Abstract
Cryptosporidium parvum and Cryptosporidium hominis isolates from human immunodeficiency virus-infected patients, cattle, and wild ruminants were characterized by PCR and DNA sequencing analysis of the 60-kDa glycoprotein gene. Seven alleles were identified, three corresponding to C. hominis and four corresponding to C. parvum. One new allele was found (IId), and one (IIb) had only been found in Portugal. Isolates from cattle and wild ruminants clustered in two alleles. In contrast, human isolates clustered in seven alleles, showing extensive allelic diversity.
Human cryptosporidiosis is mainly caused by Cryptosporidium parvum and Cryptosporidium hominis (previously known as the C. parvum human genotype) (7, 13). C. hominis is found almost exclusively in humans, whereas C. parvum is found in domestic livestock, wild animals, and humans (1, 3, 6, 9, 20, 21, 23, 24). The occurrence in humans of both Cryptosporidium parasites has provided evidence that both anthroponotic and zoonotic cycles can occur in human infections (12, 14, 17).
The GP60 gene (also known as Cpgp15/45) encodes a precursor protein that is proteolytically cleaved to yield mature cell surface glycoproteins gp45 and gp15 (also known as Cp17), both of which are implicated in zoite attachment to and invasion of enterocytes (4, 16). An important feature of this gene is its high degree of sequence polymorphism, particularly among C. hominis isolates, which is far greater than any other Cryptosporidium genetic loci examined to date (8, 18, 19). Recently, Strong et al. described five alleles based on extensive sequence polymorphisms in the GP60 gene, one containing all C. parvum isolates and four containing C. hominis isolates (18). Within each allele, there are different subgenotypes based on the number of a trinucleotide repeat. These results highlight the usefulness of the subgenotype analysis for fingerprinting Cryptosporidium isolates.
In this study we characterized Cryptosporidium isolates from human immunodeficiency virus (HIV)-infected patients, cattle, and zoo ruminants from Portugal by PCR restriction fragment length polymorphism (RFLP) and DNA sequencing analysis of the small-subunit rRNA (SSU rRNA) and GP60 genes. Results of the study showed extensive genetic diversity in C. parvum and C. hominis isolates from humans but only limited genetic diversity in C. parvum from cattle and zoo ruminants.
Cryptosporidium isolates.
A total of 75 Cryptosporidium isolates from human HIV-infected patients from the Lisbon area with diarrhea, calves from four different geographic areas in Portugal (central, central/southern, southern, and Azores Islands), and wild ruminants from the Lisbon Zoo were used in this study (Table 1). Cryptosporidium oocysts were detected in stool specimens by modified Ziehl-Neelsen staining.
TABLE 1.
Cryptosporidium isolates and PCR RFLP analysis at the SSU rRNA locus
| Isolate source | Total no. of isolates studied | No. of isolates of species:
|
|||
|---|---|---|---|---|---|
| C. parvum (bovine genotype) | C. hominis | C. felis | C. meleagridis | ||
| HIV-infecteda humans | 30b | 16 | 7 | 3 | 3 |
| Calves | 35 | 35 | 0 | 0 | 0 |
| Zoo ruminantsc | 10 | 10 | 0 | 0 | 0 |
One isolate was a sporadic case from a non-HIV-infected person.
Due to amplification failure, no RFLP data was obtained for 1 isolate.
The 10 ruminants were 4 Arabian oryxes, 3 gemsboks, and 3 addaxes.
Molecular analysis.
Oocysts were concentrated from whole feces by a modified water-ether sedimentation method (2). Genomic DNA was isolated from concentrated oocysts by a KOH/QIAamp DNA stool mini kit protocol (QIAGEN, Valencia, Calif.) as previously described (22). Cryptosporidium species and the C. parvum genotype were determined by nested PCR of a SSU rRNA gene fragment and RFLP analysis with the endonucleases VspI and SspI, as described previously (24).
Subgenotyping was achieved by sequence analysis of the GP60 gene. A fragment of the GP60 gene (800 to 850 bp) was amplified by a nested PCR, with the primers AL3531 and AL3535 (5′-GGA AGG AAC GAT GTA TCT-3′) in the primary PCR and AL3532 and AL3534 in the secondary PCR (15). PCRs were performed as previously described (5). PCR products were sequenced in both directions on an ABI Prism 3100 automated sequencer (Applied Biosystems, Foster City, Calif.) with the primers AL3532 and AL3534. Nucleotide sequences obtained from various isolates were aligned with each other and published sequences by using the GCG program (Genetics Computers Group, Madison, Wis.) with manual adjustment. A neighbor-joining tree was constructed by using the Treecom W program, based on genetic distance calculated by Kimura two-parameter model (25). The reliability of the groupings was assessed by bootstrapping analysis, with 1,000 pseudo replicates.
Nucleotide sequence accession number.
The GP60 nucleotide sequences of seven alleles were deposited in the GenBank database under accession no. AY166804 to AY166810.
Cryptosporidium species.
PCR products of the SSU rRNA locus were obtained for all the animal isolates (35 calves and 10 wild ruminants) and for 29 of the 30 human isolates. RFLP analyses with SspI and VspI showed that all animals were infected with C. parvum bovine genotype while humans were infected mostly with C. parvum bovine genotype (16 of 29 isolates) and C. hominis (7 of 29 isolates). Three patients were infected with Cryptosporidium felis, and another three were infected with Cryptosporidium meleagridis (Table 1).
C. parvum bovine genotype parasites account for most of the cases of cryptosporidiosis in Portuguese HIV-infected patients. Other authors have also reported in Europe (United Kingdom, Switzerland, and France) that the C. parvum bovine genotype is responsible for more human infections than C. hominis (3, 6, 9, 10). In the United States, Australia, Kenya, Thailand, and South Africa, anthroponotic parasites are responsible for the majority of cases of human cryptosporidiosis (8, 10, 11, 20, 21). Whether these results reflect the existence of some geographic variation in humans in the distribution of C. parvum and C. hominis is unknown, as only small numbers of isolates have been analyzed in most studies.
C. parvum and C. hominis subgenotypes.
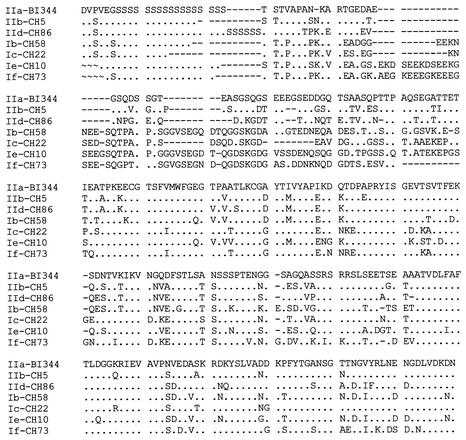
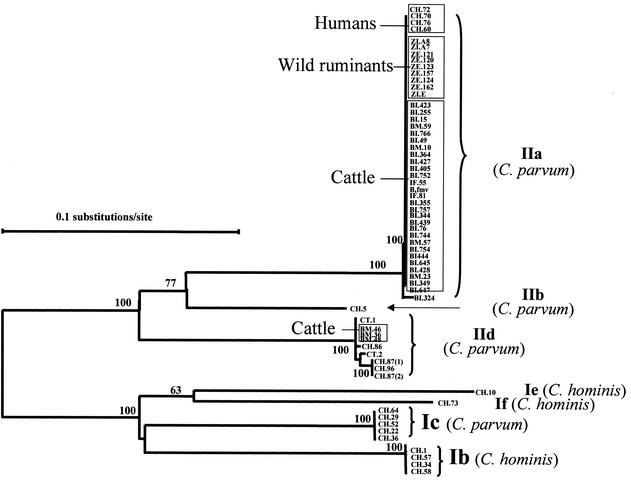
All C. parvum and C. hominis isolates plus the isolate for which no SSU rRNA data was obtained (CT.1) were analyzed at the GP60 locus. PCR amplification was obtained for all 69 isolates, only 63 of which yielded clean GP60 sequences. The remaining six isolates had underlying signals in the electropherogram, which prevented the read-out of accurate sequences. Sequence alignment revealed extensive differences in the nucleotide sequence through the entire length of the fragment and divided all C. parvum and C. hominis isolates into seven alleles (Fig. 1). The results of phylogenetic analysis of the nucleotide sequences supported the formation of these seven alleles (Fig. 2). Three alleles corresponded to C. hominis (Ib, Ie, and If) and four alleles corresponded to C. parvum (IIa, IIb, Ic, and IId). Six of them corresponded to previously described alleles, Ib, Ic, and IIa (18); Ie and IIb (15, 19); and If (same as Ie of Leav et al. [8]), and one was a new allele (IId). Alleles IIb and IId had, so far, only been found in Portugal. None of the isolates exhibited the alleles Ia and Id described before (8, 15, 18, 19).
FIG. 1.
Amino acid sequence diversity among seven GP60 alleles of Cryptosporidium in humans and animals from Portugal. Sequences are labeled as alleles followed by sample identifications. Dots denote sequence identity to the allele IIa sequence in isolate BI.344, dashes denote nucleotide deletions, and ∼ indicates that sequence information was unavailable. IIa, IIb, IId, and Ic are C. parvum alleles, and Ib, Ie, and If are C. hominis alleles.
FIG. 2.
Distribution of GP60 alleles of C. parvum and C. hominis from humans, calves, and wild ruminants as revealed by a neighbor-joining analysis of nucleotide sequences. Isolates from calves are named BI, BM, IF, and B.fmv. Human isolates are named CH and CT; isolate CH.60 is from a sporadic case of a non-HIV-infected person.
Within C. parvum alleles, allele IIa contained all the isolates from the zoo's wild ruminants, 29 of the 32 isolates from calves, and 4 human isolates. All these isolates within this allele exhibited the same subgenotype, with the exception of one isolate from a calf, which showed a different subgenotype. Similarly, allele IId also had isolates from both cattle and humans. However, this allele had four subgenotypes, with three bovine isolates belonging to a single subgenotype and six human isolates belonging to four subgenotypes. In contrast, allele Ic had only C. parvum bovine genotype isolates from humans.
Most isolates exhibited GP60 alleles concordant with the SSU rRNA results. However, allele Ic, which originally was described as a C. hominis allele (18) and contained five C. parvum bovine genotype isolates from humans in this study, had the C. parvum bovine genotype sequence in the SSU rRNA gene. Peng et al. (15) and Leav et al. (8) also found similar results with C. parvum bovine genotype isolates from humans in Guatemala, Portugal, and South Africa. Thus, allele Ic should be renamed IIc. Even though it is a C. parvum parasite, allele Ic has so far only been found in humans. Two recently identified new alleles have been named Ie, even though they are genetically distinct (8, 15, 19). Thus, the allele Ie identified in South African patients has been renamed allele If in the present study.
Epidemiological significance.
None of the Portuguese isolates displayed alleles Ia and Id, and alleles IIb and IId had so far only been found in Portugal. On the contrary, alleles Ib, Ie, If, IIa, and Ic seem to have a wide geographic distribution. Thus, geographic differences in the distribution of specific alleles may exist. However, due to the small number of isolates analyzed so far, the epidemiological significance of these results remains to be determined. Additional studies, with a larger number of isolates from various geographic areas, should be conducted to confirm these observations and to extrapolate the significance of the differences.
The isolates from animals showed limited genetic heterogeneity, with the parasites from cattle exhibiting three subgenotypes in two alleles (IIa and IId) and those from the zoo ruminants displaying only one subgenotype of one allele. Twenty-nine of 32 bovine isolates exhibited the same allele (IIa), and from these, only 28 had an identical subgenotype. Only three bovine isolates had the allele IId. The dominance of one subgenotype in calves in Portugal was probably the result of the frequent exchange of animals among farms, the relatively small size of the country, or the genetic fitness of this Cryptosporidium strain.
In contrast to the limited genetic diversity in Cryptosporidium from animals, the human isolates were found in 10 subgenotypes and in all seven alleles, including three C. parvum alleles (IIa, IIb, and Ic). These results suggest that transmission of human cryptosporidiosis in Portugal is complicated and multiple routes of transmission of human cryptosporidiosis were probably responsible for the high genetic heterogeneity of Cryptosporidium parasites seen in humans. This was also supported by the fact that C. parvum parasites from humans even had a higher genetic diversity than those from animals. Also, two of the C. parvum alleles (IIb and Ic) had only been found in human isolates. These results, though limited by the small number of isolates studied, also suggest that the occurrence of the C. parvum bovine genotype in humans is frequently not the result of zoonotic infections.
Acknowledgments
This work was supported by the project POCTI/ESP/43635/99 from Fundação para a Ciência e Tecnologia. M. Alves was supported by a Ph.D. grant (SFRH/BD/2898/2000) from Fundação para a Ciência e Tecnologia.
We thank Isabel Pereira da Fonseca, Esmeralda Delgado, and Ana Mafalda Lourenço for providing animal C. parvum isolates.
REFERENCES
- 1.Alves, M., O. Matos, and F. Antunes. 2001. Multilocus PCR-RFLP analysis of Cryptosporidium isolates from HIV-infected patients from Portugal. Ann. Trop. Med. Parasitol. 95:627-632. [DOI] [PubMed] [Google Scholar]
- 2.Alves, M., O. Matos, F. Spano, and F. Antunes. 2000. PCR-RFLP analysis of Cryptosporidium parvum isolates from HIV-infected patients in Lisbon, Portugal. Ann. Trop. Med. Parasitol. 94:291-297. [DOI] [PubMed] [Google Scholar]
- 3.Alves, M., O. Matos, I. P. Fonseca, E. Delgado, A. M. Lourenço, and F. Antunes. 2001. Multilocus genotyping of Cryptosporidium isolates from human HIV-infected and animal hosts. J. Eukarot. Microbiol. 2001 (Suppl.):17S-18S. [DOI] [PubMed]
- 4.Cevallos, A. M., X. Zhang, M. K. Waldor, S. Jaison, X. Zhou, S. Tzipori, M. R. Neutra, and H. D. Ward. 2000. Molecular cloning and expression of a gene encoding Cryptosporidium parvum glycoproteins gp40 and gp15. Infect. Immun. 68:4108-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glaberman, S., J. E. Moore, C. J. Lowery, R. M. Chalmers, I. Sulaiman, K. Elwin, P. J. Rooney, B. C. Millar, J. S. Dooley, A. A. Lal, and L. Xiao. 2002. Three drinking-water-associated cryptosporidiosis outbreaks, Northern Ireland. Emerg. Infect. Dis. 8:631-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guyot, K., A. Follet-Dumoulin, E. Lelièvre, C. Sarfati, M. Rabodonirina, G. Nevez, J. C. Cailliez, D. Camus, and E. Dei-Cas. 2001. Molecular characterization of Cryptosporidium isolates obtained from humans in France. J. Clin. Microbiol. 39:3472-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kosek, M., C. Alcantara, A. A. M. Lima, and R. L. Guerrant. 2001. Cryptosporidiosis: an update. Lancet Infect. Dis. 1:262-269. [DOI] [PubMed] [Google Scholar]
- 8.Leav, B. A., M. R. Mackay, A. Anyanwu, R. M. O'Connor, A. M. Cevallos, G. Kindra, N. C. Rollins, M. L. Bennish, R. G. Nelson, and H. D. Ward. 2002. Analysis of sequence diversity at the highly polymorphic Cpgp40/15 locus among Cryptosporidium isolates from human immunodeficiency virus-infected children in South Africa. Infect. Immun. 70:3881-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLauchlin, J., C. Amar, S. Pedraza-Díaz, and G. L. Nichols. 2000. Molecular epidemiological analysis of Cryptosporidium spp. in the United Kingdom: results of genotyping Cryptosporidium spp. in 1,705 fecal samples from humans and 105 fecal samples from livestock animals. J. Clin. Microbiol. 38:3984-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan, U., R. Weber, L. Xiao, I. Sulaiman, R. C. A. Thompson, W. Ndiritu, A. Lal, A. Moore, and P. Deplazes. 2000. Molecular characterization of Cryptosporidium isolates obtained from human immunodeficiency virus-infected individuals living in Switzerland, Kenya, and the United States. J. Clin. Microbiol. 38:1180-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan, U. M., C. C. Constantine, D. A. Forbes, and R. C. A. Thompson. 1997. Differentiation between human and animal isolates of Cryptosporidium parvum using rRNA sequencing and direct PCR analysis. J. Parasitol. 83:825-830. [PubMed] [Google Scholar]
- 12.Morgan, U. M., L. Xiao, R. Fayer, A. A. Lal, and R. C. A. Thompson. 2000. Epidemiology and strain variation of Cryptosporidium parvum. Contrib. Microbiol. 6:116-139. [DOI] [PubMed] [Google Scholar]
- 13.Morgan-Ryan, U. M., A. Fall, L. A. Ward, N. Hijjawi, I. Sulaiman, R. Fayer, R. C. Thompson, M. Olson, A. Lal, and L. Xiao. 2002. Cryptosporidium hominis n. sp. (Apicomplexa: Cryptosporidiidae) from Homo sapiens. J. Eukaryot. Microbiol. 49:433-440. [DOI] [PubMed] [Google Scholar]
- 14.Peng, M. M., L. Xiao, A. R. Freeman, M. J. Arrowood, A. A. Escalante, A. C. Weltman, C. S. L. Ong, W. R. Mackenzie, A. A. Lal, and C. B. Beard. 1997. Genetic polymorphism among Cryptosporidium parvum isolates: evidence of two distinct human transmission cycles. Emerg. Infect. Dis. 3:567-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng, M. M., O. Matos, W. Gatei, P. Das, M. Stantic-Pavlinic, C. Bern, I. M. Sulaiman, S. Glaberman, A. L. Lal, and L. Xiao. 2001. A comparison of Cryptosporidium subgenotypes from several geographic regions. J. Eukaryot. Microbiol. 2001 (Suppl.):28S-31S. [DOI] [PubMed]
- 16.Priest, J. W., J. P. Kwon, M. J. Arrowood, and P. J. Lammie. 2000. Cloning of the immunodominant 17-Kda antigen from Cryptosporidium parvum. Mol. Biochem. Parasitol. 106:261-271. [DOI] [PubMed] [Google Scholar]
- 17.Spano, F., L. Putignani, A. Crisanti, P. Sallicandro, U. M. Morgan, S. M. Le Blancq, L. Tchack, S. Tzipori, and G. Widmer. 1998. Multilocus genotypic analysis of Cryptosporidium parvum isolates from different hosts and geographical origins. J. Clin. Microbiol. 36:3255-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strong, W. B., J. Gut, and R. G. Nelson. 2000. Cloning and sequence analysis of a high polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15- and 45-kilodalton zoite surface antigen products. Infect. Immun. 68:4117-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sulaiman, I. M., A. A. Lal, and L. Xiao. 2001. A population genetic study of the Cryptosporidium parvum human genotype parasites. J. Eukaryot. Microbiol. 2001 (Suppl.):24S-27S. [DOI] [PubMed]
- 20.Sulaiman, I. M., L. Xiao, C. Yang, L. Escalante, A. Moore, C. B. Beard, M. J. Arrowood, and A. A. Lal. 1998. Differentiating human from animal isolates of Cryptosporidium parvum. Emerg. Infect. Dis. 4:681-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiangtip, R., and S. Jongwutiwes. 2002. Molecular analysis of Cryptosporidium species isolated from HIV-infected patients in Thailand. Trop. Med. Int. Health. 7:357-364. [DOI] [PubMed] [Google Scholar]
- 22.Xiao, L., I. M. Sulaiman, U. M. Ryan, L. Zhou, E. R. Atwill, M. L. Tischler, X. Zhang, R. Fayer, and A. A. Lal. 2002. Host adaptation and host-parasite co-evolution in Cryptosporidium: implications for taxonomy and public health. Int. J. Parasitol. 32:1773-1785. [DOI] [PubMed] [Google Scholar]
- 23.Xiao, L., C. Bern, M. Arrowood, I. Sulaiman, L. Zhou, V. Kawai, A. Vivar, A. A. Lal, and R. H. Gilman. 2002. Identification of the Cryptosporidium pig genotype in a human patient. J. Infect. Dis. 185:1846-1848. [DOI] [PubMed] [Google Scholar]
- 24.Xiao, L., C. Bern, J. Limor, I. Sulaiman, J. Roberts, W. Checkley, L. Cabrera, R. H. Gilman, and A. A. Lal. 2001. Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J. Infect. Dis. 183:492-497. [DOI] [PubMed] [Google Scholar]
- 25.Xiao, L., U. M. Morgan, J. Limor, A. Escalante, M. Arrowood, W. Shulaw, R. C. A. Thompson, R. Fayer, and A. A. Lal. 1999. Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl. Environ. Microbiol. 65:3386-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]