Abstract
We designed and synthesized several novel cyclic MSH analogues and tested their affinities for cells expressing the MC1, MC3, MC4 and MC5 receptors.
One of the substances HS028 (cyclic [AcCys11, dichloro-D-phenylalanine14, Cys18, Asp-NH222]-β-MSH11–22) showed high affinity (Ki of 0.95nM) and high (80 fold) MC4 receptor selectivity over the MC3 receptor. HS028 thus shows both higher affinity and higher selectivity for the MC4 receptor compared to the earlier first described MC4 receptor selective substance HS014.
HS028 antagonised a α-MSH induced increase in cyclic AMP production in transfected cells expressing the MC3 and MC4 receptors, whereas it seemed to be a partial agonist for the MC1 and MC5 receptors.
Chronic intracerebroventricularly (i.c.v.) administration of HS028 by osmotic minipumps significantly increased both food intake and body weight in a dose dependent manner without tachyphylaxis for a period of 7 days.
This is the first report demonstrating that an MC4 receptor antagonist can increase food intake and body weight during chronic administration providing further evidence that the MC4 receptor is an important mediator of long term weight homeostasis.
Keywords: Melanocortin (MC) receptor subtypes, MSH (melanocyte stimulating hormone), ligand binding, cyclic AMP, HS028, chronic i.c.v. administration, food intake
Introduction
The melanocortin (MC) receptors include five subtypes and belong to the super family of G-protein coupled receptors (Chhajlani et al., 1993; Chhajlani & Wikberg, 1992; Gantz et al., 1993a,1993b; Mountjoy et al., 1992). The MC1 receptor is well characterized and has an important role in skin and fur pigmentation in a variety of vertebrates (Cone et al., 1996). It also plays a role in immune responses (Lipton & Catania, 1997). The MC2 receptor (i.e. the ACTH (adrenocorticotropin) receptor) participates in the regulation of steroid production in the adrenal gland. The MC3 receptor is found in the brain and in placenta and gut tissues (Gantz et al., 1993a; Roselli-Refuss et al., 1993) but has still a much less defined physiological role. The MC4 receptor has only been found in the CNS, where it is expressed in several different sites, including in the cortex, thalamus, hypothalamus, brain stem and spinal cord (Gantz et al., 1993b; Mountjoy et al., 1994). Studies in a strain of mouse in which the MC4 receptor has been knocked out have related the MC4 receptor to control of weight homeostasis (Huszar et al., 1997) while work on MC5 receptor knockouts has suggested a role of this receptor in exocrine gland function (Chen et al., 1997).
The 131 amino acids long agouti protein is an antagonist at the MC receptors, including the MC3 and MC4 receptors. Ectopic over-expression of the agouti protein gives rise to an obesity syndrome. Disruption of the MC4 receptor in knockout mice caused obesity that showed similar characteristics as that found for the obesity syndrome in agouti mice (Huszar et al., 1997). Single injections of the non-selective MC3/MC4 receptor agonist MTII and the non-selective antagonist SHU9119 (Fan et al., 1997) and the selective MC4 receptor antagonist HS014 (Kask et al., 1998b) influenced food intake in experimental animals providing further evidence to the hypothesis that melanocorticergic neurones exert a tonic inhibition of feeding behaviour.
The natural melanocortic peptides (α-MSH, β-MSH, γ-MSH and ACTH) do not clearly discriminate between the different MC receptor subtypes, with the exception that α-MSH is selective for the MC1 receptor and ACTH is selective for the MC2 receptor (Schiöth et al., 1995; 1996a,1996b). Develop-ment of MC receptor antagonist and selective MC receptor active compounds for, in particular, the MC3, MC4 and MC5 receptors is still an important task as only few such substances have been forwarded.
In this study we synthesized several new cyclic MSH analogues and discovered a novel substance, HS028, which is a selective MC4 receptor antagonist showing both higher affinity and much higher selectivity for the MC4 receptor above the MC3 receptor than the earlier discovered HS014. We also report the effects of HS028 on food intake and body weight during 7 days of i.c.v. infusion in rats.
Methods
Chemicals
[Nle4, D-Phe7]α-MSH (NDP-MSH) was radio iodinated by the chloramine T method and purified by HPLC (high performance liquid chromatography). NDP-MSH and all amino acid derivatives were purchased from Neosystem, France.
Peptide synthesis
The novel peptides tested in this study were synthesized in our laboratory using the solid phase approach with subsequent purification by HPLC. The correct molecular weights of the peptides were confirmed by mass spectrometry. The peptide sequences were assembled by using Pioneer, PerSeptive Biosystems peptide synthesis system. Fmoc(9-fluorenylmethoxycarbonyl)-amino acid derivatives were used in coupling steps. When OPfp (pentafluorophenyl) esters were used the synthesis cycle was as follows: (a) the Fmoc group was removed by 20% piperidine in DMF (N,N-dimethylformamide) (5 min); (b) to form a new peptide bound side chain-protected Fmoc-amino acid OPfp ester (4 eq.) and HOAt (1-hydroxy-7-azabenzotriazole) (4 eq.) were dissolved in DMF and circulated through the column for 30–60 min; (c) to cap residual amino groups the support was treated with 0.3 M Ac2O (acetic anhydride) in DMF for 5 min. If free acids were used, then in step (b) side chain-protected Fmoc-amino acid (4 eq.), HATU (O-[7-azabenzotriazol-1-yl]1,1,3,3-tetramethyluronium hexafluorophosphate) (4 eq.) and DIEA (N,N-diisopropylethylamine) (4 eq.) were applied. For deprotection a reagent mixture (trifluoroacetic acid) – phenol – anisole – 1,2-ethanedithiol – water, 82 : 2 : 2 : 2 : 2, 2.5 h) was used. The raw peptides formed were purified by HPLC (10×250 mm column, Vydac RP C18, 90A, 201HS1010, eluent – 20–35% MeCN (acetonitrile) in water+0.1% trifluoroacetic acid, detection at 240 nm).
Expression of receptor clones
The human MC1 and human MC5 receptors (Chhajlani & Wikberg 1992; Chhajlani et al., 1993) were cloned into the expression vector pRc/CMV (InVitrogen). The human MC3 and human MC4 receptors, cloned into the expression vector pCMV/neo, were a gift from Dr Ira Gantz (Gantz et al., 1993a,1993b). For receptor expression COS-1 (CV-1 Origin, SV40) cells were grown in Dulbecco's modified Eagles medium with 10% foetal calf serum. Eighty per cent confluent cultures were transfected with the DNA (approximately 1 μg DNA for every 1×106 cells) mixed with liposomes in serum free medium (for details see Schiöth et al., 1996b). The number of receptors, using this amount of DNA was about 4,000–20,000 binding sites/cell for all of the receptor types derived from the Bmax of saturation binding studies which were performed as earlier reported (Schiöth et al., 1995). After transfection, the serum-free medium was replaced by serum-containing medium and the cells were cultivated for about 48 h. Cells were then scraped off, centrifuged, and used for radioligand binding.
Binding studies
The transfected cells were washed with binding buffer (see Schiöth et al., 1995) and distributed into 96-well non-culture-coated plates, which were centrifuged and the binding buffer was removed. The cells were then immediately incubated in the well plates for 2 h at 37°C with 0.05 ml binding buffer in each well containing a constant concentration of [125I]-NDP-MSH and appropriate concentrations of the competing unlabelled ligand. After incubation the cells were washed with 0.2 ml of ice-cold binding buffer and detached from the plates with 0.2 ml of 0.1 N NaOH. Radioactivity was counted (Wallac, Wizard automatic gamma counter) and data were analysed with a software package suitable for radioligand binding data analysis (Wan System AB, Umeå, Sweden). Data were analysed by fitting to formulas derived from the law of mass action by the method generally referred to as computer modelling. The Kd values for [125I]-NDP-MSH for the MC receptors were taken from Schiöth et al., 1995; 1996b. The binding assays were performed in duplicate wells and repeated three times. Untransfected COS-1 cells did not show any specific binding for [125I]NDP-MSH. The non-specific binding was generally low, typically being less than 5% of the total binding at 2 nM concentration of labelled NDP-MSH and 3 μM cold NDP-MSH, measured by saturation analysis which were performed as earlier reported (Schiöth et al., 1995).
cyclic AMP assay
The transfected cells were harvested and incubated for 30 min at 37°C with 0.05 ml serum free Dulbecco's modified Eagles medium in each tube, containing 0.5 mM IBMX (isobutylmethylxantine) and appropriate concentrations of α-MSH or HS028. After incubation with the indicated drugs, cyclic AMP (adenosine 3′:5′-cyclic monophosphate) was extracted with perchloric acid at final concentration 0.4 M. After centrifugation, the protein free supernatants were neutralised with 5 M KOH/1 M Tris (tris-(hydroxymethyl)aminomethane). 0.05 ml of the neutralised cyclic AMP extract or a cyclic AMP standard (dissolved in distilled water) was added to a 96 well microtiter plate. The content of cyclic AMP was then estimated essentially according to Nordstedt & Fredholm (1990), by adding to each well [3H]-cyclic AMP (0.14 pmol, approximately 11,000 c.p.m., specific activity 54 Ci/mmol−1, Amersham) and bovine adrenal binding protein and incubating at 4°C for 150 min. Standards containing non-labelled cyclic AMP were also assayed concomitantly with the samples. The standard curve ranged from 0.125–8.0 pmol/sample. Samples from each experiment were always assayed with the corresponding standard curve, using the same labelled cyclic AMP and binding protein solutions diluted from stocks directly before use. The incubates were thereafter harvested by filtration on Whatman GF/B filters using a semiautomatic Brandel cell harvester. Each filter was rinsed with 3 ml 50 mM Tris/HCl pH 7.4. The filters were punched out and put into scintillation vials with scintillation fluid and counted. The cyclic AMP assays were performed in duplicate wells and repeated three times. Both inter and intra-assay coefficients of variation were below 25%.
Drug treatment, food consumption and body weight
Thirty-four male Wistar rats weighing 200–300 g were fed a standard, commercial laboratory diet (rat and mouse main-tenance diet No. 1, Special Diets Services, Essex, U.K.) and water ad libitum under standard conditions using a 12 : 12-h light-dark cycle. One-week before the administration of drugs by i.c.v. infusion, the rats were housed individually in plastic cages. On the day the operation treatment started, the rats were assigned randomly to one of the following six treatment groups: (a) 1.0 μL h−1 i.c.v. vehicle (buffered artificial cerebrospinal fluid, ACSF, control group; n=6); (b) 0.00007 nmol h−1 i.c.v. HS028 (n=3); (c) 0.0007 nmol i.c.v. HS028 h−1 (n=6); (d) 0.007 nmol h−1 i.c.v. HS028 (n=6); (e) 0.07 nmol h−1 i.c.v. HS028 (n=5); (f) 0.7 nmol h−1 i.c.v. HS028 (n=4). An additional group received 0.1 nmol h−1 i.c.v. HS014 (n=4). HS028 and HS014 were dissolved in ACSF. Prior to the infusions, a brain infusion kit connected to an osmotic minipump (Alzet, 2001, Alza corp., Palo Alto, CA, U.S.A.) was implanted into the right lateral cerebral ventricle (1.0 mm posterior and 1.5 mm lateral to the bregma in rats anaesthetized with methohexital sodium (50 mg kg−1 i.p. Brietal®, Lilly, Indianapolis, IN, U.S.A.). Dental glas ionophor (ChemFil(R) Superior, Dentsply DeTray GmbH, D-78467 Konstanz, Germany) and a stainless steel screw were used to secure the infusion cannula in position. The osmotic minipumps were placed s.c. in the midscapular region. The osmotic minipumps were activated by immersion in 0.9% saline at room temperature 12 h before the implantation, so that the infusion started immediately upon implantation of the pumps. The room temperature was monitored at 22°±1°C and the humidity 50±5%. Food intake and body weight were recorded in the first 3 h of light phase every other day for 1 week prior to the implantation of the infusion device and for the 1-week infusion period. After the 7 days infusion, the animals were sacrificed and injected with a small amount of a dye through the infusion kit where after the brains were dissected and positive staining of the cerebral ventricles verified.
Statistical analysis
The average 24-h body weight gain and food intake for each animal was measured during the last 4 days before the operation (control period), as well as during the last 5 days of the 7 days infusion period. These values were then subtracted to obtain each animals changes in weight gain and food intake from the control period to the infusion period. In addition, each animals initial weight was defined as its weight 24 h before the first control measurement. The differences in weight gain and food intake from the control period to the infusion period were then analysed with analysis of covariance using the animals initial weight as a covariate (JMP version 3.02, SAS Institute Inc., Cary, NC, U.S.A.) calculating the least squares means for the change in each parameter for each infusion protocol. Additionally, specific preplanned contrasts were applied comparing each dose of HS028 to ACSF as well as 0.7 nmol h−1 HS028 to 0.0007 nmol h−1 HS028, utilizing Bonferroni corrections of P values keeping the error rate less than 0.05.
Animal ethics
Experimental procedures were carried out in accordance with guidelines of the European Community, local laws and policies.
Results
We designed and synthesized several new cyclic MSH analogues. The structure of the novel substances are aligned with α-MSH, NDP-MSH, MTII, SHU9119 and HS014 in Figure 1. The human DNAs for the MC1, MC3, MC4 and MC5 receptors were transiently and independently expressed in COS-1 cells for competitive receptor binding assays using [125I]-NDP-MSH as radioligand. The expression levels of the different receptor subtypes were similar (data not shown). The Ki values for the different peptides resulting from calculations of the competition curves of binding with [125I]-NDP-MSH are summarized in Table 1. For comparison, we also included in Table 1 the Ki values of α-MSH, NDP-MSH, MTII, SHU9119 and HS014, which values we recently reported (Schiöth et al., 1997b; 1998a) using the same method as in the present study.
Figure 1.

Alignment of α-MSH, NDP-MSH, MTII, SHU9119 and HS014 to the new analogues evaluated in this study. All peptides have an acetyl-group on the N-terminus and an amide group on the C-terminus. The amino acid residues which make up the ring closure in the cyclic compounds are shown underlined in italics.
Table 1.
Ki values (means±s.e.mean) obtained from competition curves, for MSH analogues on MC1, MC3, MC4 and MC5 receptor transfected COS cells

In our recent study (Schiöth et al., 1998a), we generated substances with a disulphide bridge between Cys residues in positions 4 and 11 and found them to be clearly selective for the MC4 receptor. In this study, we made several modifications on this basic ring structure (represented in HS964) as well as the previously most potent and MC4 receptor selective substance HS014 which has the same ring structure as HS964, but it is distinguished from HS964 by a C-terminal tail mimicking the C-terminal of β-MSH. We made three substances where we changed amino acids in position 6, thus in HS015, HS017 and HS023 we replaced the basic hydrophilic His6 by the non-polar and hydrophobic residue Pro6 (HS015), the polar hydrophilic residue Glu6 (HS017) and the small non-polar residue Gly6 (HS023), respectively. For HS015 (Pro6), this change cancelled out the selective character as compared with HS964. For HS017 (Glu6) the affinities were substantially reduced for all the receptor subtypes even though the MC4 receptor selective binding properties were maintained. For HS023 (Gly6) the affinities were lowered for all the receptor subtypes, except for the MC1 receptor. This peptide had about 20 fold selectivity for the MC4 receptor beyond the MC3 receptor which is similar to that of HS014. One of the peptides (HS007) originating from our earlier study (Schiöth et al., 1998a) has Arg in position 5. This peptide displayed selective character for the MC3 receptor. We here synthesized an analogue of HS007, termed HS016, having a C-terminal tail (Asp12-Arg13-Phe14) being identical to the C-terminal sequence of γ-MSH, which is a natural peptide shown to have preference for the MC3 receptor (Schiöth et al., 1996b). However, HS016 is virtually non-selective, showing equal affinities for the MC3 and MC4 receptors, and only slightly lower affinities for the MC1 and MC5 receptors. Recently, we introduced this very same sequence in NDP-MSH, also without leading to enhancement of the affinity or selectivity for the MC3 receptor (Schiöth et al., 1998b). We also made another peptide, also having a 26 membered ring as HS964, where the Gly10 was removed, and a Gly was inserted in position 6 generating a distance between the His6 and the D-Nal7-Arg8-Trp9 motifs in the peptide. This change totally abolished the binding to the MC1, MC3 and MC5 receptors, whereas the binding to the MC4 receptor was only lowered 26 fold compared with HS964. We also made four analogues based on HS014. HS019 has an N-terminal tail (Pro1-Tyr2-Arg3), which is identical to the amino acids of the corresponding positions in the β-MSH peptide. HS019 has very similar affinity for all the receptors as HS014, except that the affinity for the MC5 receptor was increased 13 fold. Moreover, we made three replacements of the D-Nal7 with three different bulky hydrophobic residues with D-conformation showing resemblance with D-Nal. The replacements of D-Nal7 by 4-parafluoro-D-phenylalanine (pF-D-Phe7) (HS029) and 4-paranitro-D-phenylalanine (pNO2-D-Phe7) (HS030) lowered the affinity for all the receptor subtypes for both the peptides.
However, when D-Nal7 was replaced by 3,4,-dichloro-D-phenylalanine (dCl-D-Phe7) (HS028), the affinity was increased for the MC4 receptor, and lowered for the MC3 receptor compared with HS014. HS028 has 78 fold selec-tivity for the MC4 receptor beyond the MC3 receptor. This is a considerably higher selectivity compared to that of HS014, which shows 17 fold affinity difference between these same receptors. The primary structure of HS028 is shown in Figure 2. Competition binding curves of HS028 for the human MC1, MC3, MC4 and MC5 receptors are shown in Figure 3.
Figure 2.
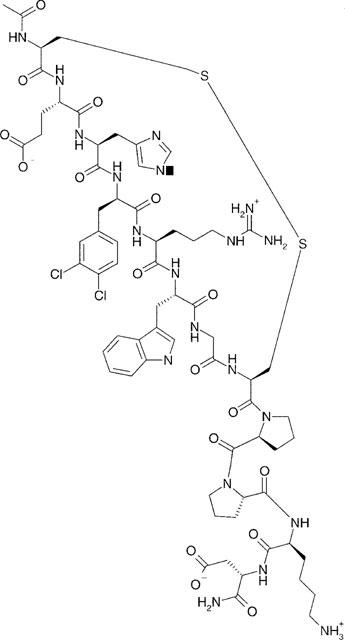
Primary structure of the MC4 receptor selective analogue; HS028.
Figure 3.

Binding data showing high selectivity of HS028 for the MC4 receptor beyond the other MC receptors. Competition curves of HS028 obtained on COS-1 cells transfected with the MC receptor clones, obtained by using a fixed concentration of [125I]-NDP-MSH and varying concentrations of the non-labelled competing peptide. Competing peptides used are indicated on abscissa for each panel. Each experiment were performed in duplicates and repeated three times.
On the basis of these results we selected HS028 for further testing and investigated the cyclic AMP response of α-MSH and HS028 in COS-1 cells expressing the human MC1, MC3, MC4 and MC5 receptors. These results are shown in Figure 4. As can be seen from the figure, α-MSH stimulated the accumulation of cyclic AMP in all the cell types. COS-1 cells which had not been transfected with any of the MC receptors did not respond to α-MSH (data not shown). HS028 did stimulate accumulation of cyclic AMP in the MC1 and MC5 receptor expressing cells but without the cyclic AMP reaching the same levels as for α-MSH. For the MC3 receptor, HS028 in concentrations up to 100 μM did not affect the cyclic AMP levels. Instead, 0.1 μM HS028 was found to completely block the cyclic AMP increase induced by α-MSH. For the MC4 receptor, HS028 blocked the α-MSH response while HS028 on itself did not affect cyclic AMP at least until a dose of 50 nM. At this dose and higher, we observed up to 2 fold increase in cyclic AMP for both HS028 itself and HS028 in combination with α-MSH.
Figure 4.
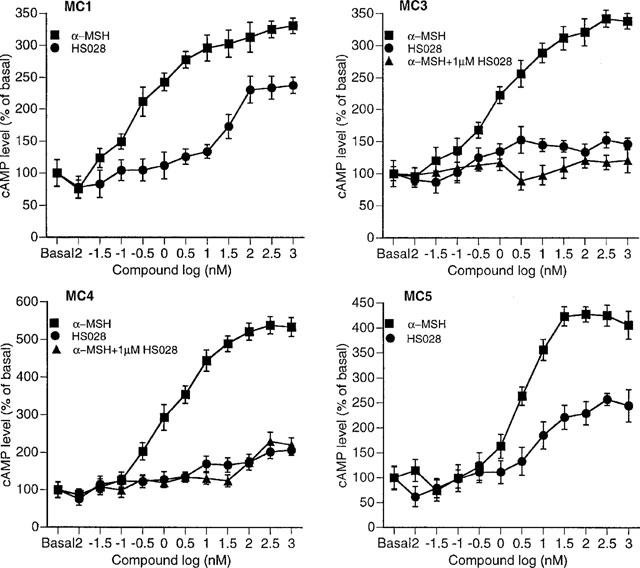
Generation of cyclic AMP in MC receptor transfected COS-1 cells. Each point represents the average±s.e.mean (n=6).
We used osmotic minipumps for continuous 7 days infusion of HS028 in free feeding rats. Figure 5 shows the body weight changes, relative to that on the operation day, during the 7 days infusion of vehicle and HS028. In all the experimental groups, including the controls, the weight of the animals decreased during the first 48 h after the start of infusion, but then the animals started to gain weight and continued to do so until the end of the infusion (Figure 5). At the end of the treatment period, the relative body weight increase was highest for the groups infused 0.007 and 0.07 nmol h−1 doses of HS028. Figure 6 shows the 24-h average change in food intake (A) and body weight gain (B) during 7 days of chronic infusion of either vehicle (ACSF), HS028 or HS014 compared with that of the baseline within each group. During 7 days of chronic infusion of either vehicle, 0.00007, 0.0007, 0.007 nmol h−1 HS028 or 0.1 nmol h−1 HS014 the 24-h food intake and body weight gain were reduced relative to that of the baseline for each group (Figure 6). HS028 at a dose of 0.07 nmol h−1 was found to significantly (P<0.05) increase the food consumption compared to the vehicle treated animals (Figure 6A). Both the doses 0.07 and 0.7 nmol h−1 of HS028 increased significantly (P<0.05) the 24-h body weight gain during 1-week chronic infusion compared with that of the vehicle (Figure 6B).
Figure 5.

The relative average body weight (%) for each group during 1-week chronic infusion of artificial cerebrospinal fluid (ACSF) or HS028 in doses 0.00007–0.7 nmol h−1. The body weight on the operation day (0) was set as 100%.
Figure 6.

24-h average change in food intake (A) and in body weight increase (B) during 1-week chronic infusion of artificial cerebrospinal fluid (ACSF), HS028 or HS014 relative to baseline period within each dose. Values are least squares means±s.e.mean during days 3 to 7 of infusion period. *P<0.05, Bonferroni corrections for multiple comparisons.
Discussion and conclusion
In a recent study we discovered that cyclic disulphide bridged MSH peptides, with 26 membered ring structure that includes the bulky hydrophobic amino acid D-Nal7, are selective for the MC4 receptor. Earlier it has been found that cyclic disulphide MSH analogues are highly stable and effective in skin tanning assays relating to the MC1 receptor (for review see Hruby et al., 1993). Discovery of the lactam analogues MTII and SHU9119 showed that replacement of D-Phe7 by D-Nal7 (in SHU9119) resulted in antagonism at the MC3 and MC4 receptors (Al Obeidi et al., 1989; Hruby et al., 1995). However, MTII is selective for the MC1 receptor and SHU9119 is virtually non-selective for all the MC receptor subtypes (Hruby et al., 1995; Schiöth et al., 1997b). A bulky hydrophobic amino acid like D-Nal7 and pI-Phe7 in position 7 gives compounds with antagonism at the MC3 and MC4 receptors and contribute also to increase the relative binding affinity for the MC4 receptor (Hruby et al., 1995; Adan et al., 1994; Schiöth et al., 1997a,1997b). This increase in binding affintity for the MC4 receptor by D-Nal7 has also recently been shown to exist for linear MSH analogues (Schiöth et al., 1998b). We speculated in our earlier report that His6 in the 26 membered cyclic ring structure might not take the same conformation as it may in the linear analogues, and that this might be the reason for the comparatively low affinity of the 26 membered ring peptides for the MC1 receptors. In our present study we elaborated further on position 6. Our present results show that none of our replacements in this position increased the affinity or the selectivity for the MC4 receptor. However, looking at the data for HS018 where the His6 was detached for the main pharmacophore (D-Nal7-Arg8-Trp9) within a 26 membered ring structure, the MC4 receptor selective property was maintained (or even increased) even though the all-over affinities were lowered. This may support the hypothesis that His6 in these cyclic peptides may not be as important for the MC4 receptor as it may be for the other subtypes.
Addition of a ‘β-MSH N-terminal' tail to HS964, giving HS014, both increased the affinity and the selectivity for the MC4 receptor. Addition of a ‘γ-MSH N-terminal tail' to our earlier MC3 receptor selective lead HS007, did not result in increased selective properties. Nor did insertion of ‘γ-MSH N-terminal tail' to the linear NDP-MSH peptide result in increase of MC3 receptor selective binding property as reported by our recent study (Schiöth et al., 1998b). It seems thus that fitting of a ‘γ-MSH N-terminal' to a specific MC3 receptor binding pocket is not as compatible as the fitting of ‘β-MSH N-terminal' tail into a MC4 receptor binding cavity which turned out to be useful when we designed HS014.
As can be deduced from earlier data, position 7 is a key position both for the selectivity and antagonism at the MC4 receptor. Our data show that replacement of D-Nal7, with three other un-natural bulky hydrophobic phenylalanine derivatives, had considerable impact on the affinity profiles even though all of the peptides made were selective for the MC4 receptor. HS028 with two chlorine atoms attached to the phenylalanine (dCl-D-Phe7) turned out to be highly potent, showing about 3 fold higher affinity than HS014. However, perhaps more importantly, it shows about 80 fold selectivity for the MC4 receptor above the MC3 receptor which is the MC receptor which is most abundantly expressed in the brain besides the MC4 receptor. We selected HS028 for further investigations. Our data on the effect of HS028 on functional coupling to intracellular cyclic AMP production indicate that HS028 is a partial antagonist at the MC1 and MC5 receptors and a potent antagonist at the MC3 and MC4 receptors. This is similar to our findings with HS014. However, at high doses HS028 showed partial agonistic activity at the MC4 receptor. Earlier lactam analogues of MSH, of which several were substituted in position 7 by bulky hydrophobic residues, displayed also both agonist and antagonistic activity at different doses (Hruby et al., 1995).
As HS028 is the most selective MC4 receptor antagonist available and there have not yet been any investigations of the long term i.c.u. administration of MSH peptides, we used HS028 to probe the chronic effects of i.c.u. infusion of a MC4 receptor antagonist. Our data show that the animals in all groups, including the controls, lose their body weight over the first 48 h after the brain operation and osmotic pump installation (Figure 5). This observation can be explained by a long-lasting effect of stress experienced by animals in association with the brain operation and osmotic pump installation (Levine et al., 1980). From day 2 the animals start gaining weight again. At day 7 of infusion the weight gain was significantly increased beyond the level of the control for the groups receiving the highest doses of HS028 (0.07 and 0.7 nmol h−1) (Figure 6B). Our data thus show that a chronic administration of a selective MC4 receptor antagonist increases food intake with concomitant increase in body weight without inducing any signs of tachyphylaxis. The orexigenic effects of HS028 are conceivably due to its antagonistic action on the MC4 receptor.
There are only few reports about orexigenic effects of MC4 receptor antagonists (Fan et al., 1997; Seeley et al., 1997; Kask et al., 1998a,1998b). It is interesting in this context that it has been observed that a single injection of the non-selective MC4 antagonist SHU9119 stimulated only nocturnal feeding in mice fed ad libitum (Fan et al., 1997). However, the MC4 receptor selective HS014 was highly effective in inducing food intake during daytime in rats (Kask et al., 1998a,1998b). We did not study the circadian feeding pattern during the long term administration of HS028. Keeping in mind that rats are nocturnal animals and that most food consumption in unstressed as well as in stressed rats occurs during the dark period of the lighting cycle (Marti et al., 1996), the effects of HS028 on feeding behaviour in our study are likely to take place during the dark period of the lighting cycle.
In summary, we have developed a novel high affinity and high selective MC4 receptor antagonist which may become an useful tool to investigate the physiology of the MC4 receptor. Moreover, we have also shown, for the first time, that chronic injection of a MC4 receptor antagonist increases food intake and body weight.
Acknowledgments
This study was supported by grants from the Swedish MRC (04X-05957), the Swedish CFN, the Groschinsky, Bergwall, and Wiberg foundations, the Royal Swedish Academy of Science, the Howard Hughes Medical Institute (HHMI 75195-548501) and the Swedish Society for Medical Research.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- ACTH
adrenocorticotropin
- cAMP
adenosine 3′:5′-cyclic monophosphate
- SV40
CV-1 Origin
- COS-1
DIEA, N,N-diisopropylethylamine
- DMF
N,N-dimethylformamide
- Fmoc
9-fluorenylmethoxycarbonyl
- HATU
O-[7-azabenzotriazol-1-yl]1,1,3,3-tetramethyluronium hexafluorophosphate pentafluorophenyl
- HOAt
OPfp-hydroxy-7-azabenzotriazole
- HPLC
high performance liquid chromatography
- MC
melanocortin
- MeCN
acetonitrile
- MSH
melanocyte stimulating hormone
- NDP-MSH
[Nle4, D-Phe7]α-MSH
- IBMX
isobutylmethylxantine
- Tris
tris-(hydroxymethyl)aminomethane
References
- ADAN R.A.H., OOSTEROM J., LUDVIGSDOTTIR G., BRAKKEE J.H., BURBACH J.P.H., GISPEN W.H. Identification of antagonists for melanocortin MC3, MC4 and MC5 receptors. Eur. J. Pharmacol. 1994;269:331–337. doi: 10.1016/0922-4106(94)90041-8. [DOI] [PubMed] [Google Scholar]
- AL-OBEIDI F., CASTRUCCI A.L., HADLEY M.E., HRUBY V.J. Potent and prolonged acting cyclic lactam analogues of α-melanotropin: Design based on molecular dynamics. J. Med. Chem. 1989;32:2555–2561. doi: 10.1021/jm00132a010. [DOI] [PubMed] [Google Scholar]
- CHEN W, , KELLY MA, , PITZ-ARAYA X, , THOMAS RE, , LOW MJ, , CONE RD. Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell. 1997;91:789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- CHHAJLANI V., MUCENIECE R., WIKBERG J.E.S. Molecular cloning of a novel human melanocortin receptor. Biochem. Biophys. Res. Commun. 1993;195:866–873. doi: 10.1006/bbrc.1993.2125. [DOI] [PubMed] [Google Scholar]
- CHHAJLANI V., WIKBERG J.E.S. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. FEBS Lett. 1992;309:417–420. doi: 10.1016/0014-5793(92)80820-7. [DOI] [PubMed] [Google Scholar]
- CONE R.D., LU D., KOPPULA S., VAGE D.I., KLUNGLAND H., BOSTON B., CHEN W., ORTH D.N., POUTON C., KESTERSON R.A. The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent. Prog. Horm. Res. 1996;51:287–317. [PubMed] [Google Scholar]
- FAN W., BOSTON B.A., KESTERSON R.A., HRUBY V.J., CONE R.D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- GANTZ I., KONDA Y., TASHIRO T., SHIMOTO Y., MIWA H., MUNZERT G., WATSON S.J., DEL VALLE J., YAMADA T. Molecular cloning of a novel melanocortin receptor. J. Biol. Chem. 1993a;268:8246–8250. [PubMed] [Google Scholar]
- GANTZ I., MIWA H., KONDA Y., SHIMOTO Y., TASHIRO T., WATSON S.J., DEL VALLE V., YAMADA T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J. Biol. Chem. 1993b;268:15174–15179. [PubMed] [Google Scholar]
- HUSZAR D., LYNCH C.A., FAIRCHILD-HUNTRESS V., DUNMORE J.H., FANG Q., BERKEMEYER L.R., GU W., KESTERSON R.A., BOSTON B.A., CONE R.D., SMITH F.J., CAMPFIELD L.A. , BURN P., LEE F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- HRUBY V.J., LU D., SHARMA S.D., CASTRUCCI A.L., KESTERSON R.A., AL-OBEIDI F.A., HADLEY M.E., CONE R.D. Cyclic lactam α-melanotropin analogues of Ac-Nle4-cyclo(Asp5, D-Phe7, Lys10)α-melanocyte-stimulating hormone-(4-10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J. Med. Chem. 1995;38:3454–3461. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- HRUBY V.J., SHARMA S.D., TOTH K., JAW J.Y., AL-OBEIDI F., SAWYER T.K., HADLEY M.E. Design, synthesis, and conformation of superpotent and prolonged acting melanotropins. Ann. N.Y. Acad. Sci. 1993;680:51–63. doi: 10.1111/j.1749-6632.1993.tb19674.x. [DOI] [PubMed] [Google Scholar]
- KASK A., RÄGO L. , KORROVITS P., WIKBERG J.E.S., SCHIÖTH H.B. Evidence that the orexigenic effects of melanocortin 4 receptor antagonist are mediated by NPY. Biochem. Biophys. Res. Commun. 1998a;248:245–249. doi: 10.1006/bbrc.1998.8961. [DOI] [PubMed] [Google Scholar]
- KASK A., RÄGO L., MUTULIS F., WIKBERG J.E.S., SCHIÖTH H.B. Selective melanocortin 4 receptor antagonist (HS014) increases food intake in free-feeding rats. Biochem. Biophys. Res. Commun. 1998b;245:90–93. doi: 10.1006/bbrc.1998.8389. [DOI] [PubMed] [Google Scholar]
- LEVINE R.L., MCINTOSH T.K, , LOTHROP D.A., JACKSON B.T. Circadian periodicity of plasma corticosterone levels in rats subjected to hemorrhagic shock and surgical trauma. Horm. Res. 1980;13:385–395. doi: 10.1159/000179306. [DOI] [PubMed] [Google Scholar]
- LIPTON J.M., CATANIA A. Anti-inflammatory actions of the neuroimmunomodulator alpha-MSH. Immunol. Today. 1997;18:140–145. doi: 10.1016/s0167-5699(97)01009-8. [DOI] [PubMed] [Google Scholar]
- MARTI O., GAVALDA A., JOLIN T., ARMARIO A. Acute stress attenuates but does not abolish circadian rhythmicity of serum thyrotrophin and growth hormone in the rat. Eur. J. Endocrinol. 1996;135:703–708. doi: 10.1530/eje.0.1350703. [DOI] [PubMed] [Google Scholar]
- MOUNTJOY K.G., MORTRUD M.T., LOW M.J., SIMERLY R.B., CONE R.D. Localization of the melanocortin-4 receptor (MC4-R) in endocrine and autonomic control circuits in the brain. Molecular Endocrinology. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- MOUNTJOY K.G., ROBBINS L.S., MORTRUD M.T., CONE R.D. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- NORDSTEDT C., FREDHOLM B.B. A modification of a protein-binding method for rapid quantification of cAMP in cell-culture supernatants and body fluid. Analytical Chemistry. 1990;189:231–234. doi: 10.1016/0003-2697(90)90113-n. [DOI] [PubMed] [Google Scholar]
- ROSELLI-REHFUSS L., MOUNTJOY K.G., ROBBINS L.S., MORTRUD M.T., LOW M.J., TARTO J.B., ENTWISTLE M.L., SIMERLY R.B., CONE R.D. Identification of a receptor for γ melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIÖTH H.B., CHHAJLANI V., MUCENIECE R., KLUSA V., WIKBERG J.E.S. Major pharmacological distinction of the ACTH receptor from the other melanocortic receptors. Life Sciences. 1996a;59:797–801. doi: 10.1016/0024-3205(96)00370-0. [DOI] [PubMed] [Google Scholar]
- SCHIÖTH H.B., MUCENIECE R., LARSSON M., MUTULIS F., SZARDENINGS M., PRUSIS P., LINDEBERG G., WIKBERG J.E.S. Selectivety of cyclic [D-Phe7] and [D-Nal7] substituted MSH analogues for the melanocortin receptors. Peptides. 1997b;18:1009–1013. doi: 10.1016/s0196-9781(97)00079-x. [DOI] [PubMed] [Google Scholar]
- SCHIÖTH H.B., MUCENIECE R., WIKBERG J.E.S., CHHAJLANI V. Characterisation of melanocortin receptor subtypes by radioligand binding analysis. Eur. J. Pharmacol., Mol. Pharm. Sect. 1995;288:311–317. doi: 10.1016/0922-4106(95)90043-8. [DOI] [PubMed] [Google Scholar]
- SCHIÖTH H.B., MUCENIECE R., WIKBERG J.E.S. Characterisation of melanocortin 4 receptor by radioligand binding analysis. Pharmacology & Toxicology. 1996b;79:161–165. doi: 10.1111/j.1600-0773.1996.tb00261.x. [DOI] [PubMed] [Google Scholar]
- SCHIÖTH H.B., MUCENIECE R., WIKBERG J.E.S. Selectivety of [Phe-I7], [Ala6] and [D-Ala4, Gln5,Tyr6] substituted ACTH(4–10) analogues for the melanocortin receptors. Peptides. 1997a;18:761–763. doi: 10.1016/s0196-9781(97)00126-5. [DOI] [PubMed] [Google Scholar]
- SCHIÖTH H.B., MUTULIS F., MUCENIECE R., PRUSIS P., WIKBERG J.E.S. Discovery of novel melanocortin 4 receptor selective MSH analogues. Br. J. Pharmacol. 1998a;124:75–82. doi: 10.1038/sj.bjp.0701804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIÖTH H.B., MUTULIS F., MUCENIECE R., PRUSIS P., WIKBERG J.E.S. Selective properties of C- and N-terminals and core residues in the MSH peptide for the melanocortin receptor subtypes. Eur. J. Pharmacol. 1998b;349:359–366. doi: 10.1016/s0014-2999(98)00212-x. [DOI] [PubMed] [Google Scholar]
- SEELEY RJ, , YAGALOFF KA, , FISHER SL, , BURN P, , THIELE TE, , VAN DIJK G, , BASKIN DG, , SCHWARTZ MW. Melanocortin receptors in leptin effects. Nature. 1997;390:349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]


