Abstract
The Duopath Verotoxin test (Merck KgaA, Darmstadt, Germany) is a newly developed immunochromatographic test for the confirmation of Shiga toxin (Stx)-producing Escherichia coli (STEC) strains from food products. This test detects both Stx 1 (Stx1)-positive and Stx2-positive samples individually with the same device. By modification of the original protocol, the present study evaluated its performance and feasibility for clinical application with human stool samples, consisting of 41 frozen samples known to contain STEC isolates (O157:H7 and non-O157 serotypes) and 250 fresh specimens. The test specimens were polymyxin B extracts of colony sweeps taken from overnight sorbitol-MacConkey agar cultures of stools containing STEC isolates and other bacteria. All 41 frozen STEC-positive stool samples were positive by the Duopath Verotoxin test, as were 2 fresh stool samples with culture-confirmed STEC O157 infection. Thus, 100% sensitivity and no false-positive results were obtained when the Premier EHEC assay (Meridian Bioscience, Cincinnati, Ohio) was used as the “gold standard.” The Duopath Verotoxin test is simple to perform and easy to interpret, providing a turnaround time of 24 h. Despite its original intended use, the Duopath Verotoxin test has a great potential for clinical application.
Verotoxins or Shiga toxin (Stx) 1 (Stx1) and Stx2 are elaborated by many serotypes of Escherichia coli that cause hemorrhagic colitis as well as the life-threatening hemolytic uremic syndrome (HUS). In the United States alone, at least 20,000 illnesses and 200 deaths occur annually due to Stx-producing E. coli (STEC) (4). Approximately 5 to 10% of infected individuals in large outbreaks develop HUS, with the mortality rate being less than 10% (9). In the United States the most common serotype reported is STEC O157:H7 (STEC O157). However, more than 50 serotypes other than STEC O157 are capable of producing hemorrhagic colitis and HUS (1). The Stx-producing non-O157 E. coli (non-O157 STEC) serotype is not uncommon in Europe and Canada (14), but the prevalence in the United States has been considered extremely low in the past. Thus, many clinical laboratories in the United States do not actively search for non-O157 STEC isolates. Moreover, the Centers for Disease Control and Prevention (CDC) did not advocate testing for non-O157 STEC strains until August 2001. Since 1993, only a handful of articles reporting sporadic cases of non-O157 STEC were published in the United States, with the proportion of non-O157 STEC isolates among all STEC isolates tested reaching as high as 31% (1, 2, 3, 5, 9, 10, 13).
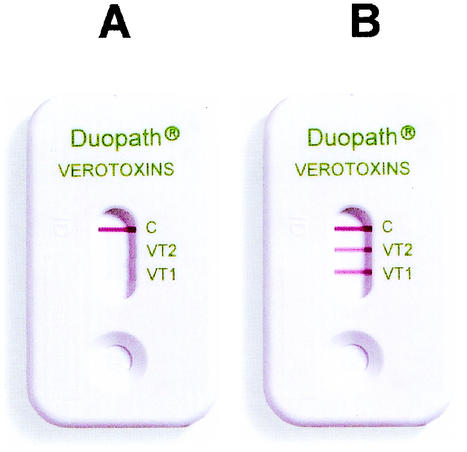
Without methods for the detection of Stx or Stx genes, many cases of non-O157 STEC infection are difficult to detect because most non-O157 STEC isolates lack the sorbitol-negative phenotype that greatly facilitates the recovery of STEC O157 strains. Therefore, excluding the PCR test, the toxin assay has become the method of choice for the detection of Stx. The commercially available methods for the detection of STEC isolates include microplate enzyme immunoassays, the Premier EHEC assay (Meridian Bioscience, Cincinnati, Ohio) and ProSpecT Shiga Toxin E. coli assay (Alexon-Trend, Ramsey, Minn.), as well as other methods, including the cell culture cytotoxicity assay and the reverse passive latex agglutination test (VTEC-RPLA; Denka-Seiken, Tokyo, Japan). The purpose of the investigation described here was to evaluate the performance of the Duopath Verotoxin (DV) immunochromatographic test (Fig. 1) and its feasibility for clinical applications with human stool specimens. The DV test is newly developed and was originally intended for use for the confirmation of STEC strains from food products. The DV test uses colloidal gold-labeled monoclonal antibodies to detect Stx1 and Stx2 independently with the same test device. Any inoculum with Stx1 and/or Stx2 complexes with antibodies in the reaction zones as the test sample migrates over a membrane, forming a sandwich complex with immobilized Stx1- and Stx2-specific antibodies in the detection zones. A positive result appears as a red band within 10 min (Fig. 1). The intensity of the color is compared to the colors on a chart provided by the manufacturer showing a scale from 0 to 10+. According to the scale, 0 (no color) is interpreted as a negative result, while 1+ (extremely faint red band) represents a very weakly positive result. However, any visible red band in the reaction zone is considered a positive result, regardless of its intensity. In this study, clinical stool samples were retrospectively and prospectively tested by the Premier EHEC assay as the “gold standard” for determination of the performance of the DV test. The methods described here were modified from the original protocol to provide a shorter turnaround time with fewer test steps.
FIG. 1.
Negative (A) and positive (B) DV test results.
In the retrospective study, 41 known Stx-positive stool specimens (31 STEC O157-positive specimens and 10 non-O157 STEC-positive specimens that had been frozen at −70°C for up to 10 years) from different patients were thawed and inoculated onto sorbitol-MacConkey agar (SMAC) plates (Becton Dickinson, Cockeysville, Md.). The serotypes of the isolates described above were confirmed by the CDC and the Commonwealth of Virginia Division of Consolidated Laboratory Services (DCLS). To prevent the inhibition of non-O157 STEC, SMAC plates lacking cefixime were used. The inoculum for the Premier EHEC tests was prepared with a Dacron swab, which was swept a few times across the area of confluent growth on the SMAC plate. If sorbitol-negative colonies were present on the SMAC plates, an effort was made to include them in the colony sweeps. The swab was suspended in 5 ml of MacConkey broth, and the mixture was incubated at 35°C for 18 to 24 h. The procedure for the Premier EHEC test was then performed according to the instructions of the manufacturer. Direct testing of stool specimens and enrichment broth by the DV test was not perfected due to an unacceptable specificity; however, further evaluation is in order. For the DV test, colony sweeps prepared in a fashion similar to that described above were suspended in 0.5 ml of distilled water containing polymyxin B (50 μg/ml). The mixture was incubated at 35°C for 30 min. Polymyxin B vials were supplied by the manufacturer of the DV test and were diluted with 1 ml of distilled water to yield a 5-mg/ml stock solution. Then, the stock solution was further diluted 1:100 to yield a working solution of 50 μg/ml. The diluted stock solution was stored at 2 to 8°C for maximum of 7 days, whereas the stock solution was kept at 2 to 8°C for 1 month. In the prospective study, all stool specimens submitted for culture between August and October 2002 (n = 250) were inoculated onto both SMAC plates and the routine media used for the isolation of normal enteric pathogens. All plates were incubated at 35°C for 18 to 24 h. The inocula for the DV and Premier EHEC tests were prepared as described above. Approximately 0.2 ml of a polymyxin B-treated inoculum was added to the sample port, the test device was incubated at room temperature, and the result was interpreted after a maximum of 10 min. The appearance of a band after 10 min of incubation is considered a negative result. Quality control was conducted daily with a stock culture of STEC O157 known to possess both Stx1 and Stx2. The Premier EHEC assay was performed according to the recommendations of the manufacturer. To test the specificity of the DV test, the following clinical isolates were inoculated onto SMAC plates and treated with polymyxin B: 10 Pseudomonas aeruginosa isolates, 10 Klebsiella isolates, 10 Enterobacter isolates, 10 Proteus isolates, 10 non-Stx-producing E. coli isolates, 3 Aeromonas isolates, 5 Serratia isolates, and 3 Shigella isolates. To determine the sensitivity of the DV test, stock cultures of 20 additional STEC isolates (4 non-O157 STEC isolates and 16 STEC O157 isolates) different from the previously tested isolates were inoculated onto SMAC plates and MacConkey broth and tested as described above.
During the retrospective study, cultures of four samples with O157:H7 lacked visible sorbitol-negative colonies due to overgrowth by other enteric flora; however, both the DV test and the Premier EHEC test produced positive reactions. To prove the validity of the positive reactions, the MacConkey broths with the four samples used for the Premier EHEC test were subcultured onto several SMAC plates. Subsequently, the colonies of STEC O157 were recovered. Thus, both the DV test and the Premier EHEC test demonstrated 100% sensitivity in detecting STEC-positive samples through the use of the colony sweep method (Table 1). All 41 samples known to be positive produced distinct red bands, as did the stock cultures of the additional 20 STEC isolates. The intensities of the red colors varied among the tests with the strains, but there were no questionable or uninterpretable bands, since all of the bands produced color intensities of 2+ to 10+ on the manufacturer's color scale.
TABLE 1.
Performance of DV test in prospective and retrospective studies
| DV test result | No. of specimens with the following result by Premier EHEC assaya:
|
|||
|---|---|---|---|---|
| Positive
|
Negative
|
|||
| Frozenb | Freshc | Frozen | Fresh | |
| Positive | 41 | 1 | NAd | 1e |
| Negative | NA | 0 | NA | 248 |
Assays were performed with samples from MacConkey broth inoculated with colony sweeps.
Retrospective study (known STEC-positive stool with specimens).
Prospective study.
NA, not applicable.
E. coli O157 was recovered from MacConkey broth inoculated with a stool specimen.
During the prospective study period, two stool samples presented with STEC O157, one of which was a bloody specimen that failed to yield sorbitol-negative colonies due to the overgrowth by enteric flora on the SMAC plate. When the colony sweeps were tested by the DV test, both the Stx1 and Stx2 bands yielded 4+ reactions, but the sample was considered negative by the Premier EHEC test. It is possible that an insufficient number of STEC O157 colonies were contained in the colony sweeps used for the Premier EHEC assay. Finally, the STEC O157 strain was isolated from MacConkey broth newly inoculated with the original stool specimen, validating the positive results originally obtained by the DV test. The two isolates were confirmed to be STEC O157 by DCLS. Five Stx-negative stool specimens produced dark bands (nonred bands) ranging from 1+ to 4+ in intensity. Although we do not know the reason for the color deviation, we interpreted bands other than red ones to be negative, since all known STEC isolates produced only red bands upon testing. Lastly, one specimen into which mucoid colonies of Klebsiella were mixed heavily did not migrate to the reaction zone due to the high degree of viscosity. The problem was resolved by centrifuging the mixture at 13,000 rpm for 2 min with an Abbott X-Systems microcentrifuge and delivering the supernatant into the test well. Other pathogens isolated during the retrospective study included seven Salmonella isolates, 2 Shigella isolates, 1 Plesiomonas isolate, 1 Vibrio parahemolyticus isolate, and 1 Campylobacter isolate, none of which demonstrated cross-reactivity. Also, the 61 isolates of other enteric bacteria that were tested for determination of the specificity of the DV test yielded negative results.
In the United States, plating of stool specimens on SMAC plates alone for the detection of STEC O157 is widely practiced because of its simplicity and the lack of a requirement for the detection of non-O157 STEC isolates. From our experience and those of other investigators, the sensitivities of SMAC plates for the detection of STEC O157 isolates ranged from 60 to 100% (8, 9, 12). Four frozen stool specimens and one prospectively evaluated stool specimen which contained STEC O157 isolates did not reveal sorbitol-negative colonies on SMAC plates due to overgrowth by normal enteric organisms. Although the colonies were invisible, they were detected by the DV test because the test requires no more than a few colonies of STEC to yield a positive reaction. Another limitation of SMAC plates is their inability to recognize the presence of sorbitol-positive non-O157 STEC colonies. Multiple occurrences of sorbitol-positive STEC O157:H− strains causing diarrheal disease and HUS were reported in Germany and other European countries (7). Unfortunately, these sorbitol-fermenting STEC strains can be detected only by testing for Stx in clinical laboratories that lack PCR testing capabilities. Recently, the importance of detection of non-O157 STEC isolates has been echoed by the Foodborne and Diarrheal Disease Branch of the CDC and by other investigators in the United States (1, 5, 10, 13).
The DV test described here demonstrated a sensitivity of 100% for the detection of clinical isolates without any cross-reactivity with other enteric organisms (Table 1). The appearance of dark bands for five of the prospectively evaluated specimens is unexplainable, and the results of the tests in which these bands appeared were interpreted as negative. Through our limited experiments, we have found that the toxins would be detectable without the addition of polymyxin B but that this would necessitate the use of a much heavier inoculum (data not shown). However, the addition of polymyxin B enhances the color intensity because it amplifies the release of toxins that are present at high concentrations in the periplasmic space of STEC isolates (8). According to Tarr et al. (15), 18% (5 of 28) of their pediatric patients infected with O157:H7 strains developed HUS, and all isolates contained Stx2. Conversely, none of 11 patients infected with non-O157 STEC strains manifested HUS, and 91% (10 of 11) of these strains possessed only Stx1. It is believed that Stx2 is found more commonly in STEC O157 isolates than in non-O157 STEC isolates. Louise and Obrig (11) observed that Stx2 possesses much greater cytotoxic potency than Stx1 against human renal glomerular microvascular endothelial cells. Although the differentiation of Stx1 and Stx2 does not alter clinical management, it has been demonstrated epidemiologically that strains expressing Stx2 are more prone to cause HUS than ones producing only Stx1 (6). If the assumptions presented above are valid, then determination of the individual toxin types may be an asset in certain epidemiological situations. It is apparent that if a specimen shows only Stx1 and sorbitol-positive colonies, the presence of non-O157 STEC strains should be suspected. In our retrospective study, 45 of 49 STEC O157 isolates (33 from frozen samples and 16 from stock cultures) produced Stx2, whereas 10 of 12 non-O157 STEC isolates (8 from frozen samples and 4 from stock cultures) produced only Stx1. For those O157:H7 isolates that did produce Stx2, it is possible that the level of toxin production was below the level detectable by the DV test. Retesting of those isolates for Stx production with different types of media may provide the answer. In order to confirm unequivocally the low level of Stx2 production, the manufacturer (Merck KGaA) recommends the use of Casamino Acids-yeast extract broth with supplement for a 6-h enrichment. This culture procedure can lead to a significant enhancement of the level of Stx production and, hence, to stronger signals, especially for Stx2 (data not shown). The use of the DV test enables the laboratory to determine the presence of STEC isolates within 24 h after specimens are plated onto SMAC plates without the use of other confirmatory tests. Other than SMAC, we have not tested other media used for the isolation of enteric organisms; therefore, we do not endorse the use of other media at this time. At present, many clinical laboratories are submitting suspected isolates of E. coli O157 to reference laboratories because the mere presence of sorbitol-negative colonies that are positive for the O157 antigen by agglutination tests does not constitute confirmation of the presence of STEC O157 isolates. Institution of a device such as the DV test will circumvent the need for confirmation of a result by a reference laboratory as well as shorten the turnaround time. Like the Premier EHEC test, the result obtained by the DV test is confirmatory for STEC, and the colonies that are isolated, especially non-O157 STEC colonies, can be submitted to a reference laboratory for specific serotyping at a later date. In conclusion, on the basis of our experience, we concur with the recommendation of Klein et al. (10) that both SMAC plates and a toxin assay be used for the optimum recovery of STEC isolates. The DV test provides a turnaround time of 24 h and demonstrates a high degree of sensitivity without false-positive results. Test results are easy to interpret, and the methodology is simple enough that laboratory personnel at all levels can perform the test. Although the DV assay was originally designed for use with food products, we believe that it has great potential for clinical applications as well.
ADDENDUM
During the editorial review process for the manuscript, three more cases of STEC were detected: two caused by STEC O157 (Stx2-producing) isolates and one caused by a non-O157 STEC (Stx2-producing) isolate. The serotypes of the former isolates were confirmed by DCLS, whereas the serotype of the latter isolate is pending. The two STEC O157 isolates were sorbitol negative, while the non-O157 STEC isolate was sorbitol positive, and all three isolates were unequivocally identified as STEC by the DV and Premier EHEC tests.
REFERENCES
- 1.Acheson, D., and G. Keusch. 1996. Which Shiga toxin-producing types of E. coli are important? ASM News 62:302-307. [Google Scholar]
- 2.Beuge, R. E., M. A. Neill, E. F. Papa, and P. H. Dennely. 1994. A prospective study of Shiga-like toxin-associated diarrhea in a pediatric population. J. Pediatr. Gastroenterol. Nutr. 19:164-169. [DOI] [PubMed] [Google Scholar]
- 3.Bokette, T. N., M. O'Callahan, C. R. Clausen, N. M. Tang, N. Tran, S. L. Moseley, T. R. Fritsche, and P. I. Tarr. 1993. Shiga-like toxin producing Escherichia coli in Seattle children: a prospective study. Gastroenterology 105:1724-1731. [DOI] [PubMed] [Google Scholar]
- 4.Boyce, T. G., D. L. Swerdlow, and P. M. Griffin. 1995. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N. Engl. J. Med. 333:364-368. [DOI] [PubMed] [Google Scholar]
- 5.Fey, P., R. Wickert, M. Rupp, T. Safranek, and S. Hinrichs. 2000. Prevalence of non-O157:H7 Shiga-toxin producing Escherichia coli in diarrheal stool samples from Nebraska. Emerg. Infect. Dis. 6:530-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 7.Karch, H., and M. Bielaszewska. 2001. Sorbitol-fermenting Shiga-toxin producing Escherichia coli strains: epidemiology, phenotypic and molecular characteristics, and microbiological diagnosis. J. Clin. Microbiol. 39:2043-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karmali, M. A. 1989. Infection by verotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kehl, S., P. Havens, C. Behnke, and D. Acheson. 1997. Evaluation of the Premier EHEC assay for detection of Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 35:2051-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein, E., J. Stapp, C. Clausen, D. Boster, J. Wells, X. Qin, D. Swerdlow, and P. Tarr. 2002. Shiga toxin-producing Escherichia coli in children with diarrhea. A prospective point of care study. J. Pediatr. 14:172-177. [DOI] [PubMed] [Google Scholar]
- 11.Louise, C. B., and T. G. Obrig. 1995. Specific interaction of Escherichia coli O157:H7 derived Shiga-like toxin II with human renal endothelial cells. J. Infect. Dis. 172:1397-1401. [DOI] [PubMed] [Google Scholar]
- 12.Park, C., K. Gates, N. Vandel, and D. Hixon. 1996. Isolation of Shiga-like toxin producing Escherichia coli (O157 and non-O157) in a community hospital. Diagn. Microbiol. Infect. Dis. 26:69-72. [DOI] [PubMed] [Google Scholar]
- 13.Park, C., H. Kim, and D. Hixon. 2002. Importance of testing stool specimens for Shiga toxin. J. Clin. Microbiol. 40:3542-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramator, K., E. Henderson, R. Szumski, and T. J. Louie. 1995. Impact of free verotoxin testing on epidemiology of diarrhea caused by verotoxin-producing Escherichia coli. J. Clin. Microbiol. 33:1114-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarr, P. I., M. A. Neill, C. R. Clausen, J. W. Newland, R. J. Neill, and S. L. Moseley. 1989. Genotypic variation in pathogenic Escherichia coli O157:H7 isolated from patients in Washington, 1984-1987. J. Infect. Dis. 159:344-347. [DOI] [PubMed] [Google Scholar]