Abstract
Jena virus (JV) is a bovine enteric calicivirus that causes diarrhea in calves. The virus is approximately 30 nm in diameter and has a surface morphology similar to the human Norwalk virus. The genome sequence of JV was recently described, and the virus has been assigned to the genus Norovirus of the family Caliciviridae. In the present study, the JV capsid gene encoded by open reading frame 2 was cloned into the baculovirus transfer vector pFastBac 1, and this was used to transform Escherichia coli to generate a recombinant bacmid. Transfection of insect cells with the recombinant baculovirus DNA resulted in expression of the JV capsid protein. The recombinant JV capsid protein undergoes self-assembly into virus-like particles (VLPs) similar to JV virions in size and appearance. JV VLPs were released into the cell culture supernatant, concentrated, and then purified by CsCl equilibrium gradient centrifugation. Purified JV VLPs were used to hyperimmunize laboratory animals. An antigen capture enzyme-linked immunosorbent assay (ELISA) was developed and characterized initially with clinical specimens containing defined human noroviruses and bovine diarrheal samples from calves experimentally infected with JV; the ELISA was specific only for JV. The ELISA was used to screen 381 diarrheal samples collected from dairy herds in Thuringia, Hesse, and Bavaria, Germany, from 1999 to 2002; 34 of these samples (8.9%) were positive for JV infection. The unexpectedly high prevalence of JV was confirmed in a seroepidemiological study using 824 serum or plasma samples screened using an anti-JV ELISA, which showed that 99.1% of cattle from Thuringia have antibodies to JV.
Caliciviruses cause a wide range of animal and human diseases. On the basis of phylogenetic variation, genome organization differences, and pathological properties, the family Caliciviridae is divided into four distinct genera: the vesiviruses, lagoviruses, noroviruses, and sapoviruses (14). Viruses in the genera Norovirus and Sapovirus cause gastroenteritis. Noroviruses have been particularly difficult to study because it has not been possible to adapt any viruses within the genus to growth in cell culture. Most studies have been performed with human noroviruses and have relied on clinical specimens or materials obtained from infected volunteers. Noroviruses are commonly associated, especially during the winter months, with large outbreaks of gastroenteritis (5) involving hospitals, homes for the elderly, and hotels. Noroviruses also cause sporadic cases and small clusters of gastroenteritis in all age groups.
Members of the family Caliciviridae possess a positive-sense, single-stranded RNA genome of 7.3 to 8.4 kb excluding the 3′ poly(A) tail (6). All of the caliciviruses are composed of a major capsid protein, and in the case of the noroviruses, this is encoded by the second of three open reading frames (ORF2) (6). A significant advance in studying the noroviruses came with the discovery that expression of the prototype Norwalk virus capsid protein in insect cells using a recombinant baculovirus led to the export of the capsid protein to the cell culture supernatant, where it undergoes self-assembly to form virus-like particles (VLPs) (22). These VLPs are antigenically indistinguishable from native Norwalk virus particles (16). Subsequently, capsid proteins from a number of different human noroviruses were expressed in insect cells as VLPs (9, 15, 20, 21, 25). VLPs are now available as an abundant and renewable source of antigen which has been used to study the seroprevalence of norovirus infections in humans as well as to generate specific antisera for use in antigen detection enzyme-linked immunosorbent assays (ELISAs) (11, 35).
Enteric caliciviruses morphologically indistinguishable from the human noroviruses have been observed in cattle in England and Germany (1, 12, 18, 36). There are no small-animal species in which noroviruses have been described; thus, bovines are the only experimental animal for viruses in this genus. The first bovine noroviruses were described in England and are known as Newbury agents 1 and 2 (NA1 and NA2) (36). Biochemical and biophysical analysis of NA1 demonstrated a major capsid protein and a characteristic electron microscopic (EM) appearance (8). Sequence analysis of NA2 (7) and Jena virus (JV) (26) has confirmed their relationship to human noroviruses, and phylogenetic analysis suggests that the bovine noroviruses belong to a distinct genetic group (10). Like the human viruses, bovine noroviruses do not grow in cell culture (36). Reports suggest that viruses with similar morphology are “commonly found in the British calf population,” (3) but as yet there are no detailed specific epidemiological data to make an assessment of the nature and extent of disease caused by these agents, although preliminary studies suggest that “calicivirus-like agents” were detected in 25% of diarrhea outbreaks among calves in southern England (4, 28).
Newborn calves and calves up to 60 days old can be experimentally infected with bovine noroviruses (2), and the target cells for virus replication are the enterocytes of the small intestine (19). Biopsy studies performed on human volunteers in the early 1970s suggest that the human viruses have a similar cell tropism (29, 30). Furthermore, reverse transcription (RT)-PCR surveillance has identified norovirus sequences in both cattle (34) and swine (32, 33), and there remains the possibility of an animal reservoir from which animal noroviruses might infect humans. Recently, an enteropathogenic bovine calicivirus was described in the United States that is morphologically indistinguishable from noroviruses but represents a potentially new genus (31). In this virus, ORF1 was fused to and contiguous with the capsid ORF. In contrast, JV has a genome organization similar to that of the human noroviruses, with three separate ORFs. The objectives of our work were to develop a diagnostic ELISA to detect JV capsid antigen in fecal specimens and an anti-JV ELISA to evaluate the seroprevalence of JV and thus to establish the importance of JV in bovine enteric disease.
MATERIALS AND METHODS
Construction of recombinant-baculovirus-expressing JV capsid protein.
A recombinant baculovirus carrying ORF2 of JV was constructed using the BAC-TO-BAC baculovirus expression system (Life Technologies, Paisley, Scotland). A sequence-verified 2,170-kb HpaI/SpeI DNA restriction fragment containing the complete JV ORF2 and part of ORF3 was excised from the subgenomic clone pJVC1 (26). The purified insert was ligated into a StuI/SpeI-digested, dephosphorylated pFastBac I vector to generate the plasmid pFastJVcapsid. Transformants were screened by PCR, and a recombinant carrying the correct insert was chosen to transform MAX Efficiency DH10Bac competent cells containing a helper plasmid and baculovirus shuttle vector (bacmid). High-molecular-weight recombinant bacmid DNA was purified and transfected into insect cells as described by the manufacturer. Recombinant baculoviruses were harvested from the transfected cells and used to generate high-titer stocks for the infection of insect cells and for protein expression. Monolayers of Sf9 (Spodoptera frugiperda) insect cells were grown in TC100 medium to ∼80% confluence and infected with recombinant baculoviruses. High-titer stocks of recombinant baculoviruses were stored as previously described (23).
Expression of JV capsid protein and purification of VLPs.
Expression studies were performed using High-Five (HF) cells from the cabbage looper (Trichoplusia ni) grown in a low-protein, serum-free medium (EX-CELL 400; JRH Biosciences, Lenexa, Kans.). Following adsorption of the recombinant baculoviruses to the cells at a multiplicity of infection of 10, the cell monolayers were washed with phosphate-buffered saline (PBS) to remove the inoculum, and viral protein expression was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Time course experiments were performed over a 5-day period to determine the optimum time to harvest the capsid proteins. EM examination of the cell culture supernatant showed that viral capsid protein undergoes self-assembly to form VLPs. JV VLPs were purified on CsCl gradients following the removal of cellular debris by low-speed centrifugation of the cell culture supernatant. The crude VLPs were pelleted by centrifugation for 2 h at 100,000 × g using a Beckman SW28 rotor, resuspended in CsCl at 1.362 g/cm3, and centrifuged for 24 h at 120,000 × g in a Beckman SW50.1 rotor to reach equilibrium. The CsCl was removed from gradient fractions containing VLPs by dialysis with 10 mM Tris-HCl- 150 mM NaCl (pH 7.5) (27). The purified VLPs were stored in aliquots at −135°C.
Production of antisera.
Hyperimmune antisera to purified JV VLPs were produced in mice and rabbits. A primary immunization with purified VLP protein (100 μg) was administered intramuscularly in complete Freund's adjuvant to a New Zealand White rabbit, followed by five boosts (100 μg each) in incomplete Freund's adjuvant at 10-day intervals. A similar protocol was followed to generate murine antisera using BALB/c mice. Hyperimmune sera from both species were used to optimize conditions to produce an antigen capture ELISA.
SDS-PAGE.
SDS-PAGE was performed on protein samples using the discontinuous buffer system method of Laemmli with 10% acrylamide gels (acrylamide-bisacrylamide, 38.5:1 [wt/wt]) (24).
Immunodetection of proteins.
Proteins separated by SDS-PAGE were electroblotted to nitrocellulose sheets in transfer buffer (Tris-HCl, 25 mM; glycine, 192 mM; SDS, 0.1% [wt/vol]; methanol, 20% [vol/vol]) using a Trans-Blot SD semidry blotter (Bio-Rad Laboratories Ltd., Hemel Hempstead, United Kingdom). After transfer of the proteins, the nitrocellulose membrane was washed in TTBS blocking solution (TBS [NaCl, 0.5 M; Tris-HCl, 20 mM; pH 7.5] plus Tween 20, 0.05% [vol/vol]) using a rotisserie system (Stuart Scientific, Stone, Staffordshire, United Kingdom) at 22°C. The blocking solution was discarded, and the primary antibody diluted 1/200 in TTBS- 1% dried skim milk was added to the tube (10 ml) for 2 to 16 h. After being washed in TTBS, the membranes were treated with the secondary-antibody- alkaline phosphatase conjugate. The alkaline phosphatase-antibody conjugates were detected with a BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium salt redox chromogenic reaction catalyzed by alkaline phosphatase in a carbonate buffer (NaHCO3, 0.1 mM; MgCl2, 1.0 mM; pH 9.8).
Specificity of antibodies determined by RIPA.
In vitro synthesis of recombinant JV (rJV) capsid protein was performed with a T7 RNA polymerase-coupled reticulocyte lysate system (TNT; Promega, Southampton, United Kingdom) in accordance with the manufacturer's instructions, using plasmid pJVC1 (which encodes JV ORF2 capsid under the control of the T7 promoter) as a template (26). [35S]methionine-labeled translation products from the TNT system were incubated with 2 μl of undiluted antiserum in 600 μl of radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA, 0.15 mM NaCl, 0.1% SDS, 0.5% Empigen BB [N-dodecyl-N,N-dimethylglycine], 0.1 mM phenylmethylsulfonyl fluoride). After incubation at 37°C for 1 h, goat anti-mouse or goat anti-rabbit immunoglobulin G attached to beaded agarose (Sigma, Poole, Dorset, United Kingdom) was added to adsorb immune complexes. For immunoprecipitation of bovine sera, protein G-beaded agarose (Sigma) was used. The beads were washed three times in RIPA buffer and once in PBS before derivatization in sample dissociating buffer and separation by SDS-PAGE. The gels were stained and prepared for autoradiography by treatment with 1 M sodium salicylate- 50% methanol for 30 min at room temperature; they were then dried under vacuum and exposed to Kodak XAR-5 film at −70°C.
JV ELISA.
Polyvinyl plates were coated by incubating 100 μl of rabbit pre- and postimmune antisera (1:500) in coupling buffer (15 mM Na2CO3, 35 mM NaHCO3 [pH 9.6], 0.02% NaN3) overnight at 4°C. The wells were washed three times in PBS- 0.05% Tween 20 (PBST) and then blocked by incubation with 100 μl of PBS- 5% skim milk for 30 min at 37°C. The wells were emptied, and 50 μl of a 20% fecal suspension in PBS was loaded into each well. Pairs of wells (pre- and postimmune) were incubated at 37°C for 2 h and then washed five times with PBST. Hyperimmune mouse antisera to JV VLPs (100 μl at 1:10,000) were loaded into each well. The plates were incubated at 37°C for 1.5 h and then washed four times with PBST. Goat anti-mouse polyvalent antibody conjugated to horseradish peroxidase (100 μl at 1:3,000 in PBS- 1% skim milk; Sigma) was applied to each well, and the plate was incubated for 1.5 h at 37°C. The plate was then washed four times in PBST before development with 100 μl of substrate (0.1% sodium acetate [pH 6.0], 1% tetramethylbenzidine, 0.01% H2O2) at room temperature for 15 min in the dark. The reaction was stopped by the addition of 50 μl of 2 M H2SO4. Specimens with postimmune optical density at 450 nm (OD450) values of >0.25 that were also at least twice the preimmune OD450 were scored as positive.
Fecal samples.
A panel of 12 characterized human fecal specimens from the routine diagnostic laboratory that had been tested by EM, ELISA, and RT-PCR and shown to contain both genogroup 1 and genogroup 2 noroviruses was used to validate the assay. A fecal specimen collected 15 h postinfection from a newborn calf experimentally infected with JV was used as a positive control sample to validate the ELISA; furthermore, this sample was also confirmed positive by RT-PCR as previously described using the primer pair GLPSG1 and YGDD1 (26). The fecal specimen contained JV particles, as assessed by direct-transmission EM. Fecal specimens were also collected from newborn calves prior to infection and used as negative control samples. Three hundred eighty-one diarrheal specimens were collected from calves aged 1 to 4 weeks from 147 dairy herds in Thuringia, Hesse, and Bavaria, Germany.
EM.
Bovine fecal samples were clarified by low-speed centrifugation at 3,000 × g for 30 min to remove large particulate material. The supernatants were adsorbed onto Zaponlack carbon-coated grids, stained with 2% phosphotungstic acid (pH 7.2), and examined using a BS 500 electron microscope (TESLA, Brno, Czech Republic). Insect cell culture supernatants were also examined by negative stain transmission EM. Samples were adsorbed directly onto carbon-coated grids, stained with 0.75% phosphotungstic acid (pH 6.0), and visualized using a Hitachi 7000 transmission electron microscope.
Anti-JV ELISA.
A solid-phase anti-JV ELISA was developed to detect bovine serum antibodies to JV. Postinfection sera collected from a colostrum-deprived newborn calf that had been experimentally infected with JV and shown to immunopreciptate rJV capsid antigen produced by in vitro transcription-translation (26) was used as a positive control and for testing and optimization of JV-specific antibody binding to JV VLPs. Polyvinyl microtiter plates were coated by incubation overnight at 4°C with 2 μg of purified JV VLPs in 15 mM Na2CO3- 35 mM NaHCO3 (pH 9.6) per well. The plates were blocked with 5% skim milk in PBS for 30 min at 37°C. Bovine sera were screened at 1:10 dilution in PBS containing 1% skim milk and incubated at 37°C for 90 min. After the plates were washed with PBS-Tween 20, 100 μl of a 1:3,000 dilution of goat anti-bovine immunoglobulin G (heavy chain plus light chain) horseradish peroxidase conjugate (Sigma) diluted in 1% skim milk in PBS was added, and the plates were incubated for 90 min at 37°C. Tetramethyl benzidine substrate (0.0036% tetramethyl benzidine, 0.1 M Na acetate, 0.0001% H2O2) was added after the plates were washed, and the reaction was stopped with 2 M H2SO4 after 5 min. The absorbance at 450 nm was recorded using an automated spectrophotometer (Anthos Labtec, Salzburg, Austria).
Serum and plasma samples.
A collection of 824 serum or plasma samples from cattle aged 10 weeks to 9 years was collected from 25 herds of cattle in Thuringia, Germany, during January and February 2002. In addition, 10 colostrum samples collected from a Thuringian dairy herd were analyzed.
RESULTS
Molecular cloning and expression of the JV capsid protein.
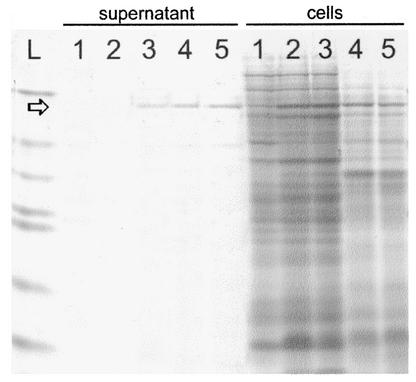
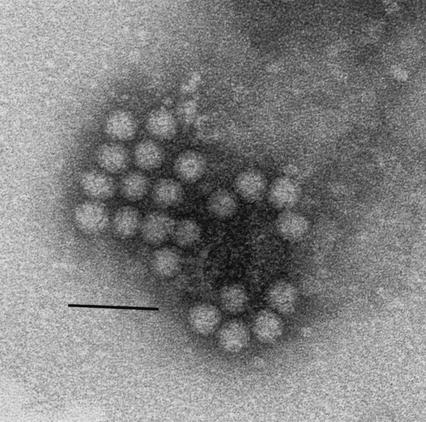
The complete ORF2 of JV was cloned into the pFASTBac1 vector under control of the baculovirus polyhedrin promoter and used in transfection experiments to produce recombinant baculoviruses. Individual recombinant baculoviruses were propagated in Sf9 cells and screened for the expression of recombinant JV capsid protein by SDS-PAGE. A single recombinant (expressing a protein of 55 kDa) was selected and used to produce a high-titer viral stock. Sf9 cells were infected with this recombinant baculovirus at a multiplicity of infection of 10 PFU/cell. The kinetics of JV capsid expression were followed in an infection time course experiment (Fig. 1). JV capsid protein could be detected in cells 24 h postinfection, with maximal synthesis occurring in 3 to 4 days. JV capsid protein was exported to the cell culture medium from 3 days postinfection and reached a maximum at 4 days. Direct negative stain transmission EM examination of the cell culture supernatant (Fig. 2) revealed that the JV capsid protein undergoes self-assembly to form VLPs. Increased yield of the protein was obtained following infection of T. ni (HF) cells, and infection of these cells was used for the bulk expression of JV capsid protein, which was purified from the cell culture supernatant.
FIG. 1.
SDS-polyacrylamide gel showing kinetics of JV capsid expression. Lane L contains molecular mass markers: bovine serum albumin (66 kDa), ovalbumin (45 kDa), glyceraldehyde-3-phosphate dehydrogenase (36 kDa), carbonic anhydrase (29 kDa), trypsinogen (24 kDa), trypsin inhibitor (20 kDa), and α-lactalbumin (14 kDa). Lanes 1 to 5 represent days 1 to 5 postinfection for cell culture supernatant and cells. The arrow indicates the JV capsid protein that is exported to the cell culture supernatant.
FIG. 2.
Electron micrograph of negatively stained JV VLPs. Bar, 50 nm.
Development of a diagnostic ELISA for JV.
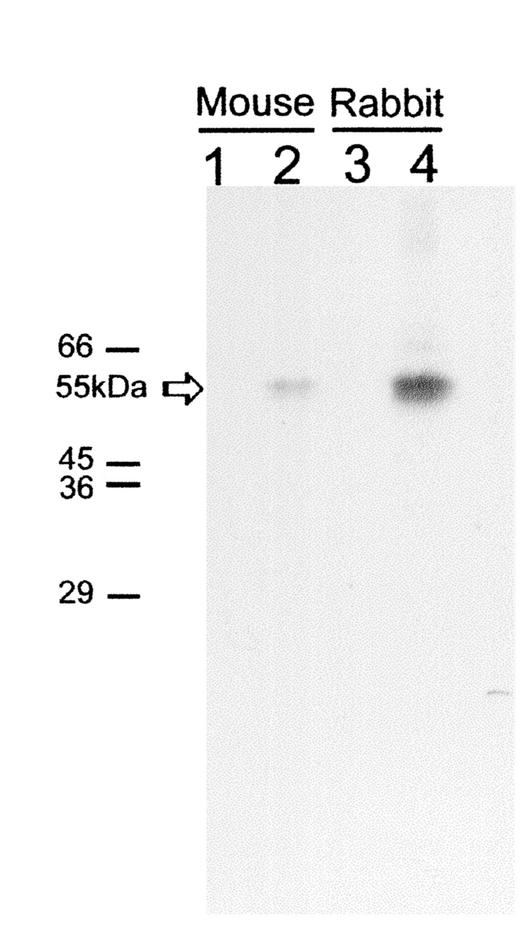
The rabbit and mice used for immunization were shown to have no preexisting antibodies to JV capsid protein as assessed by immunoblotting using purified JV VLPs (data not shown) and by RIPA using rJV capsid protein expressed in an in vitro transcription-translation system (Fig. 3). The reactivities of the hyperimmune antisera were also assessed by immunoblotting (data not shown) and RIPA with rJV capsid protein (Fig. 3). Antigen trap ELISAs using the rabbit and mouse sera were compared in two formats for the detection of JV in bovine fecal specimens. Rabbit hyperimmune serum was used as a coating antibody, and mouse serum was used as a detector; alternatively, mouse hyperimmune serum was used as a coating antibody, and rabbit hyperimmune serum was used as a detector. The ELISA showed similar sensitivities in either format. The optimal performance and dilutions of the antisera were determined using a fecal specimen from an experimentally infected newborn calf that contained JV particles, by EM, and JV genomic RNA as assessed by RT-PCR. In the final ELISA format, rabbit hyperimmune serum was used at a dilution of 1:500 as a capture antibody, and mouse serum was used at a dilution of 1:10,000 as a detector antibody. Each fecal specimen was tested with control preimmune rabbit serum and detector hyperimmune rabbit serum. To assess the specificity of the ELISA, a panel of 12 human stool specimens from patients with acute gastroenteritis caused by characterized (by RT-PCR) genogroup 1 and genogroup 2 noroviruses were screened using the JV antisera. None of the human samples was reactive in the JV ELISA (data not shown). A total of 381 bovine fecal specimens were tested in the ELISA, and of these, 34 (8.9%) were positive.
FIG. 3.
Radioimmunoprecipitation of JV capsid protein produced by in vitro transcription-translation. The positions of the molecular mass markers are indicated on the left. Lane 1, preimmunization mouse serum; lane 2, postimmunization mouse serum; lane 3, preimmunization rabbit serum; lane 4, postimmunization rabbit serum. The position of the immunoprecipitated JV capsid protein at 55 kDa is indicated by the arrow.
Antibody prevalence survey.
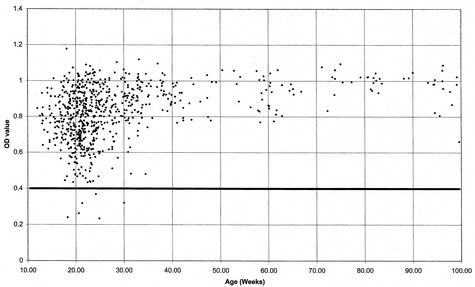
A collection of 824 bovine plasma and serum samples grouped by age were screened for antibodies to JV VLPs. Most of the samples gave high OD450 values (0.75 to 1.2) as shown in the scattergram analysis (Fig. 4). A well without sera was included on each microtiter plate. In the absence of negative control bovine sera, the cutoff value for positive samples was determined empirically. Three separate sets of bovine sera with OD450 values in the ranges 0.2 to 0.4, 0.4 to 0.75, and >0.75 were tested for their specificities for rJV capsid antigen by RIPA analysis. All samples in the 0.4 to 0.75 range immunoprecipitated the radioactively labeled rJV capsid protein produced by in vitro transcription-translation, and by contrast, none in the 0.2 to 0.4 range was able to immunoprecipitate the rJV capsid protein (data not shown). Therefore, the cutoff value for recording positive sera was taken to be an OD450 value of 0.4.
FIG. 4.
Epidemiological screen for antibodies to JV. A total of 824 serum or plasma samples collected from 25 different dairy herds in Thuringia, Germany, between January and February 2002 were screened for antibodies against rJV VLPs. The OD450 was plotted against increasing age. Each diamond represents a serum or plasma OD450 value for a sample plotted against the age of the individual animal in weeks. Positive reactivity to JV capsid was confirmed by RIPA analysis of a selection of samples. All sera with OD450 values of <0.4 did not immunoprecipitate with JV capsid protein; thus, samples below this value were designated negative.
In addition to the bovine sera from dairy herds used in the survey, preinfection serum from colostrum-deprived newborn calves and 11 separate commercially available fetal calf serum samples were tested and found to be negative. Serum samples taken at 7 and 28 days post-experimental JV infection from colostrum-deprived calves showed high levels of antibodies to JV VLPs (OD450 values of 0.731 and 0.969, respectively). Comparative analysis of a selection of different positive sera by end point dilution gave values (1:20,480) similar to those for the sera from experimentally infected calves. Colostrum was efficient at radioimmunoprecipitation of rJV capsid (data not shown), and samples tested by ELISA were shown to have extremely high titers (mean OD450 value, 1.147).
DISCUSSION
Studies of the noroviruses have been severely restricted because there is no cell culture system and no small-animal model for infection. The poor yields of bovine noroviruses from experimentally infected calves (or of human noroviruses from infected adult volunteers) has prevented purification of native viral antigen for immunization purposes. Thus, it has not been possible to produce hyperimmune antisera for ELISAs to detect the presence of viral antigen in fecal specimens by using this approach.
As an alternative, the capsid protein of JV was expressed in insect cells using a recombinant baculovirus. EM examination of the cell culture supernatant revealed that the capsid protein assembles into VLPs that resemble those of JV (Fig. 2). JV VLPs were produced in bulk from HF cells because that cell line grows without the need for fetal calf serum and gives significantly higher expression of the same proteins expressed in Sf9 cells.
JV VLPs were used to hyperimmunize laboratory animals, and an antigen capture ELISA was developed and optimized using these antisera, bovine stool samples, and JV VLPs. To determine the criteria for positivity, the ELISA was used to screen a panel of 12 characterized (EM positive and RT-PCR positive) human noroviruses, including Southampton virus (genogroup 1) and Lordsdale virus (genogroup 2). All human noroviruses were negative, showing no differences between pre- and postimmunization wells in the ELISA, which showed that the ELISA was specific for JV. This result is in close agreement with observations made for similarly designed ELISAs for the human noroviruses, where assays were found to be highly virus type specific (11, 35).
Calves are the only animals from which noroviruses have been isolated and shown to cause infection in an experimental setting (17, 36). Field observations have indicated that natural infections caused by bovine noroviruses occur in the first few days of life, and in contrast to human norovirus infections, which infect adults, bovine noroviruses have not been observed in mature cattle. Experimental infections of cattle in the early 1980s using the Newbury agents and gnotobiotic calves demonstrated that these bovine noroviruses infected enterocytes of the small intestine (3, 19). The progress of infection was followed by searching for the presence of the virus in feces or intestinal contents by EM. Alternatively viral antigens were detected in infected enterocytes by immunofluorescence using convalescent-phase sera. In gnotobiotic calves infected with NA1, viral antigens were detected in the cytoplasm of infected enterocytes at 0.5 days and remained present until 2 days postinfection (19). Virus particles within intestinal contents became detectable by EM at 0.75 days and were seen in only one calf at 3 days postinfection. The low numbers of virus particles shed during infection and the short shedding period is likely to make detection of these viruses difficult. Therefore, it was very surprising to have detected 34 of 381 (8.9%) positive samples in our field survey.
We sought to confirm the unexpectedly high positive isolation rate of JV by studying the seroprevalence of JV infection. A total of 824 age-stratified sera and plasma samples from local cattle in Thuringia, Germany, were screened for antibodies to JV. The assay was validated by the incorporation of pre- and postinfection sera from calves experimentally infected with JV and also with samples of fetal calf serum. Of the tested samples from dairy herds, 99.1% contained antibodies to JV, indicating that infection by this virus is endemic. This observation was supported by measuring the affinity of antibodies in a subset of serum samples (data not shown), which all showed highly avid antibodies indicative of longer-term exposure to JV. This approach has previously been applied to measure the avidity of sera for human noroviruses (13). Like fetal calf serum, sera from colostrum-deprived newborn calves contains no antibodies to JV. Acquisition of antibodies to JV can occur through colostrum or from infection. The six seronegative calves were all aged 10 to 30 weeks. The high seroprevalence in the other animals (99.1%) suggests a constant exposure to the virus, since there is no observable decay of serum antibody following weaning. Selected serum samples from older animals were tested by end point titration, and similar high values for both experimentally infected calves and samples from the survey suggest that the positive results are measuring the effects of JV infection specifically rather than cross-reacting antibodies to different bovine noroviruses. These results show that JV infections are common in calves and lead to the generation of long-lived specific immunoglobulin G antibodies. This situation is similar to the observations made in seroepidemiological studies with human noroviruses.
The availability of the JV ELISA as a rapid, sensitive, and reliable assay will allow us in the future to evaluate the etiological importance of JV infection for diarrheal disease in bovine populations around the world. Furthermore, it will be possible to study the biology and pathogenesis of JV infection in an experimental setting in more detail by using the ELISA to evaluate viral inoculae and viral antigen shedding. It has been suggested that human norovirus infections might originate from an animal reservoir. The availability of JV VLPs and the anti-JV ELISA will allow us to investigate whether there is evidence for JV infections in the human population and in other animal species.
Acknowledgments
We are grateful to Tony Ward for assistance with data handling and analysis.
REFERENCES
- 1.Almeida, J. D., C. R. Craig, and T. E. Hall. 1978. Multiple viruses present in the faeces of a scouring calf. Vet. Rec. 102:170-171. [DOI] [PubMed] [Google Scholar]
- 2.Bridger, J. C. 1990. Small viruses associated with gastroenteritis in animals, p. 161-182. In L. J. Saif and K. W. Theil (ed.), Viral diarrheas of man and animals. CRC Press, Boca Raton, Fla.
- 3.Bridger, J. C., G. A. Hall, and J. F. Brown. 1984. Characterization of a calici-like virus (Newbury agent) found in association with astrovirus in bovine diarrhea. Infect. Immun. 43:133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridger, J. C., G. A. Hall, D. J. Reynolds, and J. F. Brown. 1983. Calici-like viruses in calf diarrhoea, p. 155-159. In Proceedings of the 4th International Symposium on Neonatal Diarrhoea. Vaccine & Infectious Disease Organization, Saskatoon, Saskatchewan, Canada.
- 5.Chadwick, P. R., G. Beards, D. Brown, E. O. Caul, J. Cheesbrough, I. Clarke, A. Curry, S. O'Brien, K. Quigley, J. Sellwood, and D. Westmoreland. 2000. Management of hospital outbreaks of gastro-enteritis due to small round structured viruses. J. Hosp. Infect. 45:1-10. [DOI] [PubMed] [Google Scholar]
- 6.Clarke, I. N., and P. R. Lambden. 1997. The molecular biology of caliciviruses. J. Gen. Virol. 78:291-301. [DOI] [PubMed] [Google Scholar]
- 7.Dastjerdi, A. M., J. Green, C. I. Gallimore, D. W. G. Brown, and J. C. Bridger. 1999. The bovine Newbury agent-2 is genetically more closely related to human SRSVs than to animal caliciviruses. Virology 254:1-5. [DOI] [PubMed] [Google Scholar]
- 8.Dastjerdi, A. M., D. R. Snodgrass, and J. C. Bridger. 2000. Characterisation of the bovine enteric calici-like virus, Newbury agent 1. FEMS Microbiol. Lett. 192:125-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dingle, K. E., P. R. Lambden, E. O. Caul, and I. N. Clarke. 1995. Human enteric Caliciviridae: the complete genome sequence and expression of virus-like particles from a genetic group II small round structured virus. J. Gen. Virol. 76:2349-2355. [DOI] [PubMed] [Google Scholar]
- 10.Fankhauser, R. L., S. S. Monroe, J. S. Noel, C. D. Humphrey, J. S. Bresee, U. D. Parashar, T. Ando, and R. I. Glass. 2002. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J. Infect. Dis. 186:1-7. [DOI] [PubMed] [Google Scholar]
- 11.Graham, D. Y., X. Jiang, T. Tanaka, A. R. Opekun, H. P. Madore, and M. K. Estes. 1994. Norwalk virus infection of volunteers: new insights based on improved assays. J. Infect. Dis. 170:34-43. [DOI] [PubMed] [Google Scholar]
- 12.Granzow, H., and H. Schirrmeier. 1985. Identification of 32nm viruses in faeces of diarrhoeic calves by electron microscopy. Monatsh. Veterinärmed. 40:228-229. [Google Scholar]
- 13.Gray, J. J., C. Cunliffe, J. M. Ball, D. Y. Graham, U. Desselberger, and M. K. Estes. 1994. Detection of immunoglobulin M (IgM), IgA, and IgG Norwalk virus-specific antibodies by indirect enzyme-linked immunosorbent assay with baculovirus-expressed Norwalk virus capsid antigen in adult volunteers challenged with Norwalk virus. J. Clin. Microbiol. 32:3059-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green, K. Y., T. Ando, M. S. Balayan, T. Berke, I. N. Clarke, M. K. Estes, D. O. Matson, S. Nakata, J. D. Niell, M. J. Studdert, and H.-J. Thiel. 2000. Taxonomy of the caliciviruses. J. Infect. Dis. 181:S322-S330. [DOI] [PubMed] [Google Scholar]
- 15.Green, K. Y., A. Z. Kapikian, J. Valdesuso, S. Sosnovtsev, J. J. Treanor, and J. F. Lew. 1997. Expression and self-assembly of recombinant capsid protein from the antigenically distinct Hawaii human calicivirus. J. Clin. Microbiol. 35:1909-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green, K. Y., J. F. Lew, X. Jiang, A. Z. Kapikian, and M. K. Estes. 1993. Comparison of the reactivities of baculovirus-expressed recombinant Norwalk virus capsid antigen with those of the native Norwalk virus antigen in serologic assays and some epidemiologic observations. J. Clin. Microbiol. 31:2185-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Günther, H., and P. Otto. 1987. Diarrhea in young calves. 7. “Zackenvirus” (Jena-agent 117/80)—a new diarrhea pathogen in calves. Arch. Exp. Veterinarmed. 41:934-938. (In German.) [PubMed]
- 18.Günther, H., P. Otto, and P. Heilman. 1984. Diarrhea in young calves. 6. Determination of the pathogenicity of a bovine coronavirus and an unidentified icosahedral virus. Arch. Exp. Veterinarmed. 38:781-792. (In German.) [PubMed]
- 19.Hall, G. A., J. C. Bridger, B. E. Brooker, K. R. Parsons, and E. Ormerod. 1984. Lesions of gnotobiotic calves experimentally infected with a calicivirus-like (Newbury) agent. Vet. Pathol. 21:208-215. [DOI] [PubMed] [Google Scholar]
- 20.Jiang, X., D. Cubitt, J. Hu, X. M. Dai, J. J. Treanor, D. O. Matson, and L. K. Pickering. 1995. Development of an ELISA to detect MX virus, a human calicivirus in the Snow Mountain agent genogroup. J. Gen. Virol. 76:2739-2747. [DOI] [PubMed] [Google Scholar]
- 21.Jiang, X., D. O. Matson, G. M. Ruiz-Palacios, J. Hu, J. J. Treanor, and L. K. Pickering. 1995. Expression, self-assembly, and antigenicity of a Snow Mountain Agent-like calicivirus capsid protein. J. Clin. Microbiol. 33:1452-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang, X., M. Wang, D. Y. Graham, and M. K. Estes. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 66:6527-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King, L. A., and R. D. Possee. 1992. The baculovirus expression system. Chapman and Hall, London, United Kingdom.
- 24.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 25.Leite, J. P. G., T. Ando, J. S. Noel, B. Jiang, C. D. Humphrey, J. F. Lew, K. Y. Green, R. I. M. Glass, and S. S. Monroe. 1996. Characterization of Toronto virus capsid protein expressed in baculovirus. Arch. Virol. 141:865-875. [DOI] [PubMed] [Google Scholar]
- 26.Liu, B. L., P. R. Lambden, H. Günther, P. Otto, M. Elschner, and I. N. Clarke. 1999. Molecular characterization of a bovine enteric calicivirus: relationship to the Norwalk-like viruses. J. Virol. 73:819-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelosi, E., P. R. Lambden, E. O. Caul, B. L. Liu, K. E. Dingle, Y. Deng, and I. N. Clarke. 1999. The seroepidemiology of genogroup 1 and genogroup 2 Norwalk-like viruses in Italy. J. Med. Virol. 58:93-99. [PubMed] [Google Scholar]
- 28.Reynolds, D. J., J. H. Morgan, N. Chanter, P. W. Jones, J. C. Bridger, T. G. Debney, and K. J. Bunch. 1986. Microbiology of calf diarrhoea in southern Britain. Vet. Rec. 119:34-39. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber, D. S., N. R. Blacklow, and J. S. Trier. 1973. The mucosal lesion of the proximal small intestine in acute infectious nonbacterial gastroenteritis. N. Engl. J. Med. 288:1318-1323. [DOI] [PubMed] [Google Scholar]
- 30.Schreiber, D. S., N. R. Blacklow, and J. S. Trier. 1974. The small intestinal lesion induced by Hawaii agent acute infectious nonbacterial gastroenteritis. J. Infect. Dis. 129:705-708. [DOI] [PubMed] [Google Scholar]
- 31.Smiley, J. R., K. O. Chang, J. Hayes, J. Vinje, and L. J. Saif. 2002. Characterization of an enteropathogenic bovine calicivirus representing a potentially new calicivirus genus. J. Virol. 76:10089-10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugieda, M., H. Nagaoka, Y. Kakishima, T. Ohshita, S. Nakamura, and S. Nakajima. 1998. Detection of Norwalk-like virus genes in the caecum contents of pigs. Arch. Virol. 143:1-7. [DOI] [PubMed] [Google Scholar]
- 33.Sugieda, M., and S. Nakajima. 2002. Viruses detected in the caecum contents of healthy pigs representing a new genetic cluster in genogroup II of the genus ′Norwalk-like viruses'. Virus Res. 87:165-172. [DOI] [PubMed] [Google Scholar]
- 34.van der Poel, W., J. Vinjé, R. van der Heide, M. I. Herrera, A. Vivo, and M. G. Koopmans. 2000. Norwalk-like calicivirus genes in farm animals. Emerg. Infect. Dis. 6:36-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vipond, I. B., E. Pelosi, J. Williams, C. R. Ashley, P. R. Lambden, I. N. Clarke, and E. O. Caul. 2000. A diagnostic EIA for detection of the prevalent SRSV strain in United Kingdom outbreaks of gastroenteritis. J. Med. Virol. 61:132-137. [DOI] [PubMed] [Google Scholar]
- 36.Woode, G. N., and J. C. Bridger. 1978. Isolation of small viruses resembling astroviruses and caliciviruses from acute enteritis of calves. J. Med. Microbiol. 11:441-452. [DOI] [PubMed] [Google Scholar]