Abstract
The distribution and stability of human immunodeficiency virus type 1 (HIV-1) in breast milk (BM) components remain largely unknown. Inhibitory effects, if any, of BM on HIV RNA and DNA PCR amplification are poorly understood. We have addressed these issues by using virus-spiked BM samples from HIV-negative women. BM samples from HIV-negative women were spiked with HIV-1 virions or cells containing a single integrated copy of HIV DNA (8E5/LAV). After incubation under different experimental conditions, viral RNA was detected by the Roche Amplicor UltraSensitive assay in whole-milk, skim milk, and lipid fractions. We found excellent correlation between HIV-1 input copy and recovery in whole milk (r = 0.965, P < 0.0001), skim milk (r = 0.972, P < 0.0001), and the lipid fraction (r = 0.905, P < 0.001). PCR inhibition was observed in less than 10% of the spiked samples. Similar levels of inhibition were noted in BM samples collected from HIV-infected women. HIV proviral DNA was detected in BM samples using real-time PCR (linear correlation between the threshold cycle versus log DNA copy number, >0.982). The effects of incubation duration and temperature and repeated freeze-thaw cycles on HIV RNA recovery were analyzed. HIV RNA levels were remarkably stable in whole milk after three freeze-thaw cycles and for up to 30 h at room temperature. Our findings improve the understanding of the dynamics of HIV detection in BM and the conditions for BM sample collection, storage, and processing.
Transmission of human immunodeficiency virus (HIV) via breast milk is a major route of pediatric HIV infection (7). An estimated one-third to one-half of HIV-infected children in Africa acquire their infection via breast-feeding (7). Levels of HIV type 1 (HIV-1) RNA are an important determinant of transmission risk in sexual (10, 22) and perinatal transmission of HIV-1 (6, 8, 19, 21, 25, 27, 28). Recently, levels of HIV-1 RNA in breast milk have been associated with an increased risk of HIV-1 transmission via breast milk (21, 24). Thus, quantification of HIV in breast milk is an important variable in clinical trials and intervention studies of transmission of HIV-1 via breast milk. Standard procedures for the collection, processing, and storage of breast milk are critical for accurate quantification of HIV and for comparisons of data derived in different studies.
Few studies have focused on quantitation of HIV RNA and DNA in breast milk. Breast milk is a complex fluid consisting of more than 100,000 constituents, including lipids, immunoglobulins, glycoproteins, lactoferrin, and enzymes that could inhibit amplification and/or result in nucleic acid degradation (14). Using the Roche Amplicor assay, Shepard et al. reported partial inhibition, as evidenced by low recovery of the internal quantitation standard (QS), in 38% of breast milk samples (n = 5) spiked with HIV (26). However, they did not observe sufficient inhibition of their PCR product to invalidate the assay. Other groups (21, 24) using the same assay on samples from HIV-infected women did not report such inhibition, and inhibition was not noted by Lewis et al. using a quantitative competitive PCR assay (17). To date, there have been no systematic studies addressing the effects of breast milk on detection of HIV by PCR amplification.
Until recently, most HIV-1 RNA quantitation in breast milk has been performed on the acellular skim milk fraction (17, 21, 24). However, breast milk contains 1 to 10% lipid, which could harbor virus or viral nucleic acid (15). Hoffman et al. recently reported that HIV RNA could be detected in the lipid fraction of milk from 47% of HIV-infected women in Malawi (I. Hoffman, F. Martinson, S. Fiscus, P. Sohonil, C. Komoltril, D. Chilangozi, P. Kazembe, P. Stewart, and M. S. Cohen, 8th Conf. Retrovir. Opportun. Infect., Chicago, Ill., 2001). Since breastfeeding infants are exposed to whole milk, not skim milk, we designed experiments to address the sensitivity of the Roche Amplicor assay in detecting HIV in whole human milk, as well as in the skim milk and lipid fractions.
Most studies of HIV in breast milk have taken place in developing countries where access to refrigeration is limited. Thus, defining the influence of collection and storage conditions on HIV RNA and DNA stability is critical to ensure measurement accuracy, as well as to permit comparisons of data collected in multicenter clinical trials. Therefore, we sought to establish the effects of temperature and storage conditions on the stability and accuracy of HIV-1 nucleic acid detection. We examined the stability of HIV-1 RNA in whole breast milk over time at different temperatures, including the effects of freeze-thaw cycles. Finally, since HIV-infected breast milk cells are a potential source of HIV infection, we used real-time PCR (TaqMan) to quantitate HIV-1 proviral DNA burden in breast milk samples. It is anticipated that these studies will have significant impact on the design of future studies of mother-to-child transmission of HIV-1 via breast milk, particularly in areas where alternatives to breast-feeding are unsustainable and refrigeration is not commonly available.
MATERIALS AND METHODS
Study participants and procedures.
Breast milk was expressed manually or with a breast pump into sterile plastic containers and kept at 4°C. Samples were processed within 4 h of collection. For the spiking experiments, breast milk was collected from 10 healthy HIV-negative women in Birmingham, Alabama, within 8 months postdelivery. Breast milk samples were also obtained from HIV-positive women participating in the Zambia Exclusive Breastfeeding Study (ZEBS) in Lusaka, Zambia. Written informed consent was obtained from all women who participated in this study. Both studies were approved by the Institutional Review Board of the University of Alabama. Additionally, ZEBS was approved by the University of Zambia Research Ethics Committee and by the Institutional Review Boards of the University of Alabama, Boston University, and Columbia University.
Spiking of breast milk samples with viruses.
Three different sources of HIV-1 were used for the spiking experiments: (i) culture supernatant from 293T cells transfected with the HIV-1 molecular clone (NL4-3); (ii) plasma from HIV-infected persons infected with both subtype B and C viruses; and (iii) a stock obtained from the Virology Quality Assurance Laboratory of the National Institute of Allergy and Infectious Diseases. Nominal copy numbers for culture supernatant and the patient isolate were determined by the Roche Amplicor Ultrasensitive HIV-1 Monitor assay (Roche, Branchburg, N.J.) and found to have 207,760 and 53,796 copies/ml, respectively. The stock obtained from the Virology Quality Assurance Laboratory has been previously described and consists of HIV derived from two patients and quantitated by electron microscopy and p24 antigen and RNA quantitation by both Roche Molecular Diagnostics and Chiron (5).
Twofold serial dilutions (starting input, 50,000 copies/ml) of HIV-1 were added to breast milk. Spiked milk samples were incubated at room temperature for 1 h with periodic low-speed vortexing before further processing or storage at −80°C for future analysis.
8E5/LAV cells.
The 8E5/LAV cell line is a subcloned HIV-1LAV-infected human T-cell line obtained from the AIDS Research and Reference Program of the National Institutes of Health. The cells contain a single integrated copy of HIV-1LAV proviral DNA.
Aliquots of cell-free milk were spiked with log10 dilutions of 8E5/LAV cells (106 through 100). The total number of cells in each sample was kept constant (106) by adding fresh peripheral blood mononuclear cells (PBMCs) or breast milk cells obtained from HIV-negative donors. Whenever possible, breast milk cells isolated from the same donor as the milk sample were used in the experiment.
DNA extraction.
DNA was extracted using a PUREGENE DNA isolation kit (Gentra Systems, Minneapolis, Minn.) according to the manufacturer's instructions.
Fractionation of whole breast milk.
Aliquots of whole breast milk (1 ml) spiked with HIV were centrifuged at 1,600 × g for 15 min at ambient temperature to fractionate milk into the aqueous supernatant (skim milk) and an overlying lipid fraction. The underlying aqueous fraction along with the pelleted cells or cellular debris was carefully removed. Extreme care was taken to avoid carryover of the overlying lipid. The aqueous portion was added to a new microcentrifuge tube and centrifuged, and the cell-free supernatant was removed. Since the cell counts in these samples were <1,000 cells per ml, no cell pellet was visible. Equal volumes of spiked whole milk were also processed for HIV RNA detection without fractionation.
Quantitation of HIV-1 RNA.
HIV RNA levels in the spiked samples were determined by the Amplicor UltraSensitive HIV-1 Monitor version 1.0 assay (Roche Diagnostics Systems), according to the manufacturer's protocol. HIV RNA levels in the ZEBS cohort were quantitated using the Amplicor UltraSensitive HIV-1 Monitor version 1.5 (Roche Diagnostics Systems). These assays differ in the primers utilized but are otherwise comparable.
Creamatocrit.
Creamatocrit analysis was based on the original methodology of Lucas et al. (18). Approximately 75 μl of well-mixed breast milk samples were drawn into a glass capillary tube; tubes were sealed at one end and centrifuged in a hematocrit centrifuge for 15 min at 12,000 × g. To prevent the cream layer “setting” at an angle, the tubes were removed immediately after centrifugation and placed vertically with the cream layer at the top. The creamatocrit was determined using a moving stage calibrator and expressed as a percentage of the length of the milk column in the tube. Since the cream layer is opaque, it was measured to the top, rather than the bottom, of the meniscus.
Real-time PCR assay.
All standards, controls, and samples were run in triplicate; the average value of the copy number was used to quantify both HIV-1 DNA and cellular β-globin copies. Standard curves for HIV copies were generated using a 10-fold dilution series of 8E5/LAV DNA. Unstimulated, HIV-1-negative human PBMC DNA was used to determine human cell equivalents, or genomes, through the amplification of the β-globin gene. The normalized value of HIV DNA load was expressed as the number of HIV copies/105 PBMC, which was calculated as the ratio of mean HIV copy number/mean β-globin copy number.
Target mixes were made from two primers and a fluorescently labeled probe. All primers and probes were synthesized by Sigma Genosys (Sigma Genosys Ltd., Woodlands, Tex.). The primers used to detect HIV were SR1 (5′-CAA GTA GTG TGT GCC CGT CTG T-3′) and AA55 (5′-CTG CTA GAG ATT TTC CAC ACT GAC-3′). The sequence of the ZXF probe is 5′-TGT GAC TCT GGT AAC TAG AGA TCC CTC AGA CCC-3′, with 6-carboxyfluorescine linked to the 5′ end. The β-globin primers are BGF1 (5′-CAA CCT CAA ACA GAC ACC ATG G-3′) and BGR1 (5′-TCC ACG TTC ACC TTG CCC-3′), and the probe is BGX1 (5′-CTC CTG AGG AGA AGT CTG CCG TTA CTG CC-3′, with 6-carboxyfluorescine linked to the 5′ end).
TaqMan PCR Core Reagents (Applied Biosystems, Branchburg, N.J.) were used in all reaction mixtures. The PCR reagent mixture contained the following: 1× TaqMan buffer A; 5 mM MgCl2; 0.2 mM each of dATP, dCTP, dGTP, and dUTP; 0.01 U of uracil DNA glycosylase per μl; and 0.025 U of AmpliTaq Gold DNA polymerase per μl. Reaction mixture volumes were 25 μl, and both the HIV and β-globin mixtures contained 300 nM concentrations of forward primers and 150 nM concentrations of reverse complement primers and 200 nM ZXF or BGX1 probe. The reaction was run in a sequence detector (model 7700; ABI/PE Biosystems, Foster City, Calif.). Thermal cycling conditions were 50°C for 2 min for uracil DNA glycosylase activity, 95°C for 10 min for AmpliTaq Gold activation, followed by 45 cycles, each cycle consisting of 15 s at 95°C for and 60 s at 60°C.
Statistical analysis.
Spearman rank correlation coefficients (r) were computed to describe associations between input and observed viral quantities. Kruskal-Wallis tests were used to test for differences in observed viral quantity under different conditions. Paired Wilcoxon signed rank tests were used to test for differences in the observed viral quantities before and after different processing conditions.
RESULTS
Detection of HIV-1 RNA in whole milk and milk fractions.
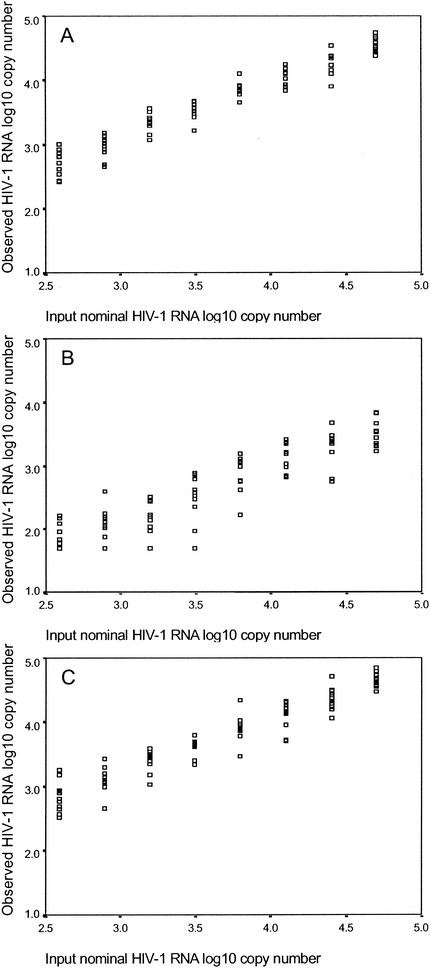
Aliquots of whole breast milk from 10 different women were spiked with serial twofold dilutions of HIV-1 stocks and then assayed using the Roche Amplicor UltraSensitive HIV-1 Monitor version 1.0 assay. The linear range of the assay is between 50 and 75,000 HIV RNA copies/ml. There was excellent correlation (r = 0.965, P < 0.0001) between input HIV-1 copy number (nominal copy number) and the number of HIV-1 RNA copies detected in whole milk. We next compared the ability to detect HIV-1 in the various fractions of breast milk. Known quantities of HIV-1 RNA were added to whole breast milk. After mixing, the spiked breast milk was spun, and the skim and lipid fractions were analyzed separately. Again, an excellent correlation was obtained between the number of HIV-1 RNA copies detected in the skim milk fraction and the input nominal copy number (r = 0.972, P < 0.0001). The correlation between the number of HIV-1 RNA copies detected in the lipid fraction and the input nominal copy number was also excellent (r = 0.905, P < 0.001), although mean values were lower (Fig. 1).
FIG. 1.
Aliquots of whole breast milk from 10 women were spiked with serial twofold dilutions of HIV-1 stocks and then assayed using the Roche Amplicor UltraSensitive version 1.0 assay. Next the spiked breast milk was spun, and the skim milk and lipid fractions were analyzed separately. The log10 numbers of HIV-1 RNA copies, measured by the nominal input copy number in the skim milk fraction (A), the lipid fraction (B), and whole milk (C), are shown.
The creamatocrit is a direct measure of the proportion of lipid within a volume of breast milk. Since the proportion of lipid in breast milk is highly variable, each milk sample was divided into two aliquots, and the creamatocrit of each aliquot was measured. The mean creamatocrits ranged from 2.5 to 8% (mean, 3.95% ± 1.74%). The relative amount of HIV-1 RNA copies detected in the lipid fraction was compared to that detected in whole milk (ratio of copy number in lipid/copy number in whole milk) for 106 samples from 17 sets of experiments with different numbers of input copies of HIV-1. The amount of HIV-1 RNA measured in the lipid fraction ranged from 2.19 to 36.35% (mean, 10.28% ± 3.74%) relative to the expected amount in an identical volume of whole milk. For example, if 1 ml of whole breast milk was spiked with 50,000 virions and the creamatocrit of that sample was 1%, the lipid fraction would be expected to contain 500 copies. There was no consistent relationship between the creamatocrit and the amount of virus recovered. As noted above, inhibition of the QS did not appear to be greater in the lipid fraction than in the other fractions. Surprisingly, comparison of the relative amount of HIV-1 RNA measured in the lipid fraction to the proportion of lipid in whole breast milk showed that lipid contains 0.75 to 6.28 times (mean, 3.09 times) more virus than expected for its volume. Studies using bovine milk spiked with NL4-3 virus also revealed that the cream layer contained, on average, over 1.6 times more virus than expected on the basis of the creamatocrit alone. Since HIV is an enveloped virus, it is likely that there are random nonpolar interactions between the virus particle and the breast milk lipid fraction. However, since the proportion of lipid in breast milk is low, the quantity of virus associated with lipid is unlikely to be significant.
Inhibition of HIV-1 RNA detection in whole breast milk.
The Roche Amplicor assay contains an internal standard (QS) to control for amplification. If the optical density for the QS is less than 0.3 in the undiluted well, the assay is invalid and no HIV RNA result is reported. A low QS can be due to PCR inhibition or loss of RNA during extraction. We observed low QS values in 24 of 249 (9.64%) of spiked whole-milk samples, 9 of 88 (10.34%) of spiked skim milk samples, and 10 of 88 (11.36%) spiked milk lipid samples. However, these results appeared to be sporadic, since different aliquots of the same breast milk sample did not consistently demonstrate a low QS. When the spiked whole-milk samples were repeated without dilution, all 24 samples had quantifiable RNA. This strongly suggests that the PCR inhibition encountered during RNA quantitation was random rather than due to the presence of a persistent inhibitory agent.
Detection of HIV-1 RNA in whole milk from HIV-infected women.
Whole-milk samples from HIV-infected women participating in ZEBS were analyzed using the Roche Amplicor UltraSensitive HIV-1 Monitor version 1.5 kit. Subtype C is the predominant circulating form of HIV in Zambia. Breast milk RNA levels ranged from <50 copies/ml to >75,000 copies/ml. HIV RNA quantitation was performed on 491 breast milk samples from HIV-infected women participating in ZEBS. Inhibition was observed in 43 samples (8.76%). All 43 samples were retested without dilution, and no inhibition was observed on the repeat test, confirming the observation among spiked breast milk samples. In contrast, none of the plasma samples from the same cohort (n = 408) had low QS values.
Stability of HIV-1 RNA in whole breast milk and breast milk fractions.
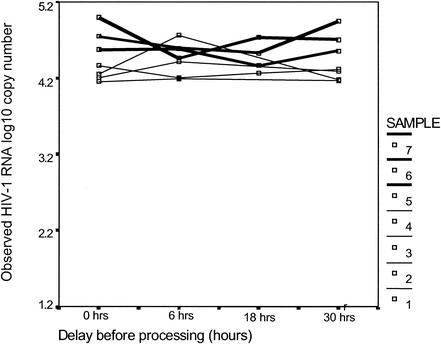
Whole breast milk samples were spiked with HIV-1 RNA and then kept at room temperature or 4°C for 6, 18, and 30 h prior to processing. Spiked samples at 0 h and replicate samples from other time points were frozen immediately at −80°C and then assayed 2 weeks later. HIV RNA levels were stable in whole milk when kept at room temperature for as long as 30 h (Fig. 2). Control samples kept at 4°C or at −80°C were equally stable (data not shown).
FIG. 2.
Whole breast milk samples were spiked with HIV-1 RNA and then were either frozen immediately or kept at room temperature for 6, 18, or 30 h prior to testing using the Roche Amplicor UltraSensitive version 1.0 assay. The observed log10 numbers of HIV-1 RNA copies per milliliter of milk measured when the input nominal copy number was 50,000 copies/ml (thick lines) or 20,776 copies/ml (thin lines) are shown.
Detection of HIV-1 RNA in whole milk subjected to freeze-thaw cycles.
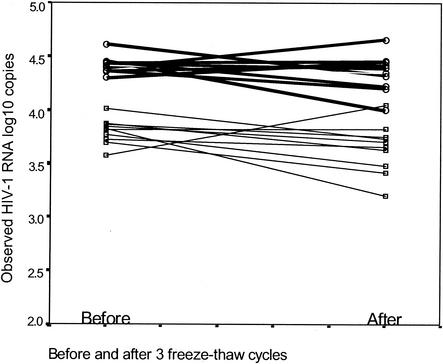
We compared the number of HIV-1 RNA copies detected in spiked whole breast milk samples after being frozen at −80°C and thawed prior to testing. Whole breast milk samples from five women were spiked in duplicate, with either 5,000 or 25,000 copies of HIV-1 RNA per ml. After the milk samples were subjected to three freeze-thaw cycles (one cycle consists of 1 h of thawing and 2 h of freezing), no statistically significant differences between the levels of HIV-1 RNA measured in whole breast milk were found. The mean reductions in the number of HIV-1 RNA copies in whole breast milk after three freeze-thaw cycles compared to the number for milk samples that were not subjected to freeze-thaw cycles were 0.15 and 0.07 log10 unit for the samples spiked with 5,000 and 25,000 copies/ml, respectively. This difference is well within the variability associated with this assay. Thus, HIV-1 RNA in whole breast milk is stable after three freeze-thaw cycles (Fig. 3). Similar results were obtained with breast milk spiked with HIV subtype C primary isolates as well as repeat measurements on breast milk from HIV-infected women (data not shown).
FIG. 3.
Whole breast milk samples from five women were spiked with either 5,000 or 25,000 copies of HIV-1 RNA per ml in duplicate. The Samples were tested immediately for HIV-1 RNA after sample collection and after three freeze-thaw cycles. The HIV-1 RNA quantities in milk spiked with 5,000 copies (thin lines) or 25,000 copies (thick lines) of HIV-1 RNA observed before and after three freeze-thaw cycles are shown.
Detection of HIV-1 proviral DNA after incubation in whole breast milk.
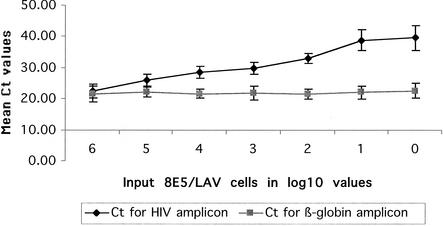
Breast milk cells or PBMCs from HIV-negative persons were used to serially dilute 8E5/LAV cells that contain one copy of HIV proviral DNA per cell. The number of 8E5/LAV cells ranged from one cell to one million cells. In order to keep the total amount of input DNA constant, various numbers of HIV-negative cells were added so that the total count was one million cells per ml of breast milk. These samples were analyzed using real-time PCR (TaqMan). One copy of HIV could be detected in 2 of 10 (20%) assays, while 10 HIV DNA copies could be detected with 80% sensitivity (8 of 10 assays). Tenfold serial dilutions ranging from 106 to 1 copy were tested in triplicate, and the threshold cycle (Ct) values were plotted against the copy number in Fig. 4. The linear correlation between the Ct and the logarithm of the DNA copy number from a total of 10 8E5/LAV spiked breast milk samples was repeatedly greater than 0.982.
FIG. 4.
Real-time PCR of HIV-1 standards. A standard curve was obtained by real-time PCR from 10-fold dilutions of 8E5/LAV cells (containing a single integrated HIV proviral copy per cell) into breast milk. The mean Ct is plotted against the logarithmic number of HIV-1 copies detected in the serial dilutions. The uniform slope of the β-globin copies detected demonstrated that an equivalent cellular background was present in each sample.
DISCUSSION
To date, studies of HIV RNA in breast milk have been principally restricted to the skim milk portion of breast milk (17, 21, 24, 26). We studied the ability of a commercial assay (Roche Amplicor UltraSensitive HIV-1 Monitor assay versions 1.0 and 1.5) to detect HIV-1 RNA in whole breast milk, skim breast milk, and breast milk lipid. We found excellent correlation between the nominal copy number and the observed copy number in whole milk as well as the skim milk portion of breast milk.
Breast milk lipid content, typically less than 10% of the total, is highly variable; maternal body fat (4) and timing of the sample collection (30) are important determinants of milk lipid. Our spiking experiments suggest that HIV may preferentially migrate into the lipid fraction of milk. However, since breast milk lipid represents such a small fraction of the total volume, sample variation in the lipid proportion does not substantially affect the amount of virus detected in whole milk. Thus, efforts to ensure standardization in the timing of breast milk sample collection (e.g., to attempt to collect only foremilk or hind-milk) may not be clinically relevant for measurement of HIV RNA quantities in breast milk. Our data do not address whether these lipid-bound virus can replicate and/or remain infectious.
Breast milk is known to contain many factors that inhibit PCR amplification including lactoferrin and immunoglobulin G. The Roche Amplicor assay uses recombinant Thermus thermophilus DNA polymerase (rTth polymerase) that has been shown to be less susceptible to amplification inhibition than other Taq polymerases (1-3). Using whole breast milk samples, we found that insufficient recovery of the QS occurred in less than 10% of the samples. This rate was similar in different milk fractions (skim milk and lipid), spiked whole-milk samples, and whole-milk samples obtained from HIV-infected women. In all cases where inhibition was observed, another aliquot of the same sample had quantifiable HIV-1 RNA detected. This strongly suggests that the insufficient QS recovery was due to errors in handling (protein carryover, loss of sample, etc.) and not necessarily due to the presence of intrinsic inhibitory factors in breast milk.
Shepard et al. reported partial inhibition, i.e., less than maximal recovery of the internal QS standard (optical density of the first QS well of <1.0), in three of five spiked skim milk samples of breast milk (26). However, in no case did the degree of decreased recovery invalidate the assay. We also observed that about one-third of the breast milk samples exhibited partial inhibition as defined above. However, in our experience, more than 90% of the samples had sufficient recovery to obtain valid results. Our results are consistent with those obtained by other groups using this assay (21, 24). These data suggest that although breast milk contains factors that could potentially impede HIV quantification by PCR, these substances do not generally affect accurate measurement.
Breast milk should be refrigerated to decrease bacterial growth and prevent activation of enzymes that could degrade virions and thereby expose HIV RNA to the degradation activities of enzymes. No significant decreases in HIV RNA levels were observed when whole breast milk was left at room temperature (25°C) or at 4°C for up to 30 h. These data suggest that HIV RNA is remarkably stable in breast milk and refrigeration does not offer any significant advantage. However, it must be stressed that we did not examine the effects of higher temperatures (30 to 40°C) that are often experienced in the field. It is therefore prudent to ensure that samples are not exposed to temperature extremes or temperatures that would support the growth of bacterial contaminants. The stability of HIV RNA at room temperature beyond 30 h needs to be evaluated to determine the maximal length of time samples can be held prior to separation or storage at −70°C.
The majority of breast milk specimens are transported and frozen at −70°C until the time of testing. Specimens must be thawed prior to RNA quantitation. Freeze-thawing has been shown to result in significant loss of plasma viral infectivity (20) but has insignificant effects on plasma RNA levels. We found that three consecutive freeze-thaw cycles had no significant effect on HIV RNA levels in breast milk. The stability of HIV RNA in breast milk is similar to that in whole blood or plasma (9, 11, 13, 16, 23, 29).
The breast-feeding infant of an HIV-infected woman is exposed not only to free virions (RNA) but also to HIV-infected breast milk cells. Breast milk lymphocytes have the ability to traverse the neonatal intestine and thus serve as a source of infection (12). The cellular pellet of breast milk often includes milk lipid globule membranes and other proteins. The majority of the cells in breast milk are fat-laden macrophages. These substances could influence PCR amplification. Using a real-time PCR methodology, we found that HIV could be accurately detected in spiked breast milk samples and samples from HIV-infected women.
Breast milk viral load is likely to be one of the chief factors associated with HIV transmission. It is critical that breast milk collection, processing, and storage methods that provide the highest degree of HIV-1 RNA and DNA stability be determined and standardized prior to implementation in studies of breast milk transmission. This standardization will ensure the accuracy of the results and allow comparison of data, especially when specimens are collected and transported from multiple sites. Our studies indicate that HIV RNA in breast milk can be accurately detected in all milk fractions (whole milk, skim milk, and lipid) and that the Roche Amplicor assay could reliably quantitate HIV in more than 90% of the breast milk samples.
Although it is always advantageous to process specimens as soon as possible, this is not always practical. Our studies indicate that HIV RNA is remarkably stable in breast milk. The RNA copy number did not significantly decrease when whole milk was maintained at room temperature or 4°C for up to 30 h or subjected to three cycles of freezing and thawing. These results should assist investigators in the development of standardized collection, transportation, processing, and storage procedures for studies involving HIV and breast milk.
Acknowledgments
This work was supported in part by grants HD396110 and HD40777 and the Elizabeth Glaser Pediatric AIDS Foundation.
We thank all the women who donated breast milk and the volunteers and staff of the ZEBS. We thank Cheryl Jennings for critical review of the manuscript, Glenda Corley for outstanding technical assistance, the Virology Quality Assurance Program of Rush Medical College (Chicago, Ill.) for providing the HIV RNA external standard, and Jerry Zack and Greg Bristol at the University of California at Los Angeles for assistance with the real-time PCR assay.
REFERENCES
- 1.Abu Al-Soud, W., and P. Rådström. 1998. Capacity of nine thermostable DNA polymerases to mediate DNA amplification in the presence of PCR-inhibiting samples. Appl. Environ. Microbiol. 64:3748-3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu Al-Soud, W., L. J. Jonsson, and P. Rådström. 2000. Identification and characterization of immunoglobulin G in blood as a major inhibitor of diagnostic PCR. J. Clin. Microbiol. 38:345-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu Al-Soud, W., and P. Rådström. 2001. Purification and characterization of PCR-inhibitory components in blood cells. J. Clin. Microbiol. 39:485-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen, J. C., R. P. Keller, P. Archer, and M. C. Neville. 1991. Studies in human lactation: milk composition and daily secretion rates of macronutrients in the first year of lactation. Am. J. Clin. Nutr. 54:69-80. [DOI] [PubMed] [Google Scholar]
- 5.Brambilla, D., S. Leung, J. Lew, J. Todd, S. Herman, M. Cronin, D. E. Shapiro, J. Bremer, C. Hanson, G. V. Hillyer, G. D. McSherry, R. S. Sperling, R. W. Coombs, and P. S. Reichelderfer. 1998. Absolute copy number and relative change in determinations of human immunodeficiency virus type 1 RNA in plasma: effect of an external standard on kit comparisons. J. Clin. Microbiol. 36:311-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns, D. N., S. Landesman, D. J. Wright, D. Waters, R. M. Mitchell, A. Rubinstein, A. Willoughby, and J. J. Goedert. 1997. Influence of other maternal variables on the relationship between maternal virus load and mother-to-infant transmission of human immunodeficiency virus type 1. J. Infect. Dis. 175:1206-1210. [DOI] [PubMed] [Google Scholar]
- 7.De Cock, K. M., M. G. Fowler, E. Mercier, I. de Vincenzi, J. Saba, E. Hoff, D. J. Alnwick, M. Rogers, and N. Shaffer. 2000. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA 283:1175-1182. [DOI] [PubMed] [Google Scholar]
- 8.Dickover, R. E., E. M. Garratty, S. A. Herman, M. S. Sim, S. Plaeger, P. J. Boyer, M. Keller, A. Deveikis, E. R. Stiehm, and Y. J. Bryson. 1996. Identification of levels of maternal HIV-1 RNA associated with risk of perinatal transmission. Effect of maternal zidovudine treatment on viral load. JAMA 275:599-605. [PubMed] [Google Scholar]
- 9.Dickover, R. E., S. A. Herman, K. Saddiq, D. Wafer, M. Dillon, and Y. J. Bryson. 1998. Optimization of specimen-handling procedures for accurate quantitation of levels of human immunodeficiency virus RNA in plasma by reverse transcriptase PCR. J. Clin. Microbiol. 36:1070-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fideli, U. S., S. A. Allen, R. Musonda, S. Trask, B. H. Hahn, H. Weiss, J. Mulenga, F. Kasolo, S. H. Vermund, and G. M. Aldrovandi. 2001. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res. Hum. Retrovir. 17:901-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginocchio, C. C., X. P. Wang, M. H. Kaplan, G. Mulligan, D. Witt, J. W. Romano, M. Cronin, and R. Carroll. 1997. Effects of specimen collection, processing, and storage conditions on stability of human immunodeficiency virus type 1 RNA levels in plasma. J. Clin. Microbiol. 35:2886-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldman, A. S., and R. M. Goldblum. 1997. Transfer of maternal leukocytes to the infant by human milk. Curr. Top. Microbiol. Immunol. 222:205-213. [DOI] [PubMed] [Google Scholar]
- 13.Griffith, B. P., M. O. Rigsby, R. B. Garner, M. M. Gordon, and T. M. Chacko. 1997. Comparison of the Amplicor HIV-1 monitor test and the nucleic acid sequence-based amplification assay for quantitation of human immunodeficiency virus RNA in plasma, serum, and plasma subjected to freeze-thaw cycles. J. Clin. Microbiol. 35:3288-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamosh, M. 1995. Enzymes in human milk, p. 388-427. In R. G. Jensen (ed.), Handbook of milk composition. Academic Press, San Diego, Calif.
- 15.Jensen, R. G., J. Bitman, S. Carlson, M. Hamosh, and D. Newburg. 1995. Human milk lipids, p. 495-542. In R. G. Jensen (ed.), Handbook of milk composition. Academic Press, San Diego, Calif.
- 16.Lew, J., P. Reichelderfer, M. Fowler, J. Bremer, R. Carrol, S. Cassol, D. Chernoff, R. Coombs, M. Cronin, R. Dickover, S. Fiscus, S. Herman, B. Jackson, J. Kornegay, A. Kovacs, K. McIntosh, W. Meyer, N. Michael, L. Mofenson, J. Moye, T. Quinn, M. Robb, M. Vahey, B. Weiser, and T. Yeghiazarian for the TUBE Meeting Workshop Attendees. 1998. Determinations of levels of human immunodeficiency virus type 1 RNA in plasma: reassessment of parameters affecting assay outcome. J. Clin. Microbiol. 36:1471-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis, P., R. Nduati, J. K. Kreiss, G. C. John, B. A. Richardson, D. Mbori-Ngacha, J. Ndinya-Achola, and J. Overbaugh. 1998. Cell-free human immunodeficiency virus type 1 in breast milk. J. Infect. Dis. 177:34-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucas, A., J. A. Gibbs, R. L. Lyster, and J. D. Baum. 1978. Creamatocrit: simple clinical technique for estimating fat concentration and energy value of human milk. Br. Med. J. 1:1018-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mofenson, L. M., J. S. Lambert, E. R. Stiehm, J. Bethel, W. A. Meyer III, J. Whitehouse, J. Moye, Jr., P. Reichelderfer, D. R. Harris, M. G. Fowler, B. J. Mathieson, G. J. Nemo, et al. 1999. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. N. Engl. J. Med. 341:385-393. [DOI] [PubMed] [Google Scholar]
- 20.Moudgil, T., and E. S. Daar. 1993. Infectious decay of human immunodeficiency virus type 1 in plasma. J. Infect. Dis. 167:210-212. [DOI] [PubMed] [Google Scholar]
- 21.Pillay, K., A. Coutsoudis, D. York, L. Kuhn, and H. M. Coovadia. 2000. Cell-free virus in breast milk of HIV-1-seropositive women. J. Acquir. Immune Defic. Syndr. 24:330-336. [DOI] [PubMed] [Google Scholar]
- 22.Quinn, T. C., M. J. Wawer, N. Sewankambo, D. Serwadda, C. Li, F. Wabwire-Mangen, M. O. Meehan, T. Lutalo, R. H. Gray, et al. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 342:921-929. [DOI] [PubMed] [Google Scholar]
- 23.Sebire, K., K. McGavin, S. Land, T. Middleton, and C. Birch. 1998. Stability of human immunodeficiency virus RNA in blood specimens as measured by a commercial PCR-based assay. J. Clin. Microbiol. 36:493-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semba, R. D., N. Kumwenda, D. R. Hoover, T. E. Taha, T. C. Quinn, L. Mtimavalye, R. J. Biggar, R. Broadhead, P. G. Miotti, L. J. Sokoll, L. van der Hoeven, and J. D. Chiphangwi. 1999. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J. Infect. Dis. 180:93-98. [DOI] [PubMed] [Google Scholar]
- 25.Shearer, W. T., T. C. Quinn, P. LaRussa, J. F. Lew, L. Mofenson, S. Almy, K. Rich, E. Handelsman, C. Diaz, M. Pagano, V. Smeriglio, L. A. Kalish, et al. 1997. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. N. Engl. J. Med. 336:1337-1342. [DOI] [PubMed] [Google Scholar]
- 26.Shepard, R. N., J. Schock, K. Robertson, D. C. Shugars, J. Dyer, P. Vernazza, C. Hall, M. S. Cohen, and S. A. Fiscus. 2000. Quantitation of human immunodeficiency virus type 1 RNA in different biological compartments. J. Clin. Microbiol. 38:1414-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sperling, R. S., D. E. Shapiro, R. W. Coombs, J. A. Todd, S. A. Herman, G. D. McSherry, M. J. O'Sullivan, R. B. Van Dyke, E. Jimenez, C. Rouzioux, P. M. Flynn, J. L. Sullivan, et al. 1996. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. N. Engl. J. Med. 335:1621-1629. [DOI] [PubMed] [Google Scholar]
- 28.Thea, D. M., R. W. Steketee, V. Pliner, K. Bornschlegel, T. Brown, S. Orloff, P. B. Matheson, E. J. Abrams, M. Bamji, G. Lambert, E. A. Schoenbaum, P. A. Thomas, M. Heagarty, M. L. Kalish, et al. 1997. The effect of maternal viral load on the risk of perinatal transmission of HIV-1. AIDS 11:437-444. [DOI] [PubMed] [Google Scholar]
- 29.Todd, J., C. Pachl, R. White, T. Yeghiazarian, P. Johnson, B. Taylor, M. Holodniy, D. Kern, S. Hamren, D. Chernoff, et al. 1995. Performance characteristics for the quantitation of plasma HIV-1 RNA using branched DNA signal amplification technology. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10(Suppl. 2):S35-S44. [PubMed] [Google Scholar]
- 30.Woodward, D. R., B. Rees, and J. A. Boon. 1989. Human milk fat content: within-feed variation. Early Hum. Dev. 19:39-46. [DOI] [PubMed] [Google Scholar]