 of 164) for cylLL+, 85% (139 of 164) for cylLS+, 84% (138 of 164) for cylLL+ and/or cylLS+, 57% (93 of 164) for cylMBA+, 53% (87 of 164) for cylM+, 46% (75 of 164) for cylA+, and 44% (72 of 164) for cylB+.
Looking at the species to which the majority of cyl+ genotypes were allocated, phenotypic-genotypic congruence (β+/cylLLLSMBA+ plus β−/at least one cyl gene absent) was also high with 94% (68 of 72) being obtained for E. faecalis, 90% (27 of 30) for E. durans, 87% (20 of 23) for E. faecium, and 100% (6 of 6) for E. hirae. For the isolates of these species assumed as carrying only a partial set of cyl genes, no correlation was found between absence of hemolysis and a particular genotype. However, a pattern emerged for the beta-hemolytic ones, since such isolates were cylBA+ in E. faecium (three clinical isolates, one of them of veterinary origin) and cylMBA+ in E. durans (reference strain) and E. faecalis (three isolates of human, veterinary, and food origin).
Phenotypic-genotypic congruence (β+/cylLLLSMBA+ plus β−/at least one cyl gene absent) was also observed in reference strains (23 of 26; 88%) and clinical (33 of 42; 79%) and food (92 of 96; 96%) isolates, with >75% of cylLLLSMBA+ isolates being beta-hemolytic. Nevertheless, a distinct pattern was found concerning the hemolytic behavior of isolates with partial cyl+ genotypes, as only 3% (2 of 63) of such food isolates were beta-hemolytic and 20% (6 of 30) were found among clinical isolates. Additionally, 40% (2 of 5) of cyl negative clinical isolates are beta-hemolytic, contrasting with 3% (1 of 29) among food isolates.
In view of the significant associations found between horse blood beta-hemolysis in anaerobiosis and the occurrence of cyl genes, the diagnostic potential of molecular detection of cytolysin-producing strains was assessed by using beta-hemolysis as the gold standard. Besides detection based on a sole cyl gene, diagnostic procedures relying on detection of two to five genes were also analyzed. As shown in Table 4, the highest sensitivity (and so the lowest false-negative rate) was observed for cyl genes involved in the maturation, excretion, and activation of cytolysin (cylM, cylB, and cylA), whereas the highest specificity (and lowest false-positive rate) was related to genes coding for cytolysin structural subunits (cylLL and cylLS). Regarding prediction ability, high similar predictive values were obtained in all methods for the negative test, whereas a high range of variation in predictive values was found for the positive test. Diagnosis based on the parallel detection of the five cyl genes showed the highest specificity and predictive values, associated with the lowest sensitivity, and seemed well correlated with cylLL-based PCR.
TABLE 4.
Reliability of cyl-based PCR diagnosis of hemolytic potential in enterococci by using beta-hemolysis in anaerobic conditions as the gold standard
| Parametera | No. of strains (% total) based on the PCR detection of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| cylLLb | cylLS | cylM | cylB | cylA | cylLL and cylLS | cylMBA | Five genes | |
| True positive | 12 (7.3) | 12 (7.3) | 19 (11.6) | 22 (13.4) | 22 (13.4) | 12 (7.3) | 18 (11.0) | 12 (7.3) |
| False positive | 3 (1.8) | 11 (6.7) | 70 (42.7) | 88 (53.7) | 85 (51.8) | 12 (7.3) | 63 (38.4) | 2 (1.2) |
| True negative | 135 (82.3) | 127 (77.4) | 68 (41.5) | 50 (30.5) | 53 (32.3) | 126 (76.8) | 75 (45.7) | 136 (82.9) |
| False negative | 14 (8.5) | 14 (8.5) | 7 (4.3) | 4 (2.4) | 4 (2.4) | 14 (8.5) | 8 (4.9) | 14 (8.5) |
| Sensitivity | 46.2 | 46.2 | 73.1 | 84.6 | 84.6 | 46.2 | 69.2 | 46.2 |
| Specificity | 97.8 | 92.0 | 49.3 | 36.2 | 38.4 | 91.3 | 54.3 | 98.6 |
| PPVa | 80.0 | 52.2 | 21.3 | 20.0 | 20.6 | 50.0 | 22.2 | 85.7 |
| NPVb | 90.6 | 90.1 | 90.7 | 92.6 | 93.0 | 90.0 | 90.4 | 90.7 |
PPV, predictive value of positive test; NPV, predictive value of negative test.
Values in parentheses refer to percentages of the total of strains (n = 164).
When a subset analysis was performed with only E. faecalis strains (data not shown), similar conclusions were obtained. A general increase in sensitivity, specificity, and predictive values was observed, owing to the reduction of false negatives and false positives, with specificity and predictive value of the positive test attaining 100% for the parallel detection of the five cyl genes.
DISCUSSION
Enterococci are opportunistic pathogenic bacteria known to be responsible for severe illness and, among virulence factors, the production of cytolysin was shown to contribute to the severity of enterococcal infection. The use of enterococci in foods and probiotics and the increasing report of other enterococcal species associated with disease, beyond E. faecalis and E. faecium, led us to investigate the hemolytic activity and the presence of cytolysin genes (cylLL, cylLS, cylM, cylB, and cylA) in a broader range of enterococci, by using clinical (from human and veterinary origin) and food isolates, as well as reference strains from 20 enterococcal species.
Although primers for amplification of cylM, cylB, and cylA have already been described by Eaton and Gasson (9), no primers for cylLL and cylLS were defined by these authors due to extensive sequence homology between both genes. Taking this in account and in order to obtain larger PCR fragments suitable for confirmatory restriction analysis, we defined new specific primers in the present study for the amplification of cyl genes by using the GenBank available sequence of cytolysin operon (16).
Hemolysin production is recognized by the development of clearing around colonies on certain blood agar media. Erythrocytes from various species were found to exhibit different levels of susceptibility to hemolysin-mediated lysis, since rabbit, human, and horse red blood cells were observed to be sensitive, whereas sheep and goose red blood cells were less susceptible or completely refractory (18, 29, 34). The selectivity of sheep erythrocytes regarding hemolysins was confirmed in our assays since only isolates from ewes' milk and cheese and the type strain of E. durans (isolated from dried milk) were hemolytic in sheep blood agar. Furthermore, a proportion of 32% of the tested strains showed discrepant results in horse and sheep blood agar. The occurrence of hemolysins with different affinity should then be taken in account when the virulence of enterococci is assayed in human and veterinary clinics.
The enterococci were also tested for the production of hemolysin in horse blood agar under aerobic and anaerobic conditions. The use of anaerobic conditions reflects our attempt to prevent oxidation and consequent lysis of the erythrocytes due to factors other than the production of enterococcal hemolysin. The high level of agreement observed for both conditions seemed indicative of a low rate of nonspecific aerobic lysis, since only 4% of the isolates were beta-hemolytic only in aerobiosis. However, a higher rate (6%) of isolates was unexpectedly beta-hemolytic only in anaerobiosis. When these discrepant strains were genotypically analyzed, the cyl negative, cylBA+, and cylMBA+ genotypes were found in both groups, and one cheese isolate negative only in anaerobiosis carried the five genes (cylLLLSMBA+). Considering that a negative cyl gene-targeted PCR may result from sequence divergence at priming sites, these findings raised questions about cyl gene variability and inorganic environmental factors on the control of the expression of cyl genes. In fact, although two regulatory genes cylR1 and cylR2 have been described as associated with the transcription control of cytolysin operon by a quorum-sensing mechanism (19), no data are yet available about other regulatory factors acting on cyl operon or cylR genes.
The occurrence of a relatively high rate of alpha-hemolysis in aerobiosis and the fact that all discrepant clinical isolates were negative in this condition also suggest that hemolytic assays performed in anaerobiosis could be more reliable when enterococcal virulence and risk associated to human health are being assessed.
Analysis of beta-hemolytic behavior among enterococcal species showed a higher prevalence in E. faecalis and E. faecium, a result that correlates well with the leading role of these species as causes of enterococcal infections (29, 30, 34). Nevertheless, the absence of significant association between hemolytic ability and species allocation of isolates points to the occurrence of this property in the genus Enterococcus, the higher prevalence found for E. faecalis and E. faecium being explained by sample size effects. Supporting evidence was also obtained by the equivalent frequencies of cyl+ genotype found in the enterococcal species analyzed in the present study.
One of our most important findings was the presence of cyl genes in species other than E. faecalis, namely, E. avium, E. casseliflavus, E. cecorum, E. durans, E. faecium, E. flavescens, E. gallinarum, E. hirae, E. malodoratus, E. raffinosus, E. saccharolyticus, E. solitarius, and L. garvieae (formerly E. seriolicida). The type strain of E. raffinosus (DSMZ 5633) carries all of the cyl genes and is beta-hemolytic under both anaerobic and aerobic conditions. For the type strains of E. avium, E. casseliflavus, and E. durans, all of which are beta-hemolytic in aerobic or anaerobic conditions, only some cyl genes were detected by PCR, suggesting divergent evolution of the gene sequences that prevented PCR amplification with the selected primer set. Absence of amplification was also observed for some cyl genes in the remaining species, all of which are nonhemolytic in anaerobiosis. These virulence determinants have not previously been described for these species. In fact, the majority of the studies on enterococcal virulence performed thus far have focused on E. faecalis and E. faecium and analyzed preferably clinical isolates. Recent studies on the incidence of several virulence factors in starter, food, and medical isolates representing the species E. faecalis, E. faecium, and E. durans (9) revealed that all of the E. faecium and E. durans were clear of cylMBA, a finding that may be related to the use of distinct primers and strains. Since some of the species referred to above have already been reported, although rarely, as causes of human infection (29, 30, 31, 37), the evidence for the presence of cytolysin determinants may be one of the explanations for such pathogenic behavior and recommends the inclusion of these species in future enterococcal virulence studies.
When clinical and food isolates were compared, a significantly higher prevalence of beta-hemolysis and cyl+ genotype was found in clinical isolates, supporting the results of previous studies (10, 11, 26) and seeming to be independent of the fact that E. faecalis isolates represent a high proportion of the strains under study (62% of clinical isolates and 38% of the remainder). However, the high frequency of cyl genes observed in food isolates (70%) points to their virulence potential and to the need of safety evaluation as stated before (9).
Analysis of congruence between blood agar hemolysis and detection of cyl genes revealed significant associations of beta-hemolysis with the presence of the complete cyl operon and also with the presence of at least one cyl determinant, strongly suggesting that all beta-hemolytic strains must have the whole set of cyl genes, the negative amplifications being probably related to gene variability. The higher phenotypic-genotypic congruence observed for cylLL and cylLS (ca. 90%) relative to cylM, cylB, and cylA (40 to 60%) associated with the higher frequencies of these last three genes in nonhemolytic strains suggests that higher levels of sequence divergence should exist in the genes coding for the structural subunits of the cytolysin, both preventing amplification and affecting their function. Nevertheless, further studies with degenerated primers or hybridization methods should be used to confirm the presence or absence of these genes.
Hemolytic activity could not be detected in two isolates (one E. durans and one unidentified at species level) in spite of the presence of all cyl genes. This lack of hemolytic activity may be explained by low levels or downregulation of gene expression or by an inactive gene product (9).
Another goal of the present study was to evaluate the diagnostic potential of molecular detection of cytolysin-producing strains. Although cylLL-based PCR and cylLLLSMBA-based PCR seemed to be the most reliable of all approaches, the low sensitivity (46%) and the gene variability indicated by our study strongly recommend the use of the phenotypic assay for the assessment of hemolytic ability in enterococci. The molecular screening of cyl genes should also be performed in nonhemolytic strains to evaluate pathogenic potential, since environmental factors may be involved in the control of cytolysin expression.
We identified both expressed and silent cyl genes in food, clinical, and reference strains. These determinants, which seem to be widespread through very different environments and in Enterococcus species not reported till now, may be determinant for the evolution of pathogenic enterococci. Such findings, associated with the natural ability of enterococci to acquire, accumulate, and share extrachromosomal elements within their genus and with other bacteria, require further studies involving species other than E. faecalis, to correctly evaluate the evolution of pathogenicity within this genus and the consequent risk for human health.
Acknowledgments
This work was supported by Fundação para a Ciência e Tecnologia (FCT), Program PRAXIS XXI project 2/2.1/BIO/1121/95 and Program SAPIENS project POCTI/AGR/36165/99. T. Semedo and M. F. Silva Lopes were also supported by research grants from FCT.
We thank Aida Duarte (Lisbon University Pharmacy Faculty), Maria Manuela Caniça (National Institute of Health), and Constança Feria (Lisbon Technical University Medical Veterinarian Faculty) for supplying clinical isolates.
REFERENCES
- 1.Aarestrup, F. M., P. Butaye, and W. Witte. 2002. Nonhuman reservoirs of enterococci, p. 55-99. In M. S. Gilmore, D. B. Clewell, P. Courvalin, G. M. Dunny, B. E. Murray, and L. B. Rice (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. American Society for Microbiology, Washington, D.C.
- 2.An, F. Y., and D. B. Clewel. 2002. Identification of the cAD1 sex pheromone precursor in Enterococcus faecalis. J. Bacteriol. 184:1880-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastos, M. C. F., K. Tanimoto, and D. B. Clewell. 1997. Regulation of transfer of the Enterococcus faecalis pheromone-responding plasmid pAD1: temperature-sensitive transfer mutants and identification of a new regulatory determinant, traD. J. Bacteriol. 179:3250-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell, M. J., and D. Machin. 1990. Medical statistics: a commonsense approach. John Wiley & Sons, Ltd., Chichester, United Kingdom.
- 5.Coburn, P. S., L. E. Hancock, M. C. Booth, and M. S. Gilmore. 1999. A novel means of self-protection, unrelated to toxin activation, confers immunity to the bacteriocidal effects of the Enterococcus faecalis cytolysin. Infect. Immun. 67:3339-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Angelis, M., A. Corsetti, N. Tosti, J. Rossi, M. R. Corbo, and M. Gobbetti. 2001. Caracterization of non-starter lactid acid bacteria from italian ewe cheeses based on phenotypic, genotypic, and cell wall protein analysis. Appl. Environ. Microbiol. 67:2021-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunny, G. M., B. A. Leonard, and P. J. Hedberg. 1995. Pheromone-inducible conjugation in Enterococcus faecalis: interbacterial and host-parasite chemical communication. J. Bacteriol. 177:871-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupont, H., P. Montravers, J. Mohler, and C. Carbon. 1998. Disparate findings on the role of virulence factors of Enterococcus faecalis in mouse and rat models of peritonitis. Infect. Immun. 66:2570-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eaton, T. J., and M. J. Gasson. 2001. Molecular screening of Enterococcus virulence determinants and potential genetic exchange between food and medical isolates. Appl. Environ. Microbiol. 67:1628-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elsner, H. A., I. Sobottka, D. Mack, M. Claussen, R. Laufs, and R. Wirth. 2000. Virulence factors of Enterococcus faecalis and Enterococcus faecium blood culture isolates. Eur. J. Clin. Microbiol. Infect. Dis. 19:39-42. [DOI] [PubMed] [Google Scholar]
- 11.Franz, C. M. A. P., A. B. Muscholl-Silberhorn, N. M. K. Yousif, M. Vancanneyt, J. Swings, and W. H. Holzapfel. 2001. Incidence of virulence factors and antibiotic resistance among enterococci isolated from food. Appl. Environ. Microbiol. 67:4385-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galli, D., and R. Wirth. 1991. Comparative analysis of Enterococcus faecalis sex pheromone plasmids identifies a single homologous DNA region which codes for aggregation substance. J. Bacteriol. 173:3029-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner, M. J., and D. G. Altman. 1989. Statistics with confidence. British Medical Journal Publications, London, England.
- 14.Gentry-Weeks, C. R., R. Karkhoff-Schweizer, A. Pikis, M. Estay, and J. M. Keith. 1999. Survival of Enterococcus faecalis in mouse peritoneal macrophages. Infect. Immun. 67:2160-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilmore, M. S., P. S. Coburn, S. R. Nallapareddy, and B. E. Murray. 2002. Enterococccal virulence, p. 301-354. In M. S. Gilmore (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press, Washington, D.C.
- 16.Gilmore, M. S., R. A. Segarra, M. C. Booth, C. P. Bogie, L. R. Hall, and D. B. Clewell. 1994. Genetic structure of the Enterococcus faecalis plasmid pAD1-encoded cytolytic toxin system and its relationship to lantibiotic determinants. J. Bacteriol. 176:7335-7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giraffa, G. 2002. Enterococci from foods. FEMS Microbiol. Rev. 744:1-9. [DOI] [PubMed] [Google Scholar]
- 18.Haas, W., and M. S. Gilmore. 1999. Molecular nature of a novel bacterial toxin: the cytolysin of Enterococcus faecalis. Med. Microbiol. Immunol. 187:183-190. [DOI] [PubMed] [Google Scholar]
- 19.Haas, W., B. D. Shepard, and M. S. Gilmore. 2002. Two-component regulator of Enterococcus faecalis cytolysin responds to quorum-sensing autoinduction. Nature 415:84-87. [DOI] [PubMed] [Google Scholar]
- 20.Huebner, J., A. Quaas, W. A. Krueger, D. A. Goldmann, and G. B. Pier. 2000. Prophylactic and therapeutic efficacy of antibodies to a capsular polysaccharide shared among vancomycin-sensitive and -resistant enterococci. Infect. Immun. 68:4631-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huycke, M. M., and M. S. Gilmore. 1995. Frequency of aggregation substance and cytolysin genes among enterococcal endocarditis isolates. Plasmid 34:152-156. [DOI] [PubMed] [Google Scholar]
- 22.Huycke, M. M., C. A. Spiegel, and M. S. Gilmore. 1991. Bacteremia caused by hemolytic, high-level gentamicin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 35:1626-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ike, Y., and D. B. Clewell. 1992. Evidence that the hemolysin/bacteriocin phenotype of Enterococcus faecalis subsp. zymogenes can be determined by plasmids in different incompatibility groups as well as by the chromosome. J. Bacteriol. 174:8172-8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ike, Y., D. B. Clewell, R. A. Segarra, and M. S. Gilmore. 1990. Genetic analysis of the pAD1 hemolysin/bacteriocin determinant in Enterococcus faecalis: Tn917 insertional mutagenesis and cloning. J. Bacteriol. 172:155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ike, Y., H. Hashimoto, and D. B. Clewell. 1984. Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect. Immun. 45:528-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ike, Y., H. Hashimoto, and D. B. Clewell. 1987. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infections. J. Clin. Microbiol. 25:1524-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ike, Y., S. E. Flannagan, and D. B. Clewell. 1992. Hyperhemolytic phenomena associated with insertions of Tn916 into the hemolysin determinant of Enterococcus faecalis plasmid pAD1. J. Bacteriol. 174:1801-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jett, B. D., H. G. Jensen, R. E. Nordquist, and M. S. Gilmore. 1992. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect. Immun. 60:2445-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jett, B. D., M. M. Huycke, and M. S. Gilmore. 1994. Virulence of enterococci. Clin. Microbiol. Rev. 7:462-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, A. P. 1994. The pathogenicity of enterococci. J. Antimicrob. Chemother. 33:1083-1089. [DOI] [PubMed] [Google Scholar]
- 31.Kurup, A., W. S. N. Tee, L. H. Loo, and R. Lin. 2001. Infection of central nervous system by motile Enterococcus: first case report. J. Clin. Microbiol. 39:820-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lányí, B. 1987. Classical and rapid identification methods for medically important bacteria. Methods Microbiol. 19:1-67. [Google Scholar]
- 33.Lopes, M. F. S., C. I. Pereira, F. M. S. Rodrigues, M. P. Martins, M. C. Mimoso, T. C. Barros, J. J. Figueiredo Marques, R. P. Tenreiro, J. S. Almeida, and M. T. Barreto Crespo. 1999. Registered designation of origin areas of fermented food products defined by microbial phenotypes and artificial neural networks. Appl. Environ. Microbiol. 65:4484-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mundy, L. M., D. F. Sahm, and M. Gilmore. 2000. Relationships between enterococcal virulence and antimicrobial resistance. Clin. Microbiol. Rev. 13:513-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park, Y., E. Oh, B. K. Kim, S. M Kim, and S. Shim. 1999. Phenotypic characteristics of Enterococcus faecium variants confirmed by intergenic ribosomal polymerase chain reaction and E. faecium polymerase chain reaction. Diagn. Microbiol. Infect. Dis. 34:269-273. [DOI] [PubMed] [Google Scholar]
- 36.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 37.Sandoe, J. A., I. R. Witherden, and C. Settle. 2001. Vertebral osteomyelitis caused by Enterococcus raffinosus. J. Clin. Microbiol. 39:1678-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shankar, N., A. S. Bagdhdayan, and M. S. Gilmore. 2002. Modulation of virulence within a pathogenicity island in vancomycin-resistant Enterococcus faecalis. Nature 417:746-750. [DOI] [PubMed] [Google Scholar]
- 39.Suzzi, G., M. Caruso, F. Gardini, A. Lombardi, L. Vannini, M. E. Guerzoni, C. Andrighetto, and M. T. Lanorte. 2000. A survey of the enterococci isolated from an artisanal italian goat's cheese (semicotto caprino). J. Appl. Microbiol. 89:267-274. [DOI] [PubMed] [Google Scholar]
- 40.Svec, P., L. A. Devriese, I. Sedlacek, M. Baele, M. Vancanneyt, F. Haesebrouck, J. Swings, and J. Doskar. 2001. Enterococcus haemoperoxidus sp. nov. and Enterococcus moraviensis sp. nov., isolated from water. Int. J. Syst. E vol. Microbiol. 51:1567-1574. [DOI] [PubMed] [Google Scholar]
- 41.Tanimoto, K., and D. B. Clewell. 1993. Regulation of the pAD1-encoded sex pheromone response in Enterococcus faecalis: expression of the positive regulator TraE1. J. Bacteriol. 175:1008-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanimoto, K., F. Y. An, and D. B. Clewell. 1993. Characterization of the traC determinant of the Enterococcus faecalis: hemolysin/bacteriocin plasmid pAD1: binding of the sex pheromone. J. Bacteriol. 175:5260-5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teixeira, L. M., M. G. S. Carvalho, M. M. B. Espinola, A. G. Steigerwalt, M. P. Douglas, D. J. Brenner, and R. R. Facklam. 2001. Enterococcus porcinus sp. nov. and Enterococcus ratti sp. nov., associated with enteric disorders in animals. Int. J. Syst. E vol. Microbiol. 51:1737-1743. [DOI] [PubMed] [Google Scholar]
- 44.Tyrrell, G. J., L. Turnbull, L. M. Teixeira, J. Lefebvre, M. G. S. Carvalho, R. R. Facklam, and M. Lovgren. 2002. Enterococcus gilvus sp. nov. and Enterococcus pallens sp. nov. isolated from human clinical specimens. J. Clin. Microbiol. 40:1140-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vancanneyt, M., C. Snauwaert, I. Cleenwerck, M. Baele, P. Descheemaeker, H. Goossens, B. Pot, P. Vandamme, J. Swings, F. Haesebrouck, and L. A. Devriese. 2001. Enterococcus villorum sp. nov., an enteroadherent bacterium associated with diarrhoea in piglets. Int. J. Syst. E vol. Microbiol. 51:393-400. [DOI] [PubMed] [Google Scholar]
- 46.Wirth, R. 1994. The sex pheromone system of Enterococcus faecalis: more than just a plasmid-collection mechanism? Eur. J. Biochem. 222:235-246. [DOI] [PubMed] [Google Scholar]
- 47.Zar, J. H. 1996. Biostatistical analysis, 3rd ed. Prentice-Hall International Editions, London, England.