Abstract
On the basis of earlier reports associating Epstein-Barr Virus (EBV) with half of the cases of idiopathic pulmonary fibrosis (IPF), we hypothesized that chronic infection with EBV or a closely related herpesvirus would be detected in all cases of IPF. We tested lung specimens from 33 IPF patients (8 patients with familial IPF and 25 patients with sporadic IPF) and 25 patients with other diseases as controls for the presence of eight herpesviruses using PCR-based techniques. One or more of four herpesviruses (cytomegalovirus [CMV], EBV, human herpesvirus 7 [HHV-7], and HHV-8) were detected in 32 of 33 (97%) subjects with IPF and in 9 of 25 (36%) controls (P < 0.0001). CMV, EBV, and HHV-8 were found more frequently in IPF patients than in controls (P < 0.05, P < 0.001, and P < 0.01 respectively). Two or more herpesviruses were detected in 19 of 33 (57%) IPF patients and in 2 of 25 (8%) controls (P < 0.001). Two or more herpesviruses and HHV-8 were found more frequently in patients with sporadic IPF than in patients with familial IPF (P < 0.05 for both comparisons), and CMV was found less frequently in patients with sporadic IPF than in patients with familial IPF (P < 0.05). Immunohistochemistry for EBV or HHV-8 antigen showed viral antigen primarily in airway epithelial cells. These data support the concept that a herpesvirus could be a source of chronic antigenic stimulation in IPF.
Idiopathic pulmonary fibrosis (IPF) is a destructive lung disease of unknown cause characterized by progressive fibrosis with variable amounts of inflammation (13, 26). There is no truly effective therapy, and most patients with IPF die within 3 to 5 years following diagnosis (12). Clinically, IPF is characterized by the insidious onset of progressive dyspnea on exertion, diffuse reticular infiltrates on chest radiographs, decreased lung volumes, decreased diffusion capacity, and progressive hypoxemia. Exclusion of interstitial lung diseases of known etiology and the histologic pattern of usual interstitial pneumonitis (UIP) on lung biopsy confirm the clinical diagnosis.
IPF also occurs in a familial pattern. Familial IPF is defined as the occurrence of disease in two or more members of an immediate family, and the fraction of cases that are familial has been reported to be as little as 2% and as much as 25% of all IPF cases (8, 20). The clinical picture, pathophysiology, histology, and treatment outcomes are the same for familial and sporadic cases of IPF, suggesting a common etiology.
Several studies have suggested a possible role for chronic infection with Epstein-Barr virus (EBV), cytomegalovirus (CMV), adenovirus, or hepatitis C virus (9, 15, 21, 27, 31-33) as a persistent antigenic stimulus in IPF. However, even the most frequently identified virus, EBV, is detected by PCR or immunohistochemistry in the lungs of only half of the cases of IPF (27).
Over the last few years, several new herpesviruses have been identified. Some, such as human herpesvirus 8 (HHV-8), are clearly associated with human disease (i.e., Kaposi's sarcoma), and others, such as HHV-7, are only tenuously linked to human pathology (7, 16). On the basis of earlier reports associating EBV with half of the cases of IPF, we hypothesized that evidence of chronic infection with EBV or a closely related herpesvirus would be found in all cases of IPF. The aim of this study was to determine whether expanding the search for viruses in the lung tissues of IPF patients to include the more recently discovered herpesviruses would identify a potentially pathogenic virus in all subjects.
(This study was presented in part at the Thomas L. Petty Aspen Lung Conference, the 43rd Annual Meeting, Aspen, Colo., 31 May to 3 June, 2000.)
MATERIALS AND METHODS
Specimen collection.
Lung tissue for herpesvirus PCR was obtained from 33 patients with IPF (8 patients with familial IPF and 25 patients with sporadic IPF) and 25 patients with other diseases (patients with a lobectomy for single pulmonary nodule, n = 9; patients with pulmonary hypertension, n = 4; patients with sarcoidosis, n = 4; unused donor lungs for transplantation, n = 4; patients with bronchiolitis obliterans, n = 3; patients with spontaneous pneumothorax, n = 1). A diagnosis of IPF was made by using clinical criteria and was confirmed by lung histology, as shown in Table 1. Lung tissue was initially collected as part of the diagnostic evaluation or at the time of lung transplant or autopsy. All histologic sections from the IPF subjects were reviewed by an experienced pulmonary pathologist. This project was approved by the Institutional Review Board at Vanderbilt University.
TABLE 1.
Clinical characteristics and herpesvirus PCR results for IPF subjectsa
| IPF type and patient no. | Age (yr) | Sex | Tissue | Immunosuppressive therapy | Histology | Virus(es) for which PCR was positive |
|---|---|---|---|---|---|---|
| Familial | ||||||
| 1 | 56 | M | Transplant | Prednisone, Cytoxan | ES fibrosis | CMV |
| 2 | 59 | M | Autopsy | Prednisone, Imuran | ES fibrosis | CMV, HHV-7 |
| 3 | 53 | M | Transplant | Prednisone | UIP | EBV |
| 4 | 62 | F | Autopsy | Prednisone, Imuran | NSIP | CMV, EBV, HHV-7, HHV-8 |
| 5 | 66 | F | Autopsy | Prednisone, Imuran, Cytoxan | UIP | CMV |
| 6 | 50 | M | Autopsy | Prednisone, Neoral | UIP | EBV |
| 7 | 46 | M | Biopsy | None | UIP | EBV |
| 8 | 43 | M | Autopsy | Prednisone, Imuran | UIP | EBV |
| Sporadic | ||||||
| 1 | 57 | M | Autopsy | Prednisone | UIP | EBV, HHV-8 |
| 2 | 61 | F | Autopsy | Prednisone, Imuran | UIP | EBV |
| 3 | 54 | F | Transplant | Prednisone, Cytoxan | UIP | CMV, EBV, HHV-7 |
| 4 | 56 | F | Transplant | Prednisone | UIP | EBV, HHV-7 |
| 5 | 43 | M | Transplant | Prednisone, Imuran | UIP | EBV, HHV-8 |
| 6 | 54 | M | Transplant | Prednisone, Cytoxan | UIP | EBV, HHV-7 |
| 7 | 53 | M | Transplant | Prednisone, Imuran | UIP | EBV |
| 8 | 61 | F | Transplant | Prednisone, Imuran | UIP | EBV, HHV-7, HHV-8 |
| 9 | 51 | M | Transplant | Prednisone, Imuran | UIP | EBV, HHV-7, HHV-8 |
| 10 | 68 | M | Biopsy | None | UIP | EBV, HHV-7 |
| 11 | 55 | M | Biopsy | None | UIP | EBV, HHV-8 |
| 12 | 59 | F | Transplant | Prednisone | UIP | HHV-7, HHV-8 |
| 13 | 54 | M | Autopsy | None | UIP | CMV HHV-8 |
| 14 | 46 | M | Transplant | None | UIP | |
| 15 | 58 | F | Transplant | Prednisone, Imuran | ES fibrosis | EBV, HHV-8 |
| 16 | 55 | M | Transplant | Prednisone, Cytoxan | UIP | HHV-8 |
| 17 | 50 | M | Biopsy | Prednisone | NSIP | EBV, HHV-7 |
| 18 | 62 | M | Biopsy | Prednisone | UIP | HHV-7 |
| 19 | 56 | F | Transplant | Prednisone, Cytoxan | UIP | HHV-8 |
| 20 | 62 | M | Transplant | Prednisone, Cytoxan | UIP | HHV-8 |
| 21 | 60 | M | Transplant | None | UIP | EBV, HHV-7 |
| 22 | 63 | M | Transplant | Prednisone | ES fibrosis | HHV-8 |
| 23 | 54 | M | Biopsy | Prednisone, Actimmune | UIP | EBV, HHV-8 |
| 24 | 54 | F | Autopsy | Prednisone | UIP | CMV, EBV, HHV-7 |
| 25 | 43 | M | Transplant | Prednisone | UIP | HHV-7, HHV-8 |
Abbreviations: F, female; M, male; ES, end stage.
In the majority of instances, fresh lung tissue was obtained, rapidly frozen in liquid nitrogen, and stored at −80°C for later PCR analysis. For six of the controls and two of the IPF patients, formalin-fixed, paraffin-embedded lung tissue was used for PCR analysis. Serial morning or induced sputum specimens for estimation of the EBV load were collected from two IPF patients whose case histories are summarized.
DNA extraction and PCR detection.
Nucleic acids were extracted from lung tissue and sputum specimens with a QIAamp DNA Mini Kit according to the protocol of the manufacturer (Qiagen Inc., Valencia, Calif.). For the formalin-fixed, paraffin-embedded lung specimens, approximately 25 mg of each specimen was treated with xylene in order to remove the paraffin before tissue lysis and DNA extraction were performed. A colorimetric microtiter plate PCR system was used to detect the DNA of eight herpesviruses (herpes simplex virus type 1 [HSV-1], HSV-2, CMV, EBV, varicella-zoster virus, HHV-6, HHV-7, and HHV-8), as described previously (29). Briefly, the isolated DNA was subjected to amplification in 50 μl of the PCR mixture, with the addition of dignoxigenin-11-dUTP during the amplification so that the digoxigenin-11-dUTP was incorporated into the PCR product. After PCR amplification, a portion of the denatured amplification reaction was mixed with a hybridization solution containing a DNA capture probe specific for the relevant herpesvirus, with the probe labeled at the 5′ end with biotin. The probe hybridized to the corresponding target DNA sequence, and the resulting biotinylated DNA complexes were captured on the streptavidin-coated microtiter plate wells. Specific DNA complexes were detected with an anti-digoxigenin-peroxidase conjugate, which recognized digoxigenin-11-dUTP substitutions incorporated into the amplicon during PCR (29). The primers and probes used in the detection were adapted from previously published articles (3, 23, 28, 29).
A microtiter plate format similar to that described above was used to amplify the human β-actin gene to ensure the quality of the extracted DNA samples (17). To avoid amplification product contamination, reagent preparation and PCR setup were performed in a “dead-air” box in a room separated from the amplification area. In addition, a uracil-N-glycosylase-based inactivation system was adapted to control for possible amplicon carryover contamination (19, 29).
EBV quantitation.
A real-time PCR assay (5) was developed to monitor anti-EBV therapy in two patients (see below) by quantitation of EBV DNA loads in sputum specimens. A standard curve was constructed by determination of the amounts of DNA in six 10-fold serial dilutions of a plasmid standard whose sequence contained the primer-spanning region of the gene for EBV capsid protein gp220 (1). The 277-bp amplicon was generated by PCR (28) and was subsequently cloned into the pCR2.1 vector (Invitrogen, Carlsbad, Calif.) in order to construct plasmid pCR-gp220. The DNA concentration of the plasmid standard was determined by spectrophotometry at 260 nm.
For accurate quantitation, DNA was extracted from 0.1 ml of each sputum specimen. Quantitative PCR was performed on a 7700 ABI Prism sequence detector (Applied Biosystems, Foster City, Calif.). An aliquot of 5 μl of the extracted nucleic acid was added to 20 μl of reaction mixture containing 0.8 μM concentration of each primer and 0.4 μM fluorophore probe (final concentration) and was mixed with 25 μl of the TaqMan Universal PCR master mix (Applied Biosystems). The sequences of the primers and probes were the same as those used in the PCR performed in the microtiter plate format (28). The fluorogenic probe had a reporter dye (6-carboxyfluorescein) and a quencher dye (6-carboxytetramethylrhodomine) covalently linked to its 5′ and 3′ ends, respectively (Applied Biosystems). The TaqMan cycling conditions were 2 min of degradation of preamplified templates at 50°C and then 40 cycles of denaturation at 95°C for 15 s and annealing and extension at 58°C for 60 s.
Statistics.
Statistical analysis of the differences in the rates of positive PCR results among the groups was performed with Epiinfo software (version 6; Centers for Disease Control and Prevention, Atlanta, Ga.) by using two-tailed comparisons and Fisher's exact probability calculation. A P value <0.05 was considered statistically significant.
RESULTS
Clinical characteristics of study subjects.
The clinical characteristics of the IPF subjects are summarized in Table 1, and those of the control subjects with other diseases are summarized in Table 2. There were six males and two females with familial IPF (defined as occurrence of the disease in two or more first-degree relatives). The average age for the group with familial IPF was 54.4 ± 2.8 (standard error of the mean [SEM]) years. Lung specimens were obtained at autopsy from five patients, at the time of lung transplantation from two patients, and at the time of open lung biopsy from one patient. All but one of the subjects with familial IPF were on some form of immunosuppressive therapy at the time that tissue was obtained. Also, as shown in Table 1, there were 17 males and 8 females with sporadic IPF, and the average age for this group was 55.4 ± 1.2 (SEM) years. Lung specimens were obtained at autopsy from 4 patients, at the time of lung transplantation from 16 patients, and at the time of open lung biopsy from 5 patients. Five subjects with sporadic IPF were on no immunosuppressive treatment at the time that lung tissue was obtained.
TABLE 2.
Clinical characteristics and herpesvirus PCR results for disease control subjects with other diseases
| Control subject no. | Age (yr) | Sex | Tissue | Immunosuppressive therapy | Diagnosis | Virus(es) for which PCR was positive |
|---|---|---|---|---|---|---|
| 1 | UK | M | Lobectomy | None | Metastatic cancer | |
| 2 | 27 | F | Donor lung | UK | Organ donor | |
| 3 | 66 | M | Lobectomy | None | Lung cancer | HHV-7 |
| 4 | UK | UK | Autopsy | UK | PPH | EBV |
| 5 | UK | UK | Donor lung | UK | Organ donor | |
| 6 | UK | M | Lobectomy | UK | Lung cancer | |
| 7 | 48 | M | Biopsy | None | Sp pneumo | HHV-7, HHV-8 |
| 8 | UK | M | Lobectomy | UK | Lung cancer | |
| 9 | UK | F | Lobectomy | UK | Lung cancer | |
| 10 | 63 | F | Lobectomy | None | Lung cancer | EBV |
| 11 | 74 | UK | Lobectomy | None | Lung cancer | |
| 12 | UK | UK | Lobectomy | UK | Lung cancer | |
| 13 | UK | UK | Donor lung | UK | Organ donor | EBV, HHV-7 |
| 14 | UK | UK | Donor lung | UK | Organ donor | |
| 15 | 44 | F | Transplant | None | ASD + Sec PH | |
| 16 | 41 | F | Transplant | None | PPH | |
| 17 | 29 | M | Transplant | None | PPH | HHV-8 |
| 18 | 39 | M | Transplant | UK | Sarcoid | |
| 19 | 41 | F | Transplant | Prednisone | Sarcoid | HHV-7 |
| 20 | 20 | F | Biopsy | None | BOOP | |
| 21 | 49 | F | Biopsy | Prednisone | BOOP | |
| 22 | 67 | M | Biopsy | Prednisone | BOOP | HHV-7 |
| 23 | UK | UK | Lobectomy | UK | Lung cancer | |
| 24 | UK | UK | Biopsy | UK | Sarcoid | |
| 25 | UK | M | Biopsy | UK | Sarcoid | HHV-7 |
Abbreviations: F, female; M, male; UK, unknown; PPH, primary pulmonary hypertension; Sp pneumo, spontaneous pneumothorax; ASD + Sec pH, atrial septal defect and secondary pulmonary hypertension; BOOP, bronchiolitis obliterans.
The histologic diagnoses for the lungs, confirmed by an experienced pulmonary pathologist, are shown in Table 1 for each IPF subject. Until very recently, the general consensus was that the clinical diagnosis of IPF is confirmed by demonstrating the histologic pattern of UIP on lung histology, which was the histologic diagnosis for 27 of the 33 IPF subjects. For four subjects, lung histology was available only at the time of transplantation, but the fibrosis was so severe that no specific classification was possible. Finally, the histologic classification of nonspecific interstitial pneumonitis (NSIP) was made in two subjects. In the past, NSIP was not considered consistent with a clinical diagnosis of IPF. However, a recent report has shown that multiple lung biopsy specimens from the same person with a clinical diagnosis of IPF can show a histologic pattern of NSIP in one specimen and of UIP in another (11). Furthermore, the prognoses and clinical outcomes for those subjects with discordant biopsy results (NSIP and UIP) are the same as those for subjects for whom all biopsy specimens showed UIP. Thus, we included the two patients with a clinical diagnosis of IPF and a histologic diagnosis of NSIP as their disease progressed; these conditions resulted in death due to respiratory failure in one patient and the need for a lung transplant in the other.
As shown in Table 2, among the control subjects, there was an equal distribution of males and females, and the average age of the controls was 46.8 ± 4.6 (SEM) years.
Increased herpesvirus DNA detection in IPF.
A housekeeping gene, the gene for human β-actin, was detectable in all DNA samples extracted from either frozen or paraffin-embedded lung tissues, indicating that these DNA samples were free of amplification inhibitors.
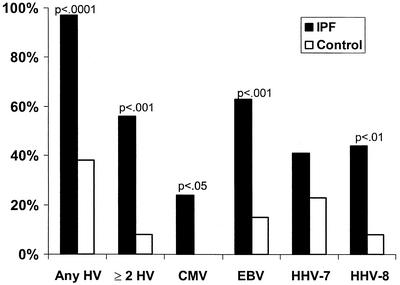
The PCR test results for the eight herpesviruses for which PCRs were conducted are summarized in Table 1 for the 33 IPF patients and Table 2 for the 25 controls with other diseases. CMV, EBV, HHV-7, and/or HHV-8 was identified in lung tissues from 32 of 33 (97%) IPF patients, 6 of whom were receiving no immunosuppressive therapy. One of these four herpesviruses was present in 9 of 25 (36%) control samples. Viruses were detected significantly more frequently in the 33 IPF subjects than in the 25 control subjects, as follows: (i) any of the four herpesviruses mentioned above, P < 0.0001; (ii) two or more herpesviruses, P < 0.001; (iii) CMV, P < 0.05; (iv) EBV, P < 0.001; and (v) HHV-8, P < 0.01 (Fig. 1).
FIG. 1.
Percentage of lung samples from 33 patients with IPF and 25 controls with other diseases positive for herpesviruses (HVs) by PCR.
Also, as shown in Fig. 1, HHV-7 was detected at similar frequencies in IPF and control subjects (14 of 33 [42%] IPF subjects and 6 of 25 [24%] control subjects [P > 0.05]). However, other herpesviruses were also detected in 13 of the 14 IPF patients positive for HHV-7, whereas in the controls with other diseases, another herpesvirus was detected in only two of the six HHV-7-positive samples. None of the 58 lung samples were positive for HSV-1, HSV-2, varicella-zoster virus, or HHV-6.
Comparison of sporadic IPF to familial IPF.
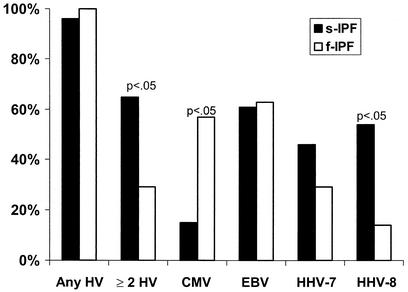
Figure 2 compares the rates of herpesvirus DNA detection in the lungs of patients with sporadic IPF versus those in patients with familial IPF. HHV-8 was detected significantly more frequently (P < 0.05) and CMV was detected significantly less frequently (P < 0.05) in patients with sporadic IPF than in patients with familial IPF. Also, two or more herpesviruses were significantly (P < 0.05) more likely to be detected in patients with sporadic IPF than in patients with familial IPF.
FIG. 2.
Percentage of lung samples from 25 patients with sporadic IPF (s-IPF) and 8 patients with familial IPF (f-IPF) positive for herpesviruses (HVs) by PCR.
The high rate of detection of HHV-8 is unusual. HHV-8 is detected consistently in patients with AIDS-associated Kaposi's sarcoma and is frequently detected in certain parts of the world where Kaposi's sarcoma is endemic, such as the eastern Mediterranean and sub-Saharan Africa. However, it is uncommon in the general population in the United States (less than 5% of the U.S. population is serologically positive for HHV-8) (6, 16, 22). Human immunodeficiency virus (HIV) infection status was not routinely determined in this study, but 10 of the 15 HHV-8-positive patients were lung transplant recipients and therefore were screened for HIV and were negative.
HHV-8 immunohistochemistry.
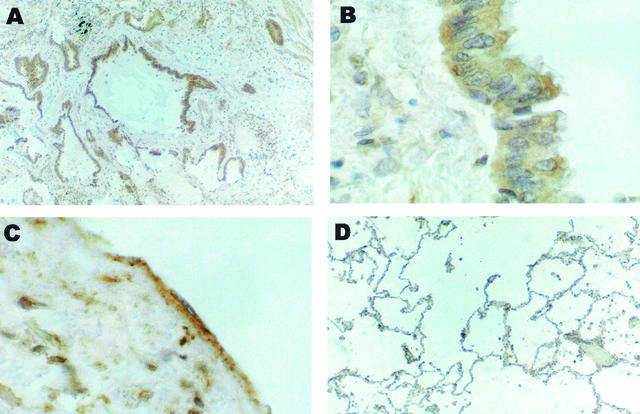
EBV antigen is seen in bronchiolar and alveolar epithelial cells of IPF patients (27). In patients with Kaposi's sarcoma, HHV-8 infects endothelium-derived spindle cells (4). Therefore, we asked, which lung cells harbor HHV-8 in IPF patients? Immunohistochemical staining with a sheep polyclonal antibody against HHV-8 cyclin showed positive staining of airway epithelial cells and minimal staining of endothelial cells (Fig. 3).
FIG. 3.
Immunohistochemical staining of lung samples from two IPF patients for HHV-8. (A) The epithelium of all airspaces in a densely fibrotic area from sporadic IPF patient 8 marks uniformly for HHV-8 k-cyclin protein. Magnification, ×62.5. (B) Epithelial cells of a larger bronchiole from sporadic IPF patient 1 are positive for HHV-8. Magnification, ×250. (C) Endothelial cells and scattered subendothelial intimal cells from sporadic IPF patient 1 express HHV-8 k-cyclin protein. Magnification, ×250. (D) Low-power view of control tissue (normal parenchyma adjacent to a carcinoma) from control patient 11 is uniformly negative for HHV-8 cyclin. Magnification, ×62.5.
Case histories of two IPF patients.
Two case histories, one for a patient with sporadic IPF and one for a patient with familial IPF, are presented to show that herpesviruses can be detected very early in the course of the disease, before any therapy is instituted, and can persist for prolonged periods.
Case patient 1 (sporadic IPF) was a 68-year-old man who was noted to have hypoxemia during a presurgical evaluation for an orthopedic procedure. In retrospect, he had noted increased shortness of breath, decreased physical activity, and cough for 2 months. His past medical history was remarkable only for systemic hypertension and benign proteinuria. Physical examination was remarkable for peripheral cyanosis, diminished breath sounds in the upper lobes, and basilar crackles. Pulmonary function tests showed a mild restrictive defect. A radiograph of the chest revealed increased peripheral lung markings, and computerized tomography of the chest revealed bibasilar subpleural reticular infiltration and honeycombing with no ground glass opacity, changes typical of IPF. Surgical lung biopsy showed UIP. PCR of fresh frozen lung tissue for herpesviruses was positive for EBV and HHV-7. Immunohistochemistry was performed and identified EBV antigen in respiratory epithelial cells and HHV-7 antigen in endothelial cells of small arteries, as well as in lymphocytes in bronchus-associated lymphoid tissue. Shortness of breath, hypoxemia, and pulmonary function deteriorated, and prednisone was prescribed, with no benefit.
Twenty months after the lung biopsy was performed, EBV and HHV-7 were detected in four induced sputum samples by PCR. By use of the quantitative PCR described in the Materials and Methods, the EBV load was calculated to range between 500 and 10,500 copies/ml in four morning sputum samples collected on 2 separate days. Over this period, the patient's forced vital capacity had decreased by 0.30 liters and his total lung capacity had decreased by 1.24 liters. Because his clinical condition had deteriorated significantly, treatment with gamma interferon at 100 g subcutaneously 3 times per week was begun, along with valacyclovir at 2,000 mg/day (valacyclovir is an antiviral agent with activity against EBV but not against HHV-7). As shown in Table 3, after this treatment EBV was no longer detected in induced sputum. However, detection of HHV-7 by PCR has persisted in multiple sputum specimens. After almost 1.5 years of therapy with valacyclovir and gamma interferon, mild liver function abnormalities developed and these drugs were discontinued (as were other potential hepatotoxic drugs, allopurinol and atorvastatin calcium [Lipitor]). Unfortunately, the patient's degree of dyspnea prevented him from performing pulmonary function testing and, therefore, prevented documentation of any response to therapy. However, his clinical symptoms have deteriorated significantly over the 3.5-year course of his disease.
TABLE 3.
EBV loads in sputum specimens of two IPF patients before and after oral valacyclovir administration
| IPF case patient no. | Time (mo) after treatment | EBV load (no. of copies/ml) |
|---|---|---|
| 1 | Before | 500-10,500 |
| 1 | Undetected | |
| 3 | Undetected | |
| 6 | Undetected | |
| 9 | Undetected | |
| 2 | Before | 40-500 |
| 1 | Undetected | |
| 3 | Undetected | |
| 6 | Undetected | |
| 9 | Undetected |
Case patient 2 (familial IPF) was a 46-year-old farmer whose brother and father had died of IPF. This patient had a history of seasonal allergic rhinitis and was self-referred to the Pulmonary Clinic at Vanderbilt University because of his family history of IPF. At the initial evaluation, he denied any symptoms except for allergic rhinitis with specific seasonal triggers. Initial physical examination was negative. Pulmonary function tests showed minimal restrictive lung disease. A radiograph of the chest showed rare scattered calcified granulomas. High-resolution computed tomography of the chest showed scattered wedge-shaped subpleural densities, reticular infiltrates, and occasional honeycomb patterns. Tests for an infectious etiology for his lung disease or an autoimmune disorder were negative.
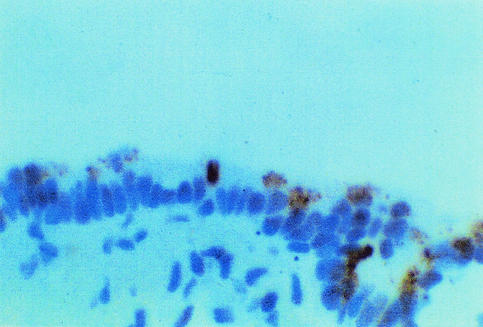
Over the subsequent 2 years, the patient remained free of respiratory symptoms; but his pulmonary function tests worsened, and a repeat high-resolution computed tomography of the chest showed increased honeycombing and subpleural densities. A diagnostic thorascopic lung biopsy was performed and showed UIP. A sample of lung tissue was positive for EBV DNA by PCR. Immunohistochemistry for EBV latent membrane protein demonstrated abundant positive staining of airway epithelial cells (Fig. 4). Serologic examination was positive for immunoglobulin G (IgG) antibodies against EBV VCA and EBNA, negative for IgG antibody to EBV EAs, and negative for EBV IgM antibody.
FIG. 4.
Immunohistochemical staining of lung tissue from case patient 2 showing airway epithelial cells in a small bronchiole staining positive for EBV latent membrane protein.
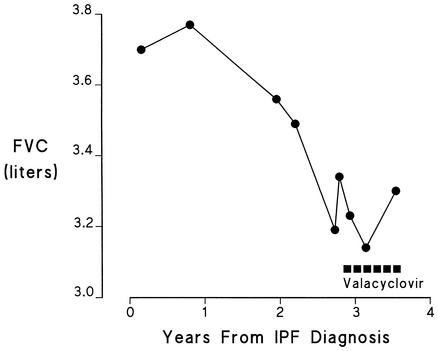
Nine months after the lung biopsy was performed, multiple induced sputum samples demonstrated 40 to 500 copies of EBV per ml. After discussions between the pulmonologist and the patient concerning the rapid progression of this disease in his family (the patient's father died at the age of 57, 1 year after being diagnosed with IPF, and the patient's brother required a lung transplant at age 48, 1 year after being diagnosed with IPF, and died 2 years later) and the lack of firm evidence that the EBV could be contributing to his disease, the patient was started on a 9-month course of valacyclovir orally (2 g twice a day). He had previously declined treatment with corticosteroids, perhaps in part related to the absence of any apparent efficacy, as well as the development of numerous side effects in his brother and father during such treatment. As in case patient 1, the EBV load diminished during this course of therapy (Table 3). Coincidental with this antiviral therapy, his pulmonary function stabilized (Fig. 5).
FIG. 5.
Serial pulmonary function testing (forced vital capacity [FVC]) in case patient 2 before and after the start of valacyclovir therapy.
DISCUSSION
The pathogenic sequences leading to the development of IPF are unclear; but one theory is that, in a genetically susceptible host, there is a “triggering agent or event induc(ing) an inflammatory reaction in the lung that perpetuates itself and causes parenchymal fibrosis” (26). One potential source for a self-perpetuating triggering event could be a chronic viral infection (10, 18). For this reason, evidence for an association between IPF and chronic viral infection has been sought for several different viruses, including EBV (9, 27, 32, 33), CMV (33), adenovirus (15), and hepatitis C virus (21, 31). Evidence for a link between an EBV infection and IPF was first suggested by a French group of investigators who tested serum and bronchoalveolar lavage specimens for antibodies against EBV in 13 patients with IPF: their data suggested active infection of the lung with EBV (32). Egan et al. (9) detected EBV lytic proteins (viral capsid antigen and gp340/220) in 70% of lung samples from 20 patients with IPF, 14 of whom had received no immunosuppressive therapy, and in only 2 of 21 (9%) control subjects with lung disease. Viral antigen was localized to alveolar epithelial cells but was also detected in some interstitial inflammatory cells (lymphocytes) and alveolar macrophages. By using PCR-based technology, EBV was detected significantly more frequently in the lungs of IPF patients than in controls in studies from Japan (30) and the United Kingdom (27) and in the present study.
One of the problems with the use of PCR-based techniques to establish a link between EBV and IPF is that the peripheral blood B cells of virtually all adults have a latent EBV infection. Although most adults do not have evidence of EBV infection in the lung, the more sensitive nested PCR techniques can detect extremely small numbers of copies of the virus, and the latently infected B cells in the circulation could give a false-positive PCR result for EBV infection of the lung. To circumvent this problem, Kelly et al. (14) examined peripheral blood and lung tissue specimens from IPF patients and controls for rearranged EBV genomic fragments (WZhet), which are associated with EBV replication and virion production in other EBV-related human diseases. They reevaluated lung samples from 18 IPF patients that they had previously found to be EBV DNA positive and found that 61% of the lung samples were also WZhet positive. For six of these IPF patients whose lung tissue was WZhet positive, buffy coats were analyzed and all six blood samples were WZhet positive. The buffy coats of a larger sample of IPF patients, transplant patients on immunosuppression therapy, and blood donors were analyzed for EBV DNA and WZhet. The analysis of buffy coats for WZhet was positive for 59% of IPF patients, none of the transplant patients, and 4% of the blood donors, indicating that about half of the patients with IPF have evidence of a pulmonary infection with EBV.
The case histories that we presented showed that the EBV gene and protein were detected in the lungs of patients with IPF prior to the institution of any therapy and, in case patient 2, prior to the appearance of any respiratory symptoms; the virus persisted in respiratory tract secretions for several months; and pulmonary function stabilized after the institution of antiviral therapy. These findings begin to provide a basis for a causal link between IPF and EBV infection. In addition, data implicating a viral etiology in analogous diseases may be relevant to building this argument. Similar to the lung pathology in patients with IPF, chronic interstitial nephritis is characterized by interstitial fibrosis and a mononuclear cell infiltrate in the kidneys (2). In one study, EBV was found by in situ hybridization in the epithelium of the proximal renal tubules in nine of nine patients with this disease but in none of the controls (2). EBV colocalized with its cellular receptor in the proximal tubular epithelium, where the receptor is expressed. The epithelial localization of the virus is of particular interest, since some evidence suggests that fibrogenesis involves transformation of epithelial cells into fibroblasts (24). This study supports the concept that EBV infection of renal epithelial cells contributes to the pathogenesis of chronic interstitial nephritis with characteristic interstitial fibrosis. If interstitial fibrosis in the lungs is pathogenetically analogous to that in the kidney, chronic viral infection of epithelial cells may be a trigger for fibrogenesis in both organs. Prior to the presentation of the data described here, that conclusion was difficult to support, since only about half of the patients with IPF had been demonstrated to have EBV in their lung tissue.
Over the last few years, several new herpesviruses have been identified. Some (e.g., HHV-8) have a clear association with significant human disease (Kaposi's sarcoma), and others (e.g., HHV-7) are linked only tenuously to human disease (7, 16). Our study extends the findings of previous studies associating EBV infection with IPF by testing for these newly discovered herpesviruses. In addition to EBV and CMV, which were detected in the lungs of 64 and 21% of the IPF patients, respectively (in each case, this rate of detection was significantly greater than those for the controls with other diseases), we also detected the DNA of other herpesviruses (HHV-7 and HHV-8) in the lungs of the IPF patients. Of these two herpesviruses, HHV-8 was found at a significantly greater frequency in the lung tissue from the IPF patients than in the lung tissue from the controls. In total, we detected herpesvirus DNA in the lungs of all but 1 of the 33 IPF patients studied, whereas we detected herpesvirus DNA in the lungs of only 36% of the controls with other diseases.
The increased frequency of HHV-8 detection in lung samples from IPF patients is particularly interesting. HHV-8 was detected in lung samples from 15 of 33 (45%) of the IPF patients (all but 1 of these patients had the sporadic form of the disease), whereas it was detected in only 8% of the control subjects. In the United States, HHV-8 infection is found almost exclusively in patients with HIV infection and Kaposi's sarcoma, multicentric-type Castleman's disease, or primary effusion lymphoma (22). Although we did not test all of the patients in our study for HIV infection status, most of the HHV-8-positive patients were lung transplant recipients and were HIV negative. Thus, occult HIV infection is unlikely to explain the high frequency of detection of HHV-8 in at least this subgroup of IPF patients. Further studies will be necessary to determine whether the striking association of HHV-8 with IPF is pathogenetically related to the lung disease.
Although the frequencies of detection of HHV-7 in the lungs of the IPF and control subjects were not significantly different, almost all of the lungs of IPF patients that were positive for HHV-7 were also positive for at least one other herpesvirus (Table 1), while another herpesvirus was detected in only one-third of the HHV-7-positive lungs of the control subjects (Table 2). HHV-7 is a ubiquitous virus (>85% of the U.S. population is infected during childhood) that is not clearly related to any human disease but may exaggerate disease due to a pathogenic herpesvirus (16). For example, HHV-7 is associated with exanthem subitum (3), but HHV-6 may be the real cause of the disease, with HHV-7 acting to reactivate HHV-6 (16). In addition, HHV-7 coinfection in renal allograft recipients infected with CMV causes progression of CMV-related disease (25).
Two or more herpesviruses were detected significantly more often in the lungs of the IPF subjects than in the lungs of the controls with other diseases (P < 0.001), and coinfection occurred significantly more frequently in the patients with the sporadic form of IPF than in those with the familial form of the disease (P < 0.05). These data suggest that patients with familial IPF may require less antigenic stimulus to trigger a progressive fibrotic response than patients with sporadic IPF. Such a conclusion would be compatible with the notion that chronic pulmonary herpesvirus infection causes IPF and that there is a genetic basis for increased host susceptibility in the familial form so that a single virus is a sufficient trigger, but that a larger antigenic stimulus resulting from infection with multiple viruses is required to trigger fibrosis in individuals who are less susceptible.
In summary, we have detected the DNA of four herpesviruses (CMV, EBV, HHV-7, and HHV-8) in the lungs of all but 1 of 33 unselected patients with familial or sporadic IPF, a frequency much greater than that for the lungs of the controls with other diseases. Although our data cannot establish herpesvirus infection as the cause of IPF, in the two patients whose case histories were presented, virus was detected early in the course of the disease, prior to the institution of any therapy (and in case patient 2, before the appearance of any symptoms), and persisted. This temporal relationship is consistent with causation. In case patient 2, the decrease in the rate of decline in pulmonary function, together with a decrease in sputum viral load following the institution of antiviral therapy, suggests an association consistent with causation.
Establishment of chronic pulmonary herpesvirus infection as the cause of IPF will require detection of a herpesvirus in the lungs before or at the beginning of the time of appearance of clinical manifestations in a large sample of untreated patients early in the course of their disease and demonstration that eradication of the viral infection stops the progression of lung fibrosis. The rapid, PCR-based method for quantitation of the number of viral DNA copies in sputum from patients with IPF that we describe awaits further evaluation as an alternative tool for the evaluation and monitoring of patients in such studies.
Acknowledgments
We thank Haijing Li, Gayle King, Sandy Olson, and James Atkinson for excellent technical assistance.
REFERENCES
- 1.Baer, R., A. T. Bankier, M. D. Biggin, P. L. Deininger, P. J. Farrell, T. J. Gibson, G. Hatfull, G. S. Hudson, S. C. Satchwell, and C. Seguin. 1984. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature 310:207-211. [DOI] [PubMed] [Google Scholar]
- 2.Becker, J. L., F. Miller, G. J. Nuovo, C. Josepovitz, W. H. Schubach, and E. P. Nord. 1999. Epstein-Barr virus infection of renal proximal tubule cells: possible role in chronic interstitial nephritis. J. Clin. Investig. 104:1673-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berneman, Z. N., D. V. Ablashi, G. Li, M. Eger-Fletcher, M. S. Reitz, Jr., C. L. Hung, I. Brus, A. L. Komaroff, and R. C. Gallo. 1992. Human herpesvirus 7 is a T-lymphotropic virus and is related to, but significantly different from, human herpesvirus 6 and human cytomegalovirus. Proc. Natl. Acad. Sci. USA 89:10552-10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Browning, P. J., J. M. Sechler, M. Kaplan, R. H. Washington, R. Gendelman, R. Yarchoan, B. Ensoli, and R. C. Gallo. 1994. Identification and culture of Kaposi's sarcoma-like spindle cells from the peripheral blood of human immunodeficiency virus-1-infected individuals and normal controls. Blood 84:2711-2720. [PubMed] [Google Scholar]
- 5.Cardullo, R. A., S. Agrawal, C. Flores, P. C. Zamecnik, and D. E. Wolf. 1988. Detection of nucleic acid hybridization by nonradiative fluorescence resonance energy transfer. Proc. Natl. Acad. Sci. USA 85:8790-8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatlynne, L. G., and D. V. Ablashi. 1999. Seroepidemiology of Kaposi's sarcoma-associated herpesvirus (KSHV). Semin. Can. Biol. 9:175-185. [DOI] [PubMed] [Google Scholar]
- 7.Clark, D. A., M. L. Freeland, L. K. Mackie, R. F. Jarrett, and D. E. Onions. 1993. Prevalence of antibody to human herpesvirus 7 by age. J. Infect. Dis. 168:251-252. [DOI] [PubMed] [Google Scholar]
- 8.Donohue, W. L. 1959. Familial fibrocystic pulmonary dysplasia and its relationship to Hamman-Rich syndrome. Pediatrics 24:786-819. [PubMed] [Google Scholar]
- 9.Egan, J. J., J. P. Stewart, P. S. Hasleton, J. R. Arrand, K. B. Carroll, and A. A. Woodcock. 1995. Epstein-Barr virus replication within pulmonary epithelial cells in cryptogenic fibrosing alveolitis. Thorax 50:1234-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan, J. J., A. A. Woodcock, and J. P. Stewart. 1997. Viruses and idiopathic pulmonary fibrosis. Eur. Respir. J. 10:1433-1437. [DOI] [PubMed] [Google Scholar]
- 11.Flaherty, K. R., W. D. Travis, T. V. Colby, G. B. Toews, E. A. Kazerooni, B. H. Gross, A. Jain, R. L. Strawderman, A. Flint, J. P. Lynch, and F. J. Martinez. 2001. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am. J. Respir. Crit. Care Med. 164:1722-1727. [DOI] [PubMed] [Google Scholar]
- 12.Hunninghake, G. W., and A. R. Kalica. 1995. Approaches to the treatment of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 151:915-918. [DOI] [PubMed] [Google Scholar]
- 13.Katzenstein, A. L., and J. L. Myers. 1998. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am. J. Respir. Crit. Care Med. 157:1301-1315. [DOI] [PubMed] [Google Scholar]
- 14.Kelly, B. G., S. S. Lok, P. S. Hasleton, J. J. Egan, and J. P. Stewart. 2002. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 166:510-513. [DOI] [PubMed] [Google Scholar]
- 15.Kuwano, K., Y. Nomoto, R. Kunitake, N. Hagimoto, T. Matsuba, Y. Nakanishi, and N. Hara. 1997. Detection of adenovirus E1A DNA in pulmonary fibrosis using nested polymerase chain reaction. Eur. Respir. J. 10:1445-1449. [DOI] [PubMed] [Google Scholar]
- 16.Levy, J. A. 1997. Three new human herpesviruses (HHV6, 7, and 8). Lancet 349:558-563. [DOI] [PubMed] [Google Scholar]
- 17.Li, H., J. S. Dummer, W. R. Estes, S. Meng, P. F. Wright, and Y. W. Tang. 2003. Measurement of human cytomegalovirus loads by quantitative real-time PCR for monitoring clinical intervention in transplant recipients. J. Clin. Microbiol. 41:187-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lok, S. S., and J. J. Egan. 2000. Viruses and idiopathic pulmonary fibrosis. Monaldi Arch. Chest Dis. 55:146-150. [PubMed] [Google Scholar]
- 19.Longo, M. C., M. S. Berninger, and J. L. Hartley. 1990. Use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene 93:125-128. [DOI] [PubMed] [Google Scholar]
- 20.Marshall, R. P., A. Puddicombe, W. O. Cookson, and G. J. Laurent. 2000. Adult familial cryptogenic fibrosing alveolitis in the United Kingdom. Thorax 55:143-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meliconi, R., P. Andreone, L. Fasano, S. Galli, A. Pacilli, R. Miniero, M. Fabbri, L. Solforosi, and M. Bernardi. 1996. Incidence of hepatitis C virus infection in Italian patients with idiopathic pulmonary fibrosis. Thorax 51:315-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore, P. S. 2000. The emergence of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8). N. Engl. J. Med. 343:1411-1413. [DOI] [PubMed] [Google Scholar]
- 23.Moore, P. S., and Y. Chang. 1995. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N. Engl. J. Med. 332:1181-1185. [DOI] [PubMed] [Google Scholar]
- 24.Okada, H., T. M. Danoff, R. Kalluri, and E. G. Neilson. 1997. Early role of Fsp1 in epithelial-mesenchymal transformation. Am. J. Physiol. 273:F563-F574. [DOI] [PubMed] [Google Scholar]
- 25.Osman, H. K., J. S. Peiris, C. E. Taylor, P. Warwicker, R. F. Jarrett, and C. R. Madeley. 1996. “Cytomegalovirus disease” in renal allograft recipients: is human herpesvirus 7 a co-factor for disease progression? J. Med. Virol. 48:295-301. [DOI] [PubMed] [Google Scholar]
- 26.Ryu, J. H., T. V. Colby, and T. E. Hartman. 1998. Idiopathic pulmonary fibrosis: current concepts. Mayo Clin. Proc. 73:1085-1101. [DOI] [PubMed] [Google Scholar]
- 27.Stewart, J. P., J. J. Egan, A. J. Ross, B. G. Kelly, S. S. Lok, P. S. Hasleton, and A. A. Woodcock. 1999. The detection of Epstein-Barr virus DNA in lung tissue from patients with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 159:1336-1341. [DOI] [PubMed] [Google Scholar]
- 28.Tang, Y. W., M. J. Espy, D. H. Persing, and T. F. Smith. 1997. Molecular evidence and clinical significance of herpesvirus coinfection in the central nervous system. J. Clin. Microbiol. 35:2869-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang, Y. W., P. N. Rys, B. J. Rutledge, P. S. Mitchell, T. F. Smith, and D. H. Persing. 1998. Comparative evaluation of colorimetric microtiter plate systems for detection of herpes simplex virus in cerebrospinal fluid. J. Clin. Microbiol. 36:2714-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsukamoto, K., H. Hayakawa, A. Sato, K. Chida, H. Nakamura, and K. Miura. 2000. Involvement of Epstein-Barr virus latent membrane protein 1 in disease progression in patients with idiopathic pulmonary fibrosis. Thorax 55:958-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueda, T., K. Ohta, N. Suzuki, M. Yamaguchi, K. Hirai, T. Horiuchi, J. Watanabe, T. Miyamoto, and K. Ito. 1992. Idiopathic pulmonary fibrosis and high prevalence of serum antibodies to hepatitis C virus. Am. Rev. Respir. Dis. 146:266-268. [DOI] [PubMed] [Google Scholar]
- 32.Vergnon, J. M., M. Vincent, G. de The, J. F. Mornex, P. Weynants, and J. Brune. 1984. Cryptogenic fibrosing alveolitis and Epstein-Barr virus: an association? Lancet ii:768-771. [DOI] [PubMed]
- 33.Yonemaru, M., I. Kasuga, H. Kusumoto, A. Kunisawa, H. Kiyokawa, S. Kuwabara, Y. Ichinose, and K. Toyama. 1997. Elevation of antibodies to cytomegalovirus and other herpes viruses in pulmonary fibrosis. Eur. Respir. J. 10:2040-2045. [DOI] [PubMed] [Google Scholar]