Abstract
The genetic relationship of 81 ampicillin-resistant and 21 ampicillin-susceptible Enterococcus faecium isolates from clinical infections and rectal screening in hospitalized patients in Norway was studied by pulsed-field gel electrophoresis (PFGE) and amplified fragment length polymorphism (AFLP). PFGE showed 55 different banding patterns, and 65 of the isolates could be grouped into one large group. With AFLP, 46 patterns were discerned, and 74 isolates clustered in one group. In general, the isolates had a higher degree of similarity than with PFGE. The purK gene, which is one of the targets of the E. faecium multilocus sequence typing scheme, was sequenced. Eleven different purK alleles could be discerned, with the majority of isolates (n = 80) harboring allele 1. With only two exceptions, all strains carrying purK-1 clustered in the same PFGE and AFLP groups, indicating a good correlation between PFGE type, AFLP type, and purK allele. Genetic polymorphism of a 571-bp PCR fragment of the C-terminal domain of the penicillin-binding protein 5 gene (pbp5) was determined, and sequence differences were associated with the level of ampicillin resistance. This study indicates that the majority of ampicillin-resistant E. faecium strains in Norway belong to a distinct genetic lineage of closely related genotypes. Rectal and clinical isolates were generally indistinguishable, and differences in clonal distribution and allele polymorphism were found mainly between ampicillin-resistant and -susceptible isolates.
Enterococci are common causes of nosocomial infections (20). Although they are considered a trivial cause of infection by some, the emergence of high-level ampicillin resistance, high-level aminoglycoside resistance, and glycopeptide resistance has forced us to reconsider the importance of these organisms (15). There have been several reports on the emergence of resistant enterococci in Scandinavia, but so far glycopeptide resistance is uncommon (10, 12, 27, 30, 31). Before 1995, acquired resistance in enterococci was rarely seen in Norway, but since then there has been an increase in ampicillin resistance, and more recently glycopeptide resistance has been reported within an outbreak of ampicillin-resistant enterococci (11). A report from Finland indicated that the vanA and vanB genes were incorporated into an endemic ampicillin-resistant strain in 1996 (28). Ampicillin resistance in E. faecium is associated with the production of low-affinity penicillin-binding protein 5 (PBP5). High-level ampicillin resistance may be due to either increased expression of PBP5 or alterations in the pbp5 gene resulting in lower affinities for ampicillin (23, 26).
In order to characterize ampicillin-resistant E. faecium in Norway among in-patients, a point prevalence investigation was performed from March to October 1999. Due to low numbers of clinical infections with these organisms in Norway (12), it was decided to screen for rectal carriage. Eight hundred fifty-four patients hospitalized in the medical, surgical, oncological, gynecological, and pediatric departments at 10 major hospitals in Norway were screened. The epidemiology of this screening has been reported previously (13). A total of 58 ampicillin-resistant E. faecium and eight ampicillin-susceptible E. faecium isolates that were initially misclassified as resistant were found. In addition to the rectal isolates, one of the participating hospitals also supplied 36 E. faecium isolates from clinical infections that had been collected during the period from 1997 to 1999.
In the present study, the genetic relationship between ampicillin-resistant and -susceptible isolates recovered by rectal screening and from clinical infections was determined by two different genotyping schemes, pulsed-field gel electrophoresis (PFGE) and amplified fragment length polymorphism (AFLP) and by sequencing of the purK housekeeping gene, which is one of the targets of the recently described E. faecium multilocus sequence typing scheme by Homan et al. (14). Furthermore, the C-terminal part of the pbp5 gene was sequenced in order to correlate sequence alterations in this region with ampicillin resistance levels. Finally, we wanted to investigate if the esp gene, a reported marker for epidemic vancomycin-resistant E. faecium strains (34), was present in our collection of glycopeptide-sensitive E. faecium strains.
MATERIALS AND METHODS
Bacterial isolates.
Isolation of E. faecium from rectal samples from inpatients at 10 geographically spread major hospitals in Norway (n = 66) was performed by the use of selective medium as described previously (13). Isolation of E. faecium from clinical samples at one of the participating hospitals (Haukeland University Hospital) was done with standard laboratory methods. During the period 1997 to 1999, 21 blood cultures yielded E. faecium, and all of these were included, as were 15 randomly selected isolates from other samples (urine [n = 13] and pus [n = 2]). The isolates were identified as E. faecium by standard biochemical tests (8), and identifications were verified by means of a PCR method described by Dutka-Malen et al. (7). E. faecium ATCC 19434 and E. faecalis ATCC 29212 were used as quality control strains.
Susceptibility testing.
MICs (milligrams per liter, translated to twofold dilutions) were determined by Etest (AB Biodisk, Solna, Sweden) as described by and interpreted as recommended by the NCCLS (22). The antimicrobial agents tested were ampicillin, ciprofloxacin, quinupristin/dalfopristin, linezolid, gentamicin, vancomycin, and teicoplanin. E. faecalis ATCC 29212 was used as a quality control strain.
PCR.
PCR was performed on crude extracts from bacterial suspensions boiled (10 min) in TE (0.01 M Tris-HCl, 0.001 M EDTA) or by adding 1 colony directly to the PCR tube. PCRs were performed in a thermal cycler (model 9600; PE Biosystems, Norwalk, Conn.) with a HotStar PCR kit (Qiagen, Hilden, Germany). Primer sequences and target regions used for amplification in this study are listed in Table 1. Amplification conditions for purK and esp were 95°C initially for 15 min; 94°C for 30 s, 52°C for 30 s, 72°C for 2 min over 30 cycles followed by a final 7 min extension period at 72°C. For pbp5 PCR HotStar PCR kit (Qiagen) with 3.5 mM MgCl2 was used and amplification conditions were as above except for an annealing temperature of 54°C. PCR products were analyzed on ethidium bromide-stained agarose gels.
TABLE 1.
PCR, AFLP, and sequence primers used in this study
| Primer | Sequence | Reference |
|---|---|---|
| espF | 5′-AGATTTCATCTTTGATTCTTGG | This study |
| espR | 5′-AATTGATTCTTTAGCATCTGG | This study |
| purKF | 5′-GCAGATTGGCACATTGAAAGT | 15 |
| purKR | 5′-TACATAAATCCCGCCTGTTTY | 15 |
| pbp5F | 5′-AACAAAATGACAAACGGG | This study |
| pbp5R | 5′-TATCCTTGGTTATCAGGG | This study |
| pbp5Fsekva | 5′-GACAAACGGGATCTCACAAG | This study |
| AFLP adapter 1b | 5′-AATTGTAAAACGACGGCCAGTAACG | 35 |
| AFLP adapter 2 | 5′-CATTTTGCTGCCGGTCATT | 35 |
| AFLP PCR 1G | 5′-CGACGGCCAGTAACGCG | 35 |
| AFLP PCR 2A | 5′-GGCCGTCGTTTTACAATTCA | 35 |
pbp5F primer used in DNA sequence analysis.
Complementary sequence is underlined.
DNA sequence analysis.
The purK and pbp5 PCR products were purified with a Qiaquick PCR purification kit (Qiagen) according to the manufacturer's instructions. The purified PCR products were sequenced with the ABI Prism BigDye cycle sequencing ready reaction kit (PE Biosystems) with the cycling profile and amount of target DNA recommended by the manufacturer. The products were analyzed on an ABI Prism 3700 DNA sequencer (PE Biosystems). The sequence primers are listed in Table 1.
Hybridization.
To verify positive PCRs, products from the ddl E. faecium PCR described by Dutka-Malen et al. (7) and the esp PCR described in this study from a known positive strain were labeled with horseradish peroxidase and used as probes on bacterial chromosomal DNA isolated as previously described (36) and digested with EcoRI and XbaI. The protocol according to the ECL direct nucleic acid labeling and detection systems protocol RPN3000PL/AA (Amersham Pharmacia Biotech AB, Uppsala, Sweden) was used.
Pulsed-field gel electrophoresis.
PFGE was performed as described previously (21) and modified by Dahl (6). The DNA banding patterns were analyzed with BioNumerics, version 2.5 (Applied Maths, Kortrijk, Belgium), and were also inspected visually. The Dice coefficient of similarity was calculated, and the unweighted pair group method with arithmetic averages (UPGMA) was used for cluster analysis.
Amplified fragment gel electrophoresis.
AFLP was performed as described previously (35). The amplification products were run on a DNA sequencer (ABI Prism 3700, PE Biosystems). For this, 1 μl of reaction mixture was mixed with 7 μl of MilliQ water, and subsequently 1 μl was diluted with 9 μl of formamide containing approximately 0.125 μl of GeneScan-500 (ROX) standard (PE Biosystems). The GeneScan collection software (PE Biosystems) was used to collect data during electrophoresis. After tracking and extraction of lanes, data were exported to the BioNumerics software version 2.5 (Applied Maths) for further analysis. Normalization was done by use of the reference positions of the internal DNA size marker GeneScan-500 (ROX). Fragments ranging in size from 50 to 500 nucleotides were used for comparison. The Pearson coefficient of similarity of AFLP curves was calculated, and UPGMA was used for cluster analysis. Two isolates were considered to have identical AFLP patterns when the similarity was ≥95% (35).
RESULTS
Bacterial isolates.
One hundred two strains from 102 patients were included. Sixty-six were from rectal samples collected from hospitalized patients in the medical, surgical, oncological, gynecological, pediatric, and intensive care unit departments at 10 major Norwegian hospitals during the point prevalence study, March to October 1999. Thirty-six were from clinical infections (blood, n = 21; urine, n = 13; and pus, n = 2) from hospitalized patients in the medical, surgical, oncological, pediatric, intensive care unit, and burn unit departments in one of the participating hospitals collected between 1997 and 1999. This hospital has had an endemic situation with ampicillin-resistant E. faecium since 1995.
Pulsed-field gel electrophoresis.
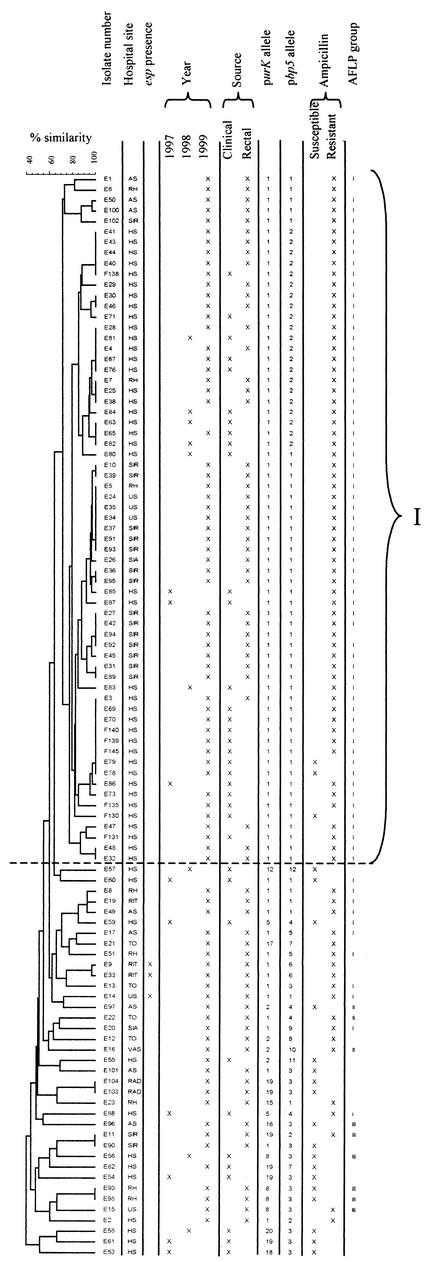
The 102 isolates had 55 different banding patterns by PFGE as discerned by visual inspection. The visual analysis revealed that isolates with over 95% similarity of the Dice coefficient were identical. The patterns comprised 13 to 17 differently sized DNA fragments between 50 and 1,000 kb. Cluster analysis revealed one large group (PFGE-I) with 65 isolates that had an intragroup band variation of up to six bands. Isolates in this group differed, with seven or more bands from the isolates outside this group. No clustering according to year or site (rectal or clinical) was found. The results of the PFGE are given in Fig. 1.
FIG.1.
PFGE dendrogram of 102 isolates produced following Dice and UPGMA analysis of SmaI-digested DNA. One large group (I) could be discerned (see text). Distribution of the isolates according to hospital site (hospital codes refer to the 10 hospitals that participated), esp positivity, year of isolation, source of isolation, purK and pbp5 alleles, ampicillin susceptibility, and AFLP groups I to III are shown.
Amplified fragment length polymorphism.
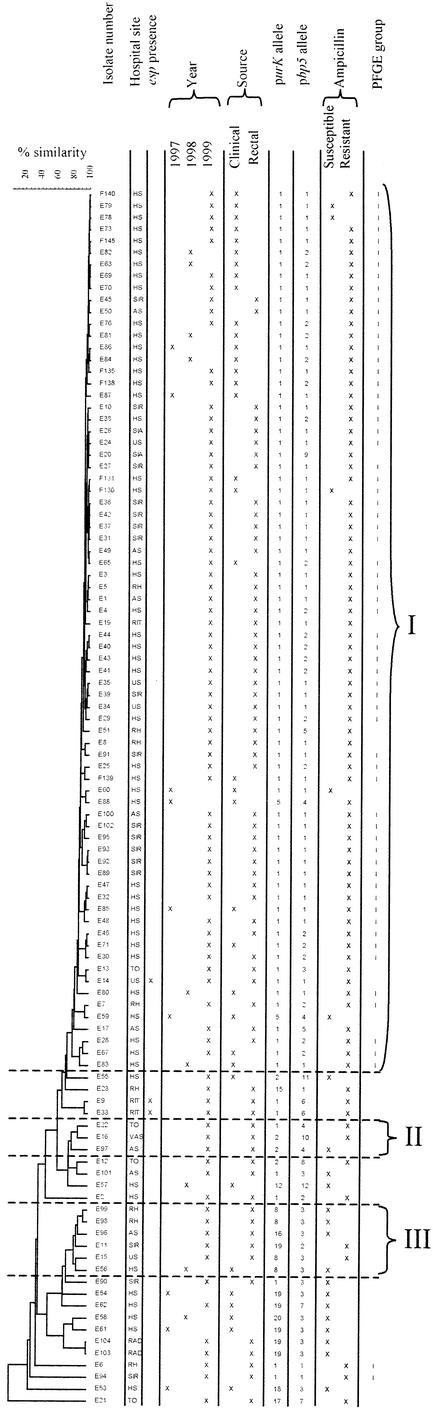
The 102 isolates comprised 46 different AFLP types with less than 95% similarity (Fig. 2). When examining isolates sharing ≥80% of the restriction fragments, a criterion used previously to discern AFLP groups (3), three groups containing three or more isolates could be discriminated. Of these, one large group contained 74 isolates. This group included the isolates grouped into the PFGE-I group except for two isolates and included 11 isolates that were not in this PFGE group.
FIG.2.
AFLP dendrogram of 102 isolates produced following Pearson and UPGMA analysis. Three AFLP groups (I to III) comprising at least three isolates were formed at ≥80% similarity. Distribution of the isolates according to hospital site (hospital codes refer to the 10 hospitals that participated), esp positivity, year of isolation, source of isolation, purK and pbp5 alleles, ampicillin susceptibility, and PFGE group I are shown.
purK alleles.
Eleven purK alleles were found (Table 2). Allele 1 dominated and was found in 80 isolates; 26 were clinical isolates and 54 were rectal isolates. Six were ampicillin susceptible and 74 were ampicillin resistant. The ampicillin-susceptible isolates had predominantly other alleles. The vast majority of isolates that grouped in PFGE-I and AFLP-I carried the purK-1 allele, while the other isolates mainly contained other purK alleles.
TABLE 2.
purK allel polymorphism of 102 isolates of E. faecium
| purK allele | Sample | No. of isolates with ampicillin phenotype:
|
|
|---|---|---|---|
| Susceptible | Resistant | ||
| 1 | Blood, urine, pus | 4 | 22 |
| Rectal swab | 2 | 52 | |
| Total | 6 | 74 | |
| 2 | Blood | 1 | |
| Rectal swab | 1 | 2 | |
| Total | 2 | 2 | |
| 5 | Blood | 1 | 1 |
| Total | 1 | 1 | |
| 8 | Blood | 1 | |
| Rectal swab | 2 | 1 | |
| Total | 3 | 1 | |
| 19 | Blood | 3 | |
| Rectal swab | 2 | 1 | |
| Total | 5 | 1 | |
| 12, 15-18, 20 | Blood | 3 | |
| Rectal swab | 1 | 2 | |
| Total | 4 | 2 | |
Antimicrobial susceptibility.
The susceptibility of all isolates to seven antibiotics was tested, and the susceptibility data are shown in Table 3. Ciprofloxacin resistance was more common among the ampicillin-resistant isolates regardless of whether they were from clinical or rectal samples. Resistance to quinupristin/dalfopristin and high-level aminoglycoside resistance were found exclusively among ampicillin-resistant rectal isolates. No resistance to linezolid or glycopeptides was detected.
TABLE 3.
Antimicrobial susceptibility of 102 E. faecium isolatesa
| Isolate type | Agent | Rectal isolates
|
Clinical isolates
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/liter)
|
No. of isolates
|
MIC (mg/liter)
|
No. of isolates
|
||||||||||
| Range | 50% | 90% | S | I | R | Range | 50% | 90% | S | I | R | ||
| Ampicillin resistant | Ampicillin | 24-256 | 64 | 128 | — | — | 58 | 6->256 | 32 | 64 | — | — | 23 |
| Ciprofloxacin | 0.75->32 | >32 | >32 | 6 | 5 | 47 | 3->32 | >32 | >32 | 0 | 1 | 22 | |
| Linezolid | 0.75-2 | 1 | 1.5 | 58 | 0 | 0 | 0.5-1.5 | 1 | 1.5 | 23 | 0 | 0 | |
| Quinupristin/dalfopristin | 0.25-64 | 0.5 | 1.5 | 50 | 4 | 4 | 0.19-0.5 | 0.5 | 0.5 | 23 | 0 | 0 | |
| Vancomycin | 0.5-2 | 1 | 2 | 58 | 0 | 0 | 0.5-2 | 1.5 | 1.5 | 23 | 0 | 0 | |
| Teicoplanin | 0.094-2 | 0.25 | 1.5 | 58 | 0 | 0 | 0.064-1.5 | 0.19 | 0.5 | 23 | 0 | 0 | |
| Gentamicin | 2->1024 | 8 | 12 | 55 | — | 3 | 2-16 | 4 | 8 | 23 | — | 0 | |
| Ampicillin susceptible | Ampicillin | 0.75-4 | 3 | 4 | 8 | — | — | 0.064-8 | 1 | 8 | 13 | — | — |
| Ciprofloxacin | 0.38->32 | 0.75 | >32 | 5 | 3 | 1 | 0.38->32 | 1.5 | >32 | 5 | 4 | 4 | |
| Linezolid | 1-1.5 | 1 | 1.5 | 8 | 0 | 0 | 0.75-1.5 | 1 | 1.5 | 13 | 0 | 0 | |
| Quinupristin/dalfopristin | 0.38-2 | 1.5 | 2 | 8 | 0 | 0 | 0.38-2 | 0.5 | 2 | 13 | 0 | 0 | |
| Vancomycin | 1-2 | 2 | 2 | 8 | 0 | 0 | 0.5-2 | 1 | 2 | 13 | 0 | 0 | |
| Teicoplanin | 1.5-3 | 3 | 3 | 8 | 0 | 0 | 0.032-2 | 0.19 | 2 | 13 | 0 | 0 | |
| Gentamicin | 3-6 | 4 | 6 | 8 | — | 0 | 0.19-8 | 4 | 8 | 13 | — | 0 | |
50% and 90%, MIC for 50 and 90% of isolates, respectively. S, sensitive; I, intermediate; R, resistant, breakpoints defined by NCCLS. For gentamicin, high-level aminoglycoside resistance = MIC ≥ 512 mg/liter. There were 58 ampicillin-resistant and 8 ampicillin-susceptible rectal isolates and 23 ampicillin-resistant and 13 ampicillin-susceptible clinical isolates. —, no value possible (for ampicillin) or no criteria established by NCCLS (for gentamicin).
pbp5 alleles.
Sequence heterogeneity of the C-terminal part of pbp5 was determined for all 102 isolates. Nineteen different C-terminal alleles encoding 12 different amino acid sequences were discerned (Table 4). Twenty-five isolates harboring an aspartic acid insertion and one isolate harboring a serine insertion just after Ser-466 (GenBank accession no. X84860) were all ampicillin resistant, but the level of resistance varied from 16 to >256 mg/liter. The association between this insertion and ampicillin resistance was statistically significant, as measured by Fisher's exact test (P = 0.0014). In addition, the 485M→T substitution in 73 of 81 resistant isolates and in 4 of 21 susceptible isolates (P < 0.0005), the 496N→K substitution in 78 of 81 resistant isolates and in 8 of 21 susceptible isolates (P < 0.0005), the 499A→T substitution in 78 of 81 resistant isolates and in 7 of 21 susceptible isolates (P < 0.0005), the 525E→D substitution in 78 of 81 resistant and in 8 of 21 susceptible isolates (P < 0.0005), the 586V→L substitution in 71 of 81 resistant isolates and in 8 of 21 susceptible isolates (P < 0.0005), and the 629E→V substitution in 78 of 81 resistant isolates and in 6 of 21 susceptible isolates (P < 0.0005) were also found significantly more often in resistant compared to susceptible strains. We considered a MIC of ampicillin of ≥16 mg/liter to indicate resistance, as recommended by the NCCLS (22).
TABLE 4.
pbp5 allele polymorphism and ampicillin susceptibility of 102 isolates of E. faeciuma
| Allele | No. of isolates | Amino acid at position:
|
MIC range of ampicillin (mg/liter) | No. of isolates
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 461 | 466′ | 470 | 485 | 496 | 497 | 499 | 524 | 525 | 558 | 582 | 586 | 593 | 607 | 629 | 631 | 634 | S | R | |||
| Q | — | H | M | N | F | A | E | E | A | G | V | L | L | E | G | N | |||||
| 1 | 50 | Q | — | Q | T | K | F | T | E | D | A | G | L | L | L | V | G | N | 6-128 | 4 | 46 |
| 2 | 23 | Q | D | Q | T | K | F | T | E | D | A | G | L | L | L | V | G | N | 16->256 | 0 | 23 |
| 3 | 14 | Q | — | Q | M | N | F | A | E | E | A | G | V | L | L | E | G | N | 0.064-96 | 12 | 2 |
| 4 | 4 | Q | — | Q | M | K | F | T | E | D | A | G | V | L | L | V | G | N | 1.5-32 | 2 | 2 |
| 5 | 2 | Q | D | Q | T | K | L | T | E | D | A | S | V | Q | L | V | G | N | 64-256 | 2 | |
| 6 | 2 | Q | — | Q | A | K | F | T | E | D | A | G | L | L | L | V | G | N | 48-64 | 2 | |
| 7 | 2 | Q | — | Q | M | N | F | A | G | E | A | G | V | L | L | E | R | N | 1-48 | 1 | 1 |
| 8 | 1 | Q | S | Q | T | K | F | T | E | D | V | G | V | L | L | V | G | N | 256 | 1 | |
| 9 | 1 | K | — | Q | A | K | F | T | E | D | A | G | L | L | L | V | G | T | 96 | 1 | |
| 10 | 1 | Q | — | Q | T | K | F | T | E | D | A | G | V | L | F | V | G | N | 64 | 1 | |
| 11 | 1 | Q | — | Q | M | K | F | I | E | D | A | G | V | L | L | E | G | N | 0.25 | 1 | |
| 12 | 1 | Q | — | Q | M | K | F | T | E | D | A | G | V | L | L | E | G | N | 0.125 | 1 | |
Amino acid positions are based on the sequence of pbp5 (GenBank and EMBL accession no. X84860). DNA corresponding to amino acids 447 to 635 was sequenced.
esp PCR and hybridization.
Three strains were repeatedly positive by esp PCR and were positive when hybridized with the esp probe. Two of the strains were closely related by PFGE and AFLP and were isolated at the same hospital on the same day. The third isolate was isolated at a different hospital and was not genetically closely related. The isolates had the purK-1 allele but different pbp5 alleles. The esp-positive strains are marked in Fig. 1 and 2.
DISCUSSION
In this study, the genetic relationship of ampicillin-resistant and ampicillin-susceptible E. faecium isolates recovered from hospitalized patients was analyzed with different molecular typing schemes. Both PFGE and AFLP clustered the majority of ampicillin-resistant isolates in a large genogroup (PFGE-I and AFLP-I), whereas the susceptible isolates seemed to be genetically more diverse. This illustrates that in this study, the grouping of isolates by both typing schemes correlates well and that the two methods have a similar discriminatory power. Earlier studies indicated that PFGE is a more discriminatory method than AFLP for studying the nosocomial spread of vancomycin-resistant enterococci (5), and PFGE has been proposed as the method of choice for epidemiological typing of vancomycin-resistant E. faecium by many researchers (16, 18, 19).
AFLP, first described by Vos and collaborators (32), is, however, much less labor intensive and makes it possible to test large numbers of isolates with an acceptable workload. Furthermore, AFLP typing, in contrast to PFGE, permits the study of genetic relationships among dissimilar, nonepidemiologically related vancomycin-resistant enterococci (35). This, together with our findings, suggests that AFLP is suitable for microepidemiological as well as global epidemiological studies to study the global spread of specific virulent or multiresistant strains. The isolates were not clustered according to origin (i.e., clinical versus rectal), indicating that the same bacterial populations were found in rectal carriers and in patients with clinical infection. This is in line with studies that have implicated colonization as one of the important factors for dissemination of these bacteria in hospitals (2, 37).
The ampicillin-susceptible isolates were genetically more diverse than the ampicillin-resistant isolates. However, one has to be cautious when interpreting this result because the number of susceptible isolates was small and the majority of these were from clinical samples from only one of the participating hospitals. The finding, however, seems to support the notion that many of the resistant strains, even though they were sampled from different hospitals and with no apparent link, were more related than the susceptible strains, and this indicates that ampicillin-resistant strains have recently spread epidemically in Norwegian hospitals. A similar finding was described for vancomycin-susceptible, ampicillin-resistant enterococci (4, 14).
The allele purK-1 was the most common purK allele and was present in 80 of the 102 isolates. The fact that purK-1 was the dominant allele among isolates that were grouped together by PFGE (PFGE-I) and AFLP (AFLP-I) supports the conclusion that a clonal lineage of highly similar ampicillin-resistant E. faecium strains is spreading in Norwegian hospitals. Earlier findings by Homan et al. showed that vancomycin-resistant enterococcal isolates related to hospital outbreaks shared the purK-1 allele (14). The finding of the purK-1 allele among highly genetically similar isolates in the present study indicates that purK-1 might also be linked to epidemicity in vancomycin-sensitive E. faecium.
Previous studies have indicated that esp could be a marker for epidemicity among vancomycin-resistant E. faecium spread in hospitals (34). Furthermore, esp was found in vancomycin-susceptible E. faecium isolates from humans (1, 38). In this study, the esp gene was found in three ampicillin-resistant rectal strains only, of which two were genetically highly similar, while the vast majority of the genetically highly similar strains in PFGE-I or AFLP-I lacked the esp gene. This suggests that additional factors besides esp must be responsible for the clinical virulence and epidemic nature of the strains described in this study. In E. faecalis, esp has previously been found to contribute to colonization, persistence in urinary tract infections, and biofilm formation (25, 29). Interestingly, esp was also recently found in E. faecalis isolated from pigs (9).
Comparison of the antibiotic susceptibility levels of rectal and clinical isolates revealed that more antibiotic resistance was found among the rectal isolates. This was illustrated by the fact that isolates with reduced susceptibility to quinupristin/dalfopristin and high-level resistance to gentamicin were only found among rectal isolates, while the level of ciprofloxacin resistance was high among both rectal (85%) and clinical (86%) isolates. The finding of quinupristin/dalfopristin resistance among E. faecium is unexpected because this drug has not been used in humans in Norway before or during the study, nor is there any veterinary use of virginiamycin in Norway that may select for quinupristin/dalfopristin resistance among E. faecium isolates (33). Whatever the reason for the resistance to this new drug among the ampicillin-resistant E. faecium strains that colonize patients in our setting, we will follow the development in clinical samples in the future. Ciprofloxacin resistance was more common among the ampicillin-resistant isolates. This is in agreement with findings by Torell et al., who concluded that over 90% of ampicillin-resistant enterococci carrier strains were resistant to fluoroquinolones (30). Ampicillin-resistant enterococci carriage in that study was correlated with the use of fluoroquinolones. However, fluoroquinolone-resistant, ampicillin-resistant enterococci have also been isolated from infections in patients in Tanzania, where fluoroquinolones are less used (Bjørn Blomberg, personal communication). Thus, coresistance to ampicillin and fluoroquinolones in E. faecium is an issue that merits further investigation.
Nucleotide polymorphism in the pbp5 gene was associated with ampicillin resistance. It was striking that all the isolates with an extra amino acid in position 466′ were resistant. This is most likely not a clonal phenomenon, since some of these were clonally unrelated as determined by PFGE and AFLP, but an indication that the aspartic acid and serine insertion at this position may affect the affinity of beta-lactam antibiotics for PBP5. Insertions of aspartic acid and serine at this position in strains with an increased level of resistance to ampicillin have been described previously (24, 39). Whether this reflects a causal association needs to be elucidated. Other studies have associated the presence of point mutations in the C-terminal region with certain levels of resistance (17, 24). We also found statistically significant associations between point mutations in ampicillin-resistant and -susceptible isolates in positions that earlier have been related to ampicillin resistance, such as 485M→T, 496N→K, 499A→T, 525E→D, 586V→L, and 629E→V. However, recent studies have indicated that specific point mutations alone do not entirely explain the differences in levels of resistance and that the mechanisms by which ampicillin resistance is expressed are more complex (23, 26).
To summarize, this study indicates that the majority of ampicillin-resistant enterococci in hospitals in Norway belong to a distinct clonal lineage of genetically highly similar strains. The purK-1 allele seems to be associated with glycopeptide-sensitive E. faecium strains with an epidemic potential similar to what was reported earlier in glycopeptide-resistant E faecium strains. Ampicillin-resistant E. faecium isolates in Norwegian hospitals are more resistant to other antibiotics than ampicillin-susceptible isolates. Rectal and clinical isolates are in general genetically indistinguishable, and the greatest differences in resistance traits, clonal distribution, and allele polymorphism seem to be between ampicillin-resistant and ampicillin-susceptible strains. The exact mechanisms of ampicillin resistance and the distribution and importance of esp in the epidemic spread of vancomycin-susceptible but ampicillin-resistant E. faecium requires further study.
Acknowledgments
We thank the Norwegian Enterococcal Study Group for supplying the rectal strains, Asbjørn Digranes at the Dept. of Microbiology and Immunology, Haukeland University Hospital, for supplying the clinical strains, and the sequence facility at HiB, University of Bergen, for technical assistance in running the ABI Prism 3700.
REFERENCES
- 1.Baldassarri, L., L. Bertuccini, M. G. Ammendolia, G. Gherardi, and R. Creti. 2001. Variant esp gene in vancomycin-sensitive Enterococcus faecium. Lancet 357:1802. [DOI] [PubMed] [Google Scholar]
- 2.Bonten, M. J., R. Willems, and R. A. Weinstein. 2001. Vancomycin-resistant enterococci: why are they here, and where do they come from? Lancet Infect. Dis. 1:314-325. [DOI] [PubMed] [Google Scholar]
- 3.Borgen, K., Y. Wasteson, H. Kruse, and R. J. Willems. 2002. Vancomycin-resistant Enterococcus faecium (VREF) from Norwegian poultry cluster with VREF from poultry from the United Kingdom and the Netherlands in an amplified fragment length polymorphism genogroup. Appl. Environ. Microbiol. 68:3133-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruinsma, N., R. J. Willems, A. E. Van Den Bogaard, M. Van Santen-Verheuvel, N. London, C. Driessen, and E. E. Stobberingh. 2002. Different levels of genetic homogeneity in vancomycin-resistant and -susceptible Enterococcus faecium isolates from different human and animal sources analyzed by amplified-fragment length polymorphism. Antimicrob. Agents Chemother. 46:2779-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Agata, E. M., M. M. Gerrits, Y. W. Tang, M. Samore, and J. G. Kusters. 2001. Comparison of pulsed-field gel electrophoresis and amplified fragment-length polymorphism for epidemiological investigations of common nosocomial pathogens. Infect. Control Hosp. Epidemiol. 22:550-554. [DOI] [PubMed] [Google Scholar]
- 6.Dahl, K. H., G. S. Simonsen, O. Olsvik, and A. Sundsfjord. 1999. Heterogeneity in the vanB gene cluster of genomically diverse clinical strains of vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 43:1105-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutka-Malen, S., S. Evers, and P. Courvalin. 1995. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 33:24-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Facklam, R. R., and J. A. Washington. 1991. Streptococcus and related catalase-negative gram-positive cocci, p. 238-257. In A. Balows, K. L. Herrmann, H. D. Isenberg, and H. J. Shadomy (ed.), Manual of clinical microbiology. American Society for Microbiology, Washington, D.C.
- 9.Hammersum, A. M., and L. B. Jensen. 2002. Prevalence of esp, encoding the enterococcal surface protein, in Enterococcus faecalis and Enterococcus faecium isolates from hospital patients, poultry, and pigs in Denmark. J. Clin. Microbiol. 40:4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanberger, H., M. Hoffmann, S. Lindgren, and L. E. Nilsson. 1997. High incidence of antibiotic resistance among bacteria in 4 intensive care units at a university hospital in Sweden. Scand. J. Infect. Dis. 29:607-614. [DOI] [PubMed] [Google Scholar]
- 11.Harthug, S., A. Digranes, O. Hope, B. E. Kristiansen, A. G. Allum, and N. Langeland. 2000. Vancomycin resistance emerging in a clonal outbreak caused by ampicillin-resistant Enterococcus faecium. Clin. Microbiol. Infect. 6:19-28. [DOI] [PubMed] [Google Scholar]
- 12.Harthug, S., G. E. Eide, and N. Langeland. 2000. Nosocomial outbreak of ampicillin-resistant Enterococcus faecium: risk factors for infection and fatal outcome. J. Hosp. Infect. 45:135-144. [DOI] [PubMed] [Google Scholar]
- 13.Harthug, S., R. Jureen, S. C. Mohn, A. Digranes, G. S. Simonsen, A. Sundsfjord, N. Langeland, and the Norwegian Enterococcal Study. 2002. The prevalence of faecal carriage of ampicillin-resistant and high-level gentamicin-resistant enterococci among inpatients at 10 major Norwegian hospitals. J. Hosp. Infect. 50:145-154. [DOI] [PubMed] [Google Scholar]
- 14.Homan, W. L., D. Tribe, S. Poznanski, M. Li, G. Hogg, E. Spalburg, J. D. Van Embden, and R. J. Willems. 2002. Multilocus sequence typing scheme for Enterococcus faecium. J. Clin. Microbiol. 40:1963-1971. (Erratum, J. Clin. Microbiol. 40:3548, 2002.) [DOI] [PMC free article] [PubMed]
- 15.Huycke, M. M., D. F. Sahm, and M. S. Gilmore. 1998. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg. Infect. Dis. 4:239-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhn, I., L. G. Burman, S. Haeggman, K. Tullus, and B. E. Murray. 1995. Biochemical fingerprinting compared with ribotyping and pulsed-field gel electrophoresis of DNA for epidemiological typing of enterococci. J. Clin. Microbiol. 33:2812-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ligozzi, M., F. Pittaluga, and R. Fontana. 1996. Modification of penicillin-binding protein 5 associated with high-level ampicillin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 40:354-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miranda, A. G., K. V. Singh, and B. E. Murray. 1991. DNA fingerprinting of Enterococcus faecium by pulsed-field gel electrophoresis may be a useful epidemiologic tool. J. Clin. Microbiol. 29:2752-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison, D., N. Woodford, S. P. Barrett, P. Sisson, and B. D. Cookson. 1999. DNA banding pattern polymorphism in vancomycin-resistant Enterococcus faecium and criteria for defining strains. J. Clin. Microbiol. 37:1084-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray, B. E. 1990. The life and times of the enterococcus. Clin. Microbiol. Rev. 3:46-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray, B. E., K. V. Singh, J. D. Heath, B. R. Sharma, and G. M. Weinstock. 1990. Comparison of genomic DNAs of different enterococcal isolates with restriction endonucleases with infrequent recognition sites. J. Clin. Microbiol. 28:2059-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Committee for Laboratory Standards. 2001. National Committee for Laboratory Standards performance standards for antimicrobial susceptibility testing (M100-S11.). National Committee for Laboratory Standards, Wayne, Pa.
- 23.Rice, L. B., L. L. Carias, R. Hutton-Thomas, F. Sifaoui, L. Gutmann, and S. D. Rudin. 2001. Penicillin-binding protein 5 and expression of ampicillin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 45:1480-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rybkine, T., J. L. Mainardi, W. Sougakoff, E. Collatz, and L. Gutmann. 1998. Penicillin-binding protein 5 sequence alterations in clinical isolates of Enterococcus faecium with different levels of beta-lactam resistance. J. Infect. Dis. 178:159-163. [DOI] [PubMed] [Google Scholar]
- 25.Shankar, N., C. V. Lockatell, A. S. Baghdayan, C. Drachenberg, M. S. Gilmore, and D. E. Johnson. 2001. Role of Enterococcus faecalis surface protein Esp in the pathogenesis of ascending urinary tract infection. Infect. Immun. 69:4366-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sifaoui, F., M. Arthur, L. Rice, and L. Gutmann. 2001. Role of penicillin-binding protein 5 in expression of ampicillin resistance and peptidoglycan structure in Enterococcus faecium. Antimicrob. Agents Chemother. 45:2594-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonsen, G. S., B. M. Andersen, A. Digranes, S. Harthug, T. Jacobsen, E. Lingaas, O. B. Natas, O. Olsvik, S. H. Ringertz, A. Skulberg, G. Syversen, and A. Sundsfjord. 1998. Low faecal carrier rate of vancomycin resistant enterococci in Norwegian hospital patients. Scand. J. Infect. Dis. 30:465-468. [DOI] [PubMed] [Google Scholar]
- 28.Suppola, J. P., E. Kolho, S. Salmenlinna, E. Tarkka, J. Vuopio-Varkila, and M. Vaara. 1999. vanA and vanB incorporated into an endemic ampicillin-resistant vancomycin-sensitive Enterococcus faecium strain: effect on interpretation of clonality. J. Clin. Microbiol. 37:3934-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toledo-Arana, A., J. Valle, C. Solano, M. J. Arrizubieta, C. Cucarella, M. Lamata, B. Amorena, J. Leiva, J. R. Penades, and I. Lasa. 2001. The enterococcal surface protein Esp is involved in Enterococcus faecalis biofilm formation. Appl. Environ. Microbiol. 67:4538-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torell, E., O. Cars, B. Olsson-Liljequist, B. M. Hoffman, J. Lindback, and L. G. Burman. 1999. Near absence of vancomycin-resistant enterococci but high carriage rates of quinolone-resistant ampicillin-resistant enterococci among hospitalized patients and nonhospitalized individuals in Sweden. J. Clin. Microbiol. 37:3509-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torfoss, D., P. Aukrust, L. Brinch, O. Mathiesen, G. S. Simonsen, A. K. Axelsen, and P. Gaustad. 1999. Carrier rate of resistant enterococci in a tertiary care hospital in Norway. APMIS 107:545-549. [DOI] [PubMed] [Google Scholar]
- 32.Vos, P., R. Hogers, M. Bleeker, M. Reijans, T. van de Lee, M. Hornes, A. Frijters, J. Pot, J. Peleman, M. Kuiper, et al. 1995. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 23:4407-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Werner, G., I. Klare, H. Heier, K. H. Hinz, G. Bohme, M. Wendt, and W. Witte. 2000. Quinupristin/dalfopristin-resistant enterococci of the satA (vatD) and satG (vatE) genotypes from different ecological origins in Germany. Microb. Drug Resist. 6:37-47. [DOI] [PubMed] [Google Scholar]
- 34.Willems, R. J., W. Homan, J. Top, M. van Santen-Verheuvel, D. Tribe, X. Manzioros, C. Gaillard, C. M. Vandenbroucke-Grauls, E. M. Mascini, E. van Kregten, J. D. van Embden, and M. J. Bonten. 2001. Variant esp gene as a marker of a distinct genetic lineage of vancomycin-resistant Enterococcus faecium spreading in hospitals. Lancet 357:853-855. [DOI] [PubMed] [Google Scholar]
- 35.Willems, R. J., J. Top, N. van Den Braak, A. van Belkum, H. Endtz, D. Mevius, E. Stobberingh, A. van Den Bogaard, and J. D. van Embden. 2000. Host specificity of vancomycin-resistant Enterococcus faecium. J. Infect. Dis. 182:816-823. [DOI] [PubMed] [Google Scholar]
- 36.Willems, R. J., J. Top, N. van den Braak, A. van Belkum, D. J. Mevius, G. Hendriks, M. van Santen-Verheuvel, and J. D. van Embden. 1999. Molecular diversity and evolutionary relationships of Tn1546-like elements in enterococci from humans and animals. Antimicrob. Agents Chemother. 43:483-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodford, N., A. P. Johnson, D. Morrison, and D. C. Speller. 1995. Current perspectives on glycopeptide resistance. Clin. Microbiol. Rev. 8:585-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodford, N., M. Soltani, and K. J. Hardy. 2001. Frequency of esp in Enterococcus faecium isolates. Lancet 358:584. [DOI] [PubMed] [Google Scholar]
- 39.Zorzi, W., X. Y. Zhou, O. Dardenne, J. Lamotte, D. Raze, J. Pierre, L. Gutmann, and J. Coyette. 1996. Structure of the low-affinity penicillin-binding protein 5 PBP5fm in wild-type and highly penicillin-resistant strains of Enterococcus faecium. J. Bacteriol. 178:4948-4957. [DOI] [PMC free article] [PubMed] [Google Scholar]