Abstract
Helicobacter pylori is chiefly acquired in childhood, but the exact timing of acquisition is not well understood. The main goal of this study was to assess H. pylori acquisition in a pediatric population. We studied two cohorts of Native American children: a birth cohort of 50 children and 58 older children (mean age, 53 months). We measured serum immunoglobulin G (IgG), IgM, and IgA antibodies to H. pylori whole-cell antigen and IgG antibodies to CagA. Among 44 birth cohort children monitored for more than 12 months, 24 (54.5%) had seroconversions, 7 (15.9%) were transient, and 17 (38.6%) were persistent. Among the older children, 49 (84.5%) of the 58 children were monitored for 1 year; 34 (69.4%) had H. pylori antibodies at study entry. During the next year, 7 (20.6%) children seroreverted, and of 15 initially negative children, 5 (33.3%) seroconverted. In both groups, evaluation of CagA antibodies increased the sensitivity of H. pylori detection. Serum pepsinogen I (PGI) levels in H. pylori-negative children rose significantly until age 6 months and remained constant for the next 19 months. At the time of H. pylori seroconversion, PGI peaked to levels significantly higher than in the never-seroconverted (P = 0.02) and the pre-seroconverted (P = 0.03) children, but then declined to levels paralleling those of H. pylori-negative children. Thus, H. pylori acquisition, accompanied by a transient PGI increase, was frequent in this population, especially in the second and third years of life, but often was brief.
Gastric colonization by Helicobacter pylori is highly prevalent throughout the world (3, 15, 41), and the organism is predominantly acquired during childhood (19, 34). In developing countries, more than 80% of adults are colonized with H. pylori, and more than 50% of children become colonized before the age of 10 years compared to 30% of the adults and 10% of children in developed countries like the United States (15-17, 50, 66). With socioeconomic development, the prevalence of H. pylori has declined (26, 48, 50). H. pylori colonization generally persists throughout life, except in persons treated with antibiotics or those who develop atrophic gastritis, usually late in life (18, 70). Although most adults have no clinical consequences of H. pylori colonization, it is a risk factor for peptic ulcer disease (7, 15, 37) and noncardia gastric adenocarcinoma (22, 38).
While colonization of adults with H. pylori almost always persists (26, 42, 48), there has been evidence for both transient and persistent colonization in children (19, 65) and in experimentally challenged nonhuman primates (12). The relative frequency of transient H. pylori acquisition among children in developing countries has been only partially characterized (29, 58, 65), and there is little information about gastric physiology in children in relation to H. pylori acquisition (58). An important question is whether pepsinogen production, reflecting gastric acidity (71), is affected by the H. pylori status of the child.
The Native American Apache peoples have high rates of infectious diseases involving the gastrointestinal tract, including childhood diarrhea and hepatitis A (39, 58), paralleling rates present in many developing countries (55, 57). The availability of two cohorts of longitudinally studied Apache children enabled us to determine the timing of H. pylori acquisition. Using stored serum samples, we used host antibody responses to assess H. pylori acquisition and measured pepsinogen I (PGI) levels to reflect gastric physiologic changes in relation to H. pylori status. Examination of this population may be representative of children living in developing countries.
MATERIALS AND METHODS
Patient populations.
All study subjects were living on the White Mountain Apache Indian Reservation in east central Arizona. The study was approved by the White Mountain Apache Tribe, the Indian Health Service Institutional Review Board, and the Committee on Human Research at the Johns Hopkins University School of Hygiene and Public Health. After written informed consent was obtained from the mothers, a birth cohort of 50 newborn infants was enrolled between 1 June 1983 and 23 April 1986 in a study of bacterial polysaccharide immunoglobulin (Ig) to prevent infections due to Haemophilus influenzae (36). During this period, 628 infants were born in the reservation. After obtaining written informed consent from their parents or guardians, a second group of 58 children, ages 2 to 11 years (mean age, 4.2 ± 0.3 years), was recruited at elementary schools as part of a study of hepatitis A vaccine in 1993 to 1994. Although these children received the hepatitis A vaccine, analysis of their responses was not included in the publication about that study (39). Sera from both groups of children have been stored at −20C since those studies. Since these sera were analyzed after removing all identifiers except the ages of the children examined, the study subjects, currently 16 to 27 years old, cannot be identified. Each child in this study was assigned a specific study number, which served as the prefix of the code for each specimen of a particular child; a suffix number indicating the timing of the sample, allowed tracking the serial specimens from each child. We also examined serum samples from 25 women (20 to 34 years old; mean age, 25.7 ± 3.7 years) from the nearby San Carlos Apache reservation to gain an estimate of the prevalence of H. pylori among women of childbearing age in this population.
Sampling of children.
A total of 638 sera from 108 Apache children were included in this study. The birth cohort group was monitored for up to 25 months (mean, 19.6 ± 0.3 months), and serum samples were obtained at 2, 4, 6, 9, 12, 15, 16, 18, 20, 24, and/or 25 months of age. For the older cohort, serum specimens were collected at the beginning of the study and at 4, 24, 28, and/or 52 weeks (mean follow-up, 10.8 ± 0.1 months). Means of 8.0 ± 0.4 and 4.2 ± 0.1 serum specimens per child were available in the birth cohort and the older cohort, respectively. The assessment of the immune response in the birth cohort group at 2, 4, and 6 months was performed to estimate the prevalence of H. pylori in their mothers and to confirm the decline of maternal antibodies as described previously (14).
Serum antibodies to H. pylori antigens.
Antibody responses were determined by antigen-specific enzyme-linked immunosorbent assays (ELISAs). The H. pylori whole-cell (WC) antigen was prepared from a pool of sonicated whole bacteria made from five different clinical H. pylori isolates, as described elsewhere (46) and validated in several ethnically diverse populations (9, 11, 13, 20, 35, 44). Levels of IgA, IgG, and IgM antibodies to the H. pylori WC antigen in serum were determined as described previously (11, 46). Serum samples were diluted 1:100, 1:800, and 1:200, respectively, and each optical density (OD) value was calculated from the mean absorbance of two assays per sample. Results for IgA and IgG were expressed as OD ratios (ODRs) (11, 42), and for IgM, the results were expressed as OD values (35). The sensitivity and specificity of testing with this antigen were >95% (11, 64), and the intra-and interassay variations have been shown to be <5% (11, 47). The presence of antibodies to the H. pylori CagA antigen was determined by an ELISA to detect serum IgG antibodies against orv220, a molecular weight 65,000 recombinant CagA protein purified from Escherichia coli (Acambis, Cambridge, Mass.), as described previously (6). Test sera were diluted 1:100 and assayed in duplicate. This ELISA has a sensitivity of 94.4% and a specificity of 92.5% (47) and also has been validated in multiple diverse populations (23, 43, 68). H. pylori culture-positive persons may be seropositive in the CagA assay in the absence of response to the WC antigen (54).
Determination of serum PGI.
Serum samples from children in both cohorts also were examined to determine serum PGI levels, using an immunoenzymatic assay for in vitro measurement of the serum concentrations of PGI (Gastroset PGI; Orion Diagnostica, Espoo, Finland) (56). The sensitivity of the assay was 5 μg/liter. Because examination of the first 81 samples examined in duplicate showed <5% interassay variation, all subsequent samples were assessed only once.
Study definitions.
The same criteria were used to define H. pylori serologic status for the birth cohort and the older cohort specimens. To determine the baseline for H. pylori seropositivity, we calculated thresholds for IgA, IgM, and IgG responses to the WC antigen and IgG to CagA by using U.S. children known to be negative for H. pylori as assessed by gastroduodenoscopy (13). Seropositivity was defined as an ODR value of >mean + 3 intervals of the standard deviation for the 27 confirmed negative children. We used this stringent criterion because serologic responses to H. pylori differ in adults and children (67). ODR values defined as positives when using the WC antigen were 0.4 for IgA and 0.7 for IgG, with an OD of 0.9 for IgM. For CagA, an ODR of >0.30 was considered positive. Seroconversion in a child was defined as a serum specimen showing a positive value in any of the four assays after a previous negative value. Seroconversion was defined as being transient when all assays in one or more follow-up samples become negative. Persistent seroconversion was defined as continued seropositivity during the follow-up period. Seroreversion was defined as the loss of positivity from previously positive values. For studies of the women of childbearing age, the definition of serostatus was based on prior definitions for adults (42, 47). Serum from 2-month-old infants in the birth cohort also was used as a proxy for maternal serum IgG levels. Infants whose serum IgG levels to either the WC or CagA antigens were positive as described above (child criterion) were defined as being born from an H. pylori-seropositive mother.
RESULTS
Maternal antibodies in the birth cohort.
For the 50 children in the birth cohort, there were 992 total person-months of follow-up, with 8.0 ± 0.4 specimens per person. A total of 44 children had at least 1 year of follow-up, and for 38 of these, follow-up was at least 21 months. The IgG results for the 2-month serum specimens from 48 of the children indicate that at least 77.1% of their mothers were H. pylori positive (62.5 and 52.1% in the WC and CagA assays, respectively). This result is similar to that found (76.0% in total; 72 and 48% in the WC and CagA assays, respectively) in 25 women of childbearing age from a nearby Apache community. As expected, the maternal IgG antibodies to both the WC and the CagA antigens declined progressively, nearing baseline levels by the time the children were 6 months old and well below the cutoff values for positivity (data not shown). None of the IgG results obtained at 2, 4, and 6 months was used in assessing H. pylori acquisition.
Serologic evidence of H. pylori acquisition.
Among the 44 birth cohort children monitored for at least 1 year (mean, 21.5 ± 0.5 months), 20 (45.5%) children had no evidence of seroconversion in any of 171 specimens studied in the four assays (34.4 ± 6 assays per child) (data not shown). In contrast, 24 (54.5%) showed evidence of H. pylori seroconversion. IgM seroconversions were frequent (15.9%), but IgG seroconversions to H. pylori WC antigens (31.8%) or to CagA (36.4%) were more common. By the end of the follow-up period, only 17 of the 24 children remained seropositive, being positive in a mean of 2.3 ± 0.2 assays; thus, a total of 38.6% of the children showed persistent seroconversion (Table 1). The seven patients who had only transient seroconversions had been positive in 1.3 ± 0.2 assays. The CagA IgG assay alone or in combination with other assays was the earliest test positive in 15 (62.5%) of the 24 children who had either persistent or transient seropositivity (data not shown).
TABLE 1.
Persistence of H. pylori seropositivity in birth cohort of Native American children
| Follow-up period (mo) | No. of children | No. (%) of seropositive children at end of follow-upa
|
||||
|---|---|---|---|---|---|---|
| WC antigen
|
CagAb | Any | ||||
| IgA | IgM | IgG | ||||
| ≥21 | 38 | 9 (23.7) | 0 (0) | 11 (28.9) | 13 (34.2) | 15 (39.5) |
| 12-18 | 6 | 0 (0) | 1 (16.7) | 1 (16.7) | 0 (0) | 2 (33.3) |
| Total | 44 | 9 (20.5) | 1 (2.3) | 12 (27.3) | 13 (29.5) | 17 (38.6) |
According to antibody assay.
IgG antibodies to recombinant CagA antigen.
H. pylori seropositivity in the cohort of older children.
Of the 58 older children (mean age, 53.2 ± 25.8 months), 39 were H. pylori seropositive (mean of 1.9 ± 0.1 positive assays) at enrollment (Table 2); thus the prevalence of seropositivity was 67.2% at the time of entry into the older cohort. There was a clear age-related trend in seropositivity, with all children over 72 months being positive. Over the next year, of 59 evaluable children, 27 (79.4%) of 34 remained seropositive, whereas 10 (66.7%) of 15 remained seronegative (Table 3). Of the 12 children who changed status during the 1-year follow-up, 5 seroconverted, whereas 7 seroreverted, representing annual rates of 33.3 and 20.6%, respectively.
TABLE 2.
H. pylori seroprevalence in 58 older Native-American children by age at time of study entry
| Age group (mo) | Mean ± SD age (mo) | No. of children | No. (%) of seropositive children by antibody assaya
|
Mean no. of positive assays | |||
|---|---|---|---|---|---|---|---|
| WC
|
CagAb | Any | |||||
| IgA | IgG | ||||||
| 23-47 | 35.5 ± 6.1 | 33 | 7 (21.2) | 12 (36.4) | 11 (33.3) | 18 (54.5) | 1.7 ± 0.2 |
| 48-71 | 59.7 ± 6.5 | 14 | 7 (50.0) | 7 (50.0) | 7 (50) | 10 (71.4) | 2.1 ± 0.3 |
| 72-142 | 97.5 ± 19.6 | 11 | 9 (81.8) | 8 (72.7) | 7 (63.6) | 11 (100) | 2.2 ± 0.3 |
| All | 53.2 ± 25.8 | 58 | 23 (39.6) | 27 (46.5) | 25 (43.1) | 39 (67.2) | 1.9 ± 0.1 |
Number in parentheses represents percentage of each group.
IgG antibodies to recombinant CagA antigen.
TABLE 3.
Persistence of H. pylori antibody status in 49 older Native American children after 1-year follow-up according to age
| Age group (mo) | No. of children monitoreda | No. (%) of persistently seropositive childrenb
|
No. (%) of persistently seronegative childrend | |||
|---|---|---|---|---|---|---|
| Total | Assay positive
|
|||||
| WC
|
CagAc | |||||
| IgA | IgG | |||||
| 23-47 | 27 | 12 (44.4) | 6 | 8 | 8 | 8 (29.6) |
| 48-71 | 12 | 6 (50.0) | 4 | 6 | 5 | 2 (16.7) |
| 72-142 | 10 | 9 (90.0) | 5 | 8 | 6 | 0 (0.0) |
| All | 49e | 27 (55.1) | 15 | 22 | 19 | 10 (20.4) |
The mean number of specimens was 4.2 + 0.1.
Positive in any class on first and last sample. Number in parentheses represents percentage of total group of that age.
IgG antibodies to recombinant CagA antigen.
Defined as never having a positive sample.
For nine children (five initially positive and four initially negative), no follow-up specimens for up to 1 year were available.
H. pylori seroincidence.
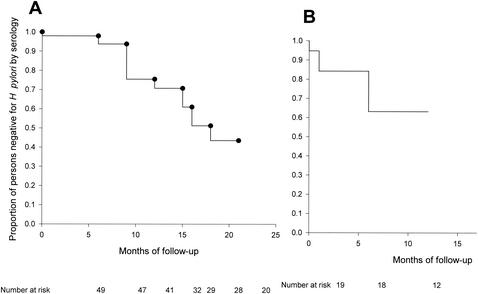
We also examined the acquisition of H. pylori as measured by seroconversion. In total, 137 seronegative children were evaluable, and 37 persistent seroconversions occurred in 23 children (1.6 ± 0.1 per child) (Table 4). In each of these children, seroconversion involved multiple specimens and persisted until the end of the observation period. Only one seroconversion occurred in a child by 6 months of age; this was not due to a failure of maternal antibodies to decline, since the seroconversions occurred in IgA and IgM, which do not cross the placenta. Until the age of 11 months, only two children seroconverted. The presence in early life of maternal antibodies did not have an impact on subsequent H. pylori acquisition (data not shown). For the first year of life, the calculated seroconversion rate was 4.1% (and would be 2.0% if the child who converted by 6 months is excluded). The peak incidence of seroconversion occurred in the second year of life and was nearly 45%. Over the subsequent years, the annual seroconversion rates declined but remained high. These data are consistent with the high H. pylori seroprevalence observed in both the cohort of older children (Table 2) and the women of childbearing age (data not shown). Among the birth cohort group, acquisition of H. pylori began at 6 months of age and continued through the follow-up period (Fig. 1A). In the older cohort group (Fig. 1B), although the follow-up period was shorter, the acquisition rate was similar to that of the younger group (Fig. 1B) at 12 months.
TABLE 4.
Calculated seroconversion rates for H. pylori persistence in Native American children by age
| Age of child (mo) | No. of children evaluablea | No. showing seroconversion by antibody assayb
|
Annualized seroconversion rate (%) | ||||
|---|---|---|---|---|---|---|---|
| WC
|
CagAc | Any | |||||
| IgG | IgA | IgM | |||||
| 1-11 | 49 | 0 | 2 | 1 | 1 | 2 | 4.1 |
| 12-17 | 41 | 5 | 3 | 1 | 6 | 10 | 48.8 |
| 18-25 | 30 | 3 | 2 | 0 | 6 | 6 | 40.0 |
| 23-47 | 12 | 1 | 3 | 0 | 2 | 4 | 33.3 |
| 48-142 | 5 | 0 | 1 | 0 | 0 | 1 | 20.0 |
Each child from whom sera were available during the indicated period was included. Children <6 months of age were not evaluable for IgG due to the presence of maternal antibodies, but could be evaluated according to IgA and IgM results.
Conversion from antibody negative to positive, with antibody positivity continuing to the end of the evaluation period.
IgG antibodies to recombinant CagA antigen.
FIG. 1.
Kaplan-Meier estimate of the proportion of Apache children remaining H. pylori negative during the follow-up period. (A) Birth cohort (24 months). (B) Older children (12 months).
Determination of transient seroconversion.
Previous reports have suggested that H. pylori colonization may be spontaneously lost during childhood (19, 65). The incidence of transient seroconversion in the birth cohort was 15.9%, and for the older children, it was 20.6%. In examining the loss of H. pylori antibody responses, age inversely correlated with seroreversion rates. All three children under 1 year of age who originally seroconverted to H. pylori lost their responses during follow-up. The rates of seroreversion gradually decreased with age (data not shown). Furthermore, children who showed seroconversion in only one serologic test were more likely to have transient seroconversion than those who became positive in two or more assays (P = 0.004) (data not shown).
Analysis of serum PGI levels.
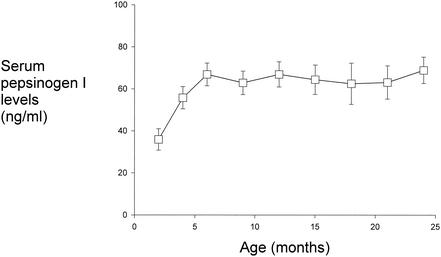
We next sought to determine the effect of H. pylori acquisition on serum PGI levels. This study was concentrated in the birth cohort only, since longitudinal sera were available before, during, and after each seroconversion event. First, to establish serum PGI levels in the absence of H. pylori, we studied 153 specimens from 17 children who had no serologic responses to H. pylori during the study period (Fig. 2). During the first 6 months of life, levels progressively rose from 35.9 ± 5.1 ng/ml in the first 2 months of life to 66.9 ± 5.4 at 6 months of age (P < 0.01). Although, PGI levels were stable after 6 months of age (mean, 64.8 ± 2.7 ng/ml), there was individual variation, and 10 sera (8.3%) showed levels of >100 ng/ml. Next, we examined 82 sera from 15 children found to be persistently H. pylori seropositive (Fig. 3). Each point represents a pool of the values for that time period for all of the children who seroconverted. With the first serologic evidence of H. pylori acquisition (defined as time 0), PGI levels peaked. Prior to time 0, levels were similar to those in H. pylori-negative children, and post-H. pylori acquisition, levels declined to the baseline as well. We also directly compared the PGI levels in the sera of the 15 children at the time of seroconversion to those in sera from 66 children over 6 months of age who were persistently H. pylori negative and those in 44 sera from children who were stably colonized with H. pylori. In this analysis, recent seroconversion again was associated with a peak in PGI levels (mean, 85.1 ± 15 ng/ml) over those present in both H. pylori-negative children (60.8 ± 2.2 ng/ml; P = 0.02) and children who were persistently H. pylori seropositive (63.3 ± 3.3 ng/ml; P = 0.03).
FIG. 2.
Serum PGI levels in the17 Native-American children who did not seroconvert to H. pylori. Mean (± standard error) PGI levels (nanograms per milliliter) in sequential sera to 24 months for the 17 persistently seronegative children from the cohort are shown.
FIG. 3.
Changes in serum PGI levels in children associated with seroconversion to H. pylori antigens. Time 0 represents the time at which acquisition of H. pylori was documented by seroconversion. Each other time point represents the interval before or after detection of H. pylori acquisition. The values shown are the mean (± standard error) for 15 birth cohort children before and after seroconversion. The numbers of specimens examined for several intervals exceed 15, since specimens from multiple times were available. The solid line represents the mean serum PGI level for the 17 children, calculated from the 121 samples obtained from the persistently seronegative children (>6 months old) (Fig. 2). The dashed lines represent the boundaries for the upper (75th) and lower (25th) quartiles.
DISCUSSION
Acquisition of H. pylori mainly occurs during childhood (19, 65, 67). However, until now, there have been few studies that prospectively assessed the acquisition rates in human populations (19, 31). Most of the longitudinal studies have been done in populations of developed countries where the annual incidence rate has been estimated to be low: in the neighborhood of 0.3% in both children and adults (1, 27). Since acquisition of H. pylori often is asymptomatic, ascertainment of symptoms does not accurately indicate when this occurs (40). To most easily assess incidence of childhood H. pylori acquisition, studying a population in which the adult H. pylori prevalence is high is most useful. Developing countries are the logical choice for this type of study; however, lack of adequate infrastructure to perform such studies often is limiting. However, within developed countries, there exist minority populations that also have high H. pylori prevalence (62), similar to that observed in developing countries (34, 44, 66).
This study of White Mountain Apache children represented an ideal opportunity to assess H. pylori incidence in a minority population in the United States, since we were able to show that H. pylori prevalence is high in the adult population. Although, we were not able to directly determine the H. pylori status of the mothers of the birth cohort children, our estimate, based on the IgG results from the 2-month-old infant serum, correlated well with that from comparable women in the community and confirmed the high endemicity of H. pylori in adult Native American populations (2).
The assays we employed have high predictive value for determining H. pylori status in both adults and children (6, 11, 13, 46, 64). Our studies of specimens frozen for more than 20 years (6, 38, 43) showed that multiple freeze-thaws had essentially no impact on serological results (5). Furthermore, our assays have documented significant declines in antibody levels in patients in whom H. pylori was eradicated by specific therapy (45). Since the purpose of this study was to estimate as accurately as possible the true seroconversion rate, we used four different assays to detect H. pylori acquisition. Commercial tests are not recommended for this type of study, since they are not capable of measuring the IgM- and IgA-specific responses to H. pylori antigens. In addition, there is no commercial test available to assess the CagA response. Advantages of serologic assays include their value for inexpensive screening of large numbers of individuals and their utility for determining H. pylori status from samples that had been stored for many years. Limitations of serological assays include the following. (i) No single antigen is recognized by all subjects. Therefore, preparations should contain multiple H. pylori strains (23). (ii) Defining threshold values that accurately separate positive from negative subjects may be difficult. (iii) Finally, assays may be sensitive to changes in reagents and laboratory conditions (8). Each of these limitations was addressed in this study. Multiple antigens were used, including the CagA antigen, which is complementary to the H. pylori group antigen in identifying H. pylori culture-positive individuals not responding to the group antigen (54). The thresholds established were more conservative than those used in adults, and all sera were studied together to minimize assay and reagent differences. A seroconversion to the CagA antigen without seroconversion in the WC assays indicates that the CagA antigen is more antigenic and immunodominant for that individual colonized with a cag+ H. pylori strain. We have documented that when antibodies to the WC antigen do not reach the level of positivity, antibodies to CagA represent true-positive CagA and false-negative WC results, based on the results of culture studies (54).
The lack of independent confirmation of H. pylori acquisition (either by endoscopy-based tests, urea breath test, or detection of H. pylori antigen in stools) (69) is another limitation. However, we used stringent criteria to define seropositivity (13): only specimens from children >9 months of age were included in the analysis of IgG seroconversion and seroreversion, and the progressive decline in maternal antibodies in each child until the age of 6 months and the consistency of seropositivity in children with H. pylori persistence further validate the serologic assays. Although studies of children have intrinsic limitations (24, 61), young children must be examined to gain insight into the events of H. pylori colonization, since they are the most relevant hosts. The results observed in the two cohorts we studied are consistent, but direct comparisons between the groups were not made. Although the two groups of children were enrolled 8 to 10 years apart, they were from the same reservation, and the mean birth years of the older and birth cohorts were 1987 and 1985, respectively. Thus, although acquisition rates are compared (Table 4), these should only be considered as illustrating broad trends. Our observation that seroconversion occurs at young ages in these Apache cohorts confirms that H. pylori acquisition happens early in life, particularly in groups of low socioeconomic status. Furthermore, the early acquisition of H. pylori has been associated with gastric cancer (60), and, as expected, this disease is highly prevalent in the community studied (72).
Previous studies of the early events surrounding H. pylori acquisition have been based on accidental and experimental challenges of adults (32, 63) and not children, despite their relevancy. The very transient IgM responses to acute H. pylori acquisition documented previously (35) and now again in this study explain the lack of utility of studying IgM responses in adults, since most have been colonized by H. pylori for decades. The pattern in this study showed that IgM seroconversion, although documented in only a small number of children, preceded the IgG and IgA seroconversion or occurred simultaneously. Among the seroconversions in which an IgM response was not observed, IgG always appeared first and IgA always appeared last, whether the seroconversion was transient or persistent. Similarities in the clinical and physiologic consequences of microbial acquisition in children and adults cannot be assumed, since the primary manifestations of varicella-zoster virus, mycoplasma, Epstein-Barr virus, and Salmonella enterica serovar Typhi infections, for example, differ between young children and adults.
Urea breath test studies among young children in developing countries suggest that urease-positive organisms often are transiently acquired during the first year of life, but that only about half of such children remain positive by 2 years of age (58, 65). However, the proportion of these events that are due to H. pylori has not been determined. We confirm transient H. pylori colonization may occur in children (29, 65) and that it is more common in younger than in older children (28, 30, 31). Our data indicate that the first childhood encounter with H. pylori is not necessarily responsible for the persistent colonization observed; transient colonization of experimentally challenged primates also is consistent with these findings (12). Our assessment of transient colonization must be considered to be a minimal estimate in both the birth and the older cohort populations, since by definition, the child first had to seroconvert. The frequency of very transient H. pylori acquisition in the absence of seroconversion is not known. Longitudinal studies of adults indicate that seroreversion in the absence of specific treatment to eradicate H. pylori is uncommon (26, 42, 48) even over long periods of follow-up. The data from this study stand in marked contrast and suggest that the niche of H. pylori immediately after acquisition is precarious and potentially quite vulnerable to the initial host immune responses. If correct, this concept suggests that effective pre-exposure vaccination is feasible. The data from this and other studies (52) indicate that once fully established, the colonizing H. pylori population is robust, resisting the ongoing immune response and consistent with phenotypic plasticity due to genetic variation within the colonizing population (4).
We determined the serum PGI levels to address whether H. pylori acquisition causes perturbations in the gastric physiology in children as it does in adults. In a prior study of 183 symptomatic children, the mean PGI value was approximately 65 μg/ml, and the values were nearly the same in H. pylori-positive and -negative children (49), similar to our findings (Fig. 2). In Korea (25), among 51 H. pylori-positive (mean age, 11.8 ± 2.1 years) children, the mean PGI value was 45 μg/ml. Our work provides three novel observations in relation to PGI physiology in childhood. First, in the absence of H. pylori, PGI levels clearly rise during the first 6 months of life and then remain at a plateau for at least 18 months (Fig. 2). Data from the two prior studies of older children (25, 49) suggest that this plateau is sustained. Second, there is a sharp but transient (<3 months) increase in PGI associated with H. pylori acquisition (Fig. 3). The return to baseline is consistent with the development of an immune response that, while not sufficient for eradication of the organism in many cases (thus, persistent colonization), protects the mucosa from acute injury.
Third, PGI levels in H. pylori-positive and -negative children are closely matched, except during the acquisition phase. There has been concern that the prolonged hypochlorhydria observed in adults who acquire H. pylori (33) also is present in children and increases the risk of diarrheal diseases (10, 59). Although we did not measure gastric acidity directly, our results suggest that the changes in gastric physiology associated with H. pylori acquisition in young children may be of short duration: for weeks rather than the many months that can occur in adults (21, 51). The concept of age-related differences in response to H. pylori acquisition parallels those associated with other organisms (e.g., varicella-zoster virus and Epstein-Barr virus), which are consistent with the hypothesis of a maturing immune response during childhood (53). This phenomenon may be of epidemiological relevance, since age at H. pylori acquisition appears to be a risk factor for development of gastric cancer decades later (6).
Acknowledgments
We thank the White Mountain Apache tribe and the Apache field workers for their cooperation and support of this study. We thank Orion Diagnostica and Peptide Therapeutics for diagnostic reagents used in this study.
This study was supported in part by RO1AI20738, AI18125, and DK53707 from the National Institutes of Health; the Medical Research Service of the Department of Veterans Affairs; Thrasher Research Funds 2792-6 and 2816-9; Indian Health Service grant 86-s/85-pc/84-Ic; and Merck Research Laboratories.
REFERENCES
- 1.Ashorn, M., M. Maki, M. Hallstrom et al. 1995. Helicobacter pylori infection in Finnish children and adolescents: a serologic cross-sectional and follow-up study. Scand. J. Gastroenterol. 30:876-879. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein, C. N., I. Mckeown, J. M. Embil, J. F. Blanchard, M. Dawood, A. Kabani, E. Kliewer, G. Smart, G. Coghl, S. MacDonald, C. Cook, and P. Orr. 1999. Seroprevalence of Helicobacter pylori, incidence of gastric cancer and peptic ulcer-associated hospitalization in a Canadian Indian population. Dig. Dis. Sci. 44:668-674. [DOI] [PubMed] [Google Scholar]
- 3.Blaser, M. J. 1998. Helicobacters are indigenous to the human stomach: duodenal ulceration is due to changes in gastric microecology in the modern era. Gut 43:721-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser, M. J., and D. E. Berg. 2001. Helicobacter pylori genetic diversity and risk of human disease. J. Clin. Investig. 107:767-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaser, M. J., G. I. Pérez-Pérez, J. Lindenbaum, D. Schneidman, G. Van Deventer, M. Marin-Sorensen, and W. M. Weinstein. 1991. Association of Helicobacter pylori infection with specific upper gastrointestinal pathology. Rev. Infect. Dis. 13:S704-S708. [DOI] [PubMed] [Google Scholar]
- 6.Blaser, M. J., G. I. Pérez-Pérez, H. Kleanthous, T. L. Cover, R. M. Peek, P. H. Chyou, G. N. Stemmermann, and A. Nomura. 1995. Infection with Helicobacter pylori strains possessing cagA associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 55:2111-2115. [PubMed] [Google Scholar]
- 7.Brenner, H., D. Rothenbacher, G. Bode, and G. Adler. 1998. The individual and joint contributions of Helicobacter pylori infection and family history to the risk for peptic ulcer disease. J. Infect. Dis. 177:1124-1127. [DOI] [PubMed] [Google Scholar]
- 8.Brown, L. M. 2000. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol. Rev. 22:283-297. [DOI] [PubMed] [Google Scholar]
- 9.Camorlinga-Ponce, M., J. Torres, G. I. Perez-Perez, Y. Leal-Herrera, B. Gonzalez-Ortiz, A. Madrazo de la Garza, A. Gomez, and O. Muñoz. 1998. Validation of a serologic test for the diagnosis of Helicobacter pylori infection and the immune response to urease and CagA in children. Am. J. Gastroenterol. 93:1264-1270. [DOI] [PubMed] [Google Scholar]
- 10.Clemens, J., M. J. Albert, M. Rao, F. Qadri, S. Huda, B. Kay, F. P. Van loon, D. Sack, P. A. Bradhan, and R. B. Sack. 1995. Impact of infection by Helicobacter pylori on the risk and severity of endemic cholera. J. Infect. Dis. 171:1653-1656. [DOI] [PubMed] [Google Scholar]
- 11.Drumm, B., G. I. Pérez-Pérez, M. J. Blaser, and P. Sherman. 1990. Intrafamilial clustering of Helicobacter pylori infection. N. Engl. J. Med. 322:359-363. [DOI] [PubMed] [Google Scholar]
- 12.Dubois, A., D. E. Berg, E. T. Incecik, N. Fiala, L. M. Heman-Ackah, G. I. Pérez-Pérez, and M. J. Blaser. 1996. Transient and persistent experimental infection of nonhuman primates with Helicobacter pylori: implications for human disease. Infect. Immun. 64:2885-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glassman, M. S., S. Dallal, S. H. Berenzin, H. E. Bostwick, L. Newman, G. I. Pérez-Pérez, and M. J. Blaser. 1990. Helicobacter pylori related gastroduodenal disease in children. Diagnostic utility of enzyme-linked immunosorbent assay (ELISA). Dig. Dis. Sci. 35:993-997. [DOI] [PubMed] [Google Scholar]
- 14.Gold, B. D., B. Khanna, L. M. Huang, C. Y. Lee, and N. Banatvala. 1997. Helicobacter pylori acquisition in infancy after decline of maternal passive immunity. Pediatr. Res. 41:641-646. [DOI] [PubMed] [Google Scholar]
- 15.Goodman, K. J., and P. Correa. 1995. The transmission of Helicobacter pylori: a critical review of the evidence. Int. J. Epidemiol. 24:875-877. [DOI] [PubMed] [Google Scholar]
- 16.Goodman, K. J., and P. Correa. 2000. Transmission of Helicobacter pylori among siblings. Lancet 355:358-362. [DOI] [PubMed] [Google Scholar]
- 17.Goodman, K. J., P. Correa, H. J. T. Aux, J. P. DeLany, and T. Collazos. 1997. Nutritional factors and Helicobacter pylori infection in Colombian children. J. Pediatr. Gastroenterol. Nutr. 25:507-515. [DOI] [PubMed] [Google Scholar]
- 18.Graham, D. Y., G. M. Lew, P. D. Klein, D. G. Evans, D. J. Evans, Jr., Z. A. Saeed, and H. M. Malaty. 1992. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer: a randomized, controlled study. Ann. Intern. Med. 116:705-708. [DOI] [PubMed] [Google Scholar]
- 19.Granstrom, M., Y. Tindberg, and M. Blennow. 1997. Seroepidemiology of Helicobacter pylori infection in a cohort of children monitored from 6 months to 11 years of age. J. Clin. Microbiol. 35:468-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groves, F. D., L. Zhang, J. Y. Li, W. C. You, Y. S. Chang, L. Zhao, W. D. Liu, C. S. Rabkin, G. I. Pérez-Pérez, M. J. Blaser, and M. H. Gail. 1997. Comparison of two enzyme-linked immunosorbent assay tests for diagnosis of Helicobacter pylori infection in China. Cancer Epidemiol. Biomark. Prev. 6:551-552. [PubMed] [Google Scholar]
- 21.Harford, W. W., C. Barnett, E. Lee, G. I. Perez-Perez, M. J. Blaser, and W. L. Peterson. 2000. Acute gastritis with hypochlorhydria: report of 35 cases with long term follow-up. Gut 47:467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helicobacter and Cancer Collaborative Group. 2001. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut 49:1347-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Höök-Nikanne, J., G. I. Perez-Perez, and M. J. Blaser. 1997. Antigenic characterization of Helicobacter pylori strains from different parts of the world. Clin. Diagn. Lab. Immunol. 4:592-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khanna, B., A. Cutler, and N. R. Israel. 1998. Use caution with serologic testing for Helicobacter pylori in children. J. Infect. Dis. 178:460-461. [DOI] [PubMed] [Google Scholar]
- 25.Kim, J. W., and K. S. Chung. 1998. Serum gastrin and pepsinogen I, II concentrations in children with Helicobacter pylori infection: the role of CagA and VacA. Yonsei Med. J. 39:159-165. [DOI] [PubMed] [Google Scholar]
- 26.Kosunen, T. U., A. Aromaa, P. Knekt, A. Salomaa, H. Rautelin, P. Lohi, and O. P. Heinonen. 1997. Helicobacter antibodies in 1973 and 1994 in the adult population of Vammala, Finland. Epidemiol. Infect. 119:29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuipers, E. J., A. S. Pena, G. van Kamp, A. M. Uyterlinde, G. Pals, N. F. Pels, E. Kutz-Pohlmann, and S. G. Meuwissen. 1993. Seroconversion for Helicobacter pylori. Lancet 342:328-331. [DOI] [PubMed] [Google Scholar]
- 28.Kumagai, T., H. M. Malaty, D. Y. Graham, S. Hosogaya, K. Misawa, K. Furihata, H. Ota, C. Sei, E. Tanaka, T. Akamatsu, T. Shimizu, K. Kiyosawa, and T. Katsuyama. 1998. Acquisition versus loss of Helicobacter pylori infection in Japan: results from an 8-year birth cohort study. J. Infect. Dis. 178:717-721. [DOI] [PubMed] [Google Scholar]
- 29.Lindkvist, P., F. Enquselassie, D. Asrat, L. Multe, I. Nilsson, and J. Giesecke. 1998. Risk factors for infection with Helicobacter pylori—a study of children in rural Ethiopia. Scand. J. Infect. Dis. 30:371-376. [DOI] [PubMed] [Google Scholar]
- 30.Malaty, H. M., G. Y. Graham, W. A. Wattigney, S. R. Srinivasan, M. Osato, and G. S. Berenson. 1999. Natural history of Helicobacter pylori infection in childhood: 12-year follow-up cohort study in a biracial community. Clin. Infect. Dis. 28:79-82. [DOI] [PubMed] [Google Scholar]
- 31.Malaty, H. M., A. El-Kasabany, D. Y. Graham, C. C. Miller, S. G. Reddy, S. R. Srinivasan, Y. Yamaoka, and G. S. Berenson. 2002. Age at acquisition of Helicobacter pylori infection: a follow-up study from infancy to adulthood. Lancet 359:931-935. [DOI] [PubMed] [Google Scholar]
- 32.Marshall, B. J., J. A. Armstrong, D. B. McGechie, and R. J. Glancy. 1985. Attempt to fulfill Koch's postulates for pyloric Campylobacter. Med. J. Aust. 142:436-439. [DOI] [PubMed] [Google Scholar]
- 33.McColl, K. E., E. El-Omar, and D. Guillen. 1998. Interaction between H. pylori infection, gastric acid secretion and anti-secretory therapy. Br. Med. Bull. 54:121-138. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell, H. M., Y. Y. Li, P. J. Hu, Q. Liu, M. Chen, G. G. Du, Z. J. Wang, A. Lee, and S. L. Hazell. 1992. Epidemiology of Helicobacter pylori in southern China: identification of early childhood as the critical period for acquisition. J. Infect. Dis. 166:149-153. [DOI] [PubMed] [Google Scholar]
- 35.Morris, A. J., A. M. Rafiq, G. I. Nicholson, G. I. Pérez-Pérez, and M. J. Blaser. 1991. Long-term follow-up of voluntary ingestion of Helicobacter pylori. Ann. Intern. Med. 114:662-663. [DOI] [PubMed] [Google Scholar]
- 36.Newcomer, W., B. Rivin, R. Reid, L. H. Moulton, M. C. Wolff, J. Croll, C. Johnson, L. Brown, D. Nalin, and M. Santosham. 1994. Immunogenicity, safety and tolerability of varying doses and regimens of inactivated hepatitis A virus vaccine in Navajo children. Pediatr. Infect. Dis. J. 13:640-642. [DOI] [PubMed] [Google Scholar]
- 37.Nomura, A. M., G. I. Perez-Perez, J. Lee, G. N. Stemmermann, and M. J. Blaser. 2002. Relation between Helicobacter pylori cagA status and the risk of peptic ulcer disease. Am. J. Epidemiol. 155:1054-1059. [DOI] [PubMed] [Google Scholar]
- 38.Nomura, A. M., G. N. Stemmermann, P.-H. Chyou, I. Kato, G. I. Pérez-Pérez, and M. J. Blaser. 1993. Helicobacter pylori infection and gastric carcinoma in a population of Japanese-Americans in Hawaii. N. Engl. J. Med. 325:1132-1136. [DOI] [PubMed] [Google Scholar]
- 39.Owen, G. M., P. H. Garry, R. D. Seymoure, G. G. Harrison, and P. B. Acosta. 1991. Nutrition studies with White Mountain Apaches preschool children in 1976 and 1969. Am. J. Clin. Nutr. 34:266-267. [DOI] [PubMed] [Google Scholar]
- 40.Parsonnet, J. 1995. The incidence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 9:45-51. [PubMed] [Google Scholar]
- 41.Parsonnet, J. 1998. Helicobacter pylori: the size of the problem. Gut. 43(Suppl. 1):S6-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parsonnet, J., M. J. Blaser, G. I. Pérez-Pérez, N. Hargrett-Bean, and R. V. Tauxe. 1992. Symptoms and risk factors of Helicobacter pylori infection in a cohort of epidemiologists. Gastroenterology 102:41-46. [DOI] [PubMed] [Google Scholar]
- 43.Pérez-Pérez, G. I., N. Bhat, J. Gaensbauer, A. Fraser, D. N. Taylor, E. J. Kuipers, L. Zhang, W. C. You, and M. J. Blaser. 1997. Country-specific constancy by age in cagA+ proportion of Helicobacter pylori infections. Int. J. Cancer 72:453-456. [DOI] [PubMed] [Google Scholar]
- 44.Pérez-Pérez, G. I., L. Bodhidatta, J. Wongsrichanalai, D. N. Taylor, W. B. Baze, B. E. Dunn, P. D. Echeverria, and M. J. Blaser. 1990. Seroprevalence of Helicobacter pylori infections in Thailand. J. Infect. Dis. 161:1237-1241. [DOI] [PubMed] [Google Scholar]
- 45.Pérez-Pérez, G. I., A. Cutler, and M. J. Blaser. 1997. Value of serology as a noninvasive method for evaluating the efficacy of treatment of Helicobacter pylori infection. Clin. Infect. Dis. 25:1038-1043. [DOI] [PubMed] [Google Scholar]
- 46.Pérez-Pérez, G. I., B. M. Dworkin, J. E. Chodos, and M. J. Blaser. 1988. Campylobacter pylori antibodies in humans. Ann. Intern. Med. 109:11-17. [DOI] [PubMed] [Google Scholar]
- 47.Pérez-Pérez, G. I., T. Marrie, H. Inouye, T. Shimoyama, G. Marshall, G. Meiklejohn, and M. J. Blaser. 1992. Effect of age and occupation on the seroprevalence of Helicobacter pylori infection. Can. J. Infect. Dis. 3:134-138. [Google Scholar]
- 48.Perez-Perez, G. I., A. Salomaa, T. U. Kosunen, B. Daverman, H. Rautelin, A. Aromaa, P. Knekt, and M. J. Blaser. 2001. Evidence that cagA+ Helicobacter pylori strains are disappearing more rapidly than cagA− strains. Gut 50:295-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plebani, M., G. Guanso, P. Foger, D. Basso, N. Gallo, C. F. Zambon, R. Mozrzymas, M. Celadin, and F. Zacchello. 1999. Efect of cagA status on the sensitivity of enzyme immunoassay in diagnosing Helicobacter pylori-infected children. Helicobacter 4:226-232. [DOI] [PubMed] [Google Scholar]
- 50.Pounder, R. E., and D. Ng. 1995. The prevalence of Helicobacter pylori infection in different countries. Aliment. Pharmacol. Ther. 9:S33-S39. [PubMed] [Google Scholar]
- 51.Ramsey, E. J., K. V. Carey, W. L. Peterson, J. J. Jackson, F. K. Murphy, N. W. Read, K. B. Taylor, J. S. Trier, and J. S. Fordtram. 1979. Epidemic gastritis with hypochlorhydria. Gastroenterology 76:1449-1457. [PubMed] [Google Scholar]
- 52.Rehnberg-Laiho, L., H. Rautelin, M. Valle, and T. U. Kosunen. 1998. Persisting Helicobacter antibodies in Finnish children and adolescents between two and twenty years of age. Pediatr. Infect. Dis. J. 17:796-799. [DOI] [PubMed] [Google Scholar]
- 53.Ridge, J. P., E. Fuchs, and P. Matzinger. 2002. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science 271:1723-1726. [DOI] [PubMed] [Google Scholar]
- 54.Romero-Gallo, J., G. I. Pérez-Pérez, R. P. Novick, P. Kamath, T. Norbu, and M. J. Blaser. 2002. Responses of endoscopy patients in Ladakh, India, to Helicobacter pylori whole cell and CagA antigens. Clin. Diagn. Lab. Immunol. 9:1313-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sack, R. B., M. Santosham, R. Reid, R. Black, J. Croll, R. Yolken, L. Aurelian, M. Wolff, E. Chan et al. 1995. Diarrhoeal diseases in the White Mountain: clinical studies. J. Diarrhoeal Dis. Res. 13:12-17. [PubMed] [Google Scholar]
- 56.Sande, N., M. Nikulin, I. Nilsson, T. Wadstrom, F. Laxen, M. Harkonen, O. Suovaniemi, and P. Sipponen. 2001. Increased risk of developing atrophic gastritis in patients infected with CagA+ Helicobacter pylori. Scand. J. Gastroenterol. 36:928-933. [DOI] [PubMed] [Google Scholar]
- 57.Santosham, M., R. Reid, D. M. Ambrasino, M. C. Wolff, J. Almeido-Hill, C. Priehs, K. M. Aspery, S. Garrett, L. Croll, S. Foster et al. 1987. Prevention of Haemophilus influenzae type B infections in high-risk infants treated with bacterial polysaccharide immune globulin. N. Engl. J. Med. 317:923-924. [DOI] [PubMed] [Google Scholar]
- 58.Sarker, S. A., D. Mahalanabis, P. Hildebrand, M. M. Rahaman, P. K. Bardhan, G. Fuchs, C. Beglinger, and K. Gyr. 1997. Helicobacter pylori: prevalence, transmission, and serum pepsinogen II concentrations in children of a poor periurban community in Bangladesh. Clin. Infect. Dis. 25:990-995. [DOI] [PubMed] [Google Scholar]
- 59.Shahinian, M. L., D. J. Passaro, D. L. Swerdlow, E. D. Mintz, M. Rodriguez, and J. Parsonnet. 2000. Helicobacter pylori and epidemic Vibrio cholerae O1 infection in Peru. Lancet 355:377-378. [DOI] [PubMed] [Google Scholar]
- 60.Sipponen, P., and K. Kimura. 1994. Intestinal metaplasia, atrophic gastritis, and stomach cancer: trends over time. Eur. J. Gastroenterol. Hepatol. 6(Suppl. 1):79-83. [PubMed] [Google Scholar]
- 61.Snyder, J. D., and S. Veldhuyzen van Zanten. 1999. Novel diagnostic tests to detect Helicobacter pylori infection: a pediatric prospective. Can. J. Gastroenterol. 13:585-589. [DOI] [PubMed] [Google Scholar]
- 62.Staat, M. A., D. M. Kruszov-Moran, G. M. McCuillan, and R. A. Kaslow. 1996. A population-based serologic survey of Helicobacter pylori infection in children and adolescents in the United States. J. Infect. Dis. 174:1120-1123. [DOI] [PubMed] [Google Scholar]
- 63.Takata, T., T. Shirotani, M. Okada, M. Kanda, S. Fujimoto, and J. Ono. 1998. Acute hemorrhagic gastropathy with multiple shallow ulcers and duodenitis caused by a laboratory infection of Helicobacter pylori. Gastrointest. Endosc. 47:291-294. [DOI] [PubMed] [Google Scholar]
- 64.Talley, N. J., D. G. Newell, J. E. Ormand, H. A. Carpenter, W. R. Wilson, A. R. Zinsmeister, G. I. Perez-Perez, and M. J. Blaser. 1991. Serodiagnosis of Helicobacter pylori: comparison of enzyme-linked immunosorbent assays. J. Clin. Microbiol. 29:1635-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas, J. E., A. Dale, M. Harding, W. A. Coward, T. J. Cole, and L. T. Weaver. 1995. Helicobacter pylori colonization in early life. Pediatr. Res. 45:218-223. [DOI] [PubMed] [Google Scholar]
- 66.Torres, J., Y. Leal-Herrera, G. I. Perez-Perez, A. Gomez, M. Camorlinga-Ponce, R. Cedillo-Ribera, R. Tapia-Conyer, and O. Munoz. 1998. A community based seroepidemiologic study of Helicobacter pylori infection in Mexico. J. Infect. Dis. 178:1089-1094. [DOI] [PubMed] [Google Scholar]
- 67.Torres, J., G. I. Perez-Perez, K. J. Goodman, J. C. Atherton, B. D. Gold, P. R. Harris, A. Madrazo de la Garza, J. Guarner, and O. Muñoz. 2000. A comprehensive review of the natural history of Helicobacter pylori infection in children. Arch. Med. Res. 31:431-469. [DOI] [PubMed] [Google Scholar]
- 68.Torres, J., G. I. Perez-Perez, Y. Leal-Herrera, and O. Muñoz. 1998. Infection with CagA+ Helicobacter pylori strains as a possible predictor of risk in the development of gastric adenocarcinoma in Mexico. Int. J. Cancer 78:298-300. [DOI] [PubMed] [Google Scholar]
- 69.Vaira, D., P. Malfertheiner, F. Megraud, A. T. R. Axon, M. Deltenre, A. Hirschl, G. Gasbarini, C. O'Morain, J. M. Garcia, M. Quina, and G. N. That. 1999. Diagnosis of Helicobacter pylori infection with a new noninvasive antigen based assay. Lancet 354:30-33. [DOI] [PubMed] [Google Scholar]
- 70.Valle, J., M. Kekki, P. Sipponen, T. Ihamaki, and M. Siurala. 1996. Long-term course and consequences of Helicobacter pylori gastritis—results of a 32-year follow-up study. Scand. J. Gastroenterol. 31:546-550. [DOI] [PubMed] [Google Scholar]
- 71.Walker, M. M. 2001. Gastric mucosa immune response in Helicobacter pylori infection in children. Men are not mice and more paediatric studies are needed. Dig. Liver Dis. 33:14-20. [DOI] [PubMed] [Google Scholar]
- 72.Wiggins, C. L., T. M. Becker, C. R. Key, and J. M. Samet. 1989. Stomach cancer among New Mexico's American Indians, Hispanic whites and non-Hispanic whites. Cancer Res. 49:1595-1599. [PubMed] [Google Scholar]